ABSTRACT
Heavy metal contamination has severe impacts on the natural environment. The currently existing physico-chemical methods have certain limitations, restricting their wide-scale application. The use of biological agents like bacteria, algae, and fungi can help eliminate heavy metals without adversely affecting flora and fauna. Due to their inherent ability to withstand adverse environmental conditions, nowadays, mycoremediation approaches are receiving considerable attention for heavy metal removal from contaminated sites. In this review, we emphasised the role of white rot fungi in remediation of heavy metal along with different factors influencing biosorption, effects on exposed fungi, and the mechanisms involved. Bibliometric analysis tools have been applied to literature search and trend analysis of the research on white rot fungi-mediated heavy metal removal. Annual growth rates and average citations per document are 5.08% and 35.48, respectively. Phanerochaete chrysosporium, Pleurotus ostreatus, and Trametes versicolor have been widely explored for the remediation of heavy metals. In addition to providing some prospects, the review also highlighted a few limitations, including inconsistent removal and effects of environmental factors influencing the functioning of white rot fungi. Overall, white rot fungi have been found to have immense potential to be widely utilised for sustainable remediation of heavy metal-contaminated environments.
KEYWORDS: Adsorption, biomass, bioremediation, environmental hazard, non-target organisms
1. Introduction
Continuously rising human population and industrial development has led to massive wastewater production, causing deterioration of soil, water, and atmosphere (Ahmed et al. 2021). The wastewater originating from agrochemical, electroplating, mining operations and ore processing, plastics, textiles, fertiliser, and pesticide industries are laden with different non-biodegradable heavy metals having toxicity, carcinogenicity, and reactivity (Santos et al. 2021; Ab Rhaman et al. 2021; Xiong et al. 2023; Waqas and Ahmad 2024). In addition, several geogenic factors also contribute to the release of heavy metals in different ecosystems (Rajan and Nandimandalam 2024). Different heavy metals of environmental concern are lead, nickel, iron, zinc, cobalt, copper, chromium, cadmium, arsenic, and manganese (Singh and Singh 2018; Al-Huqail and El-Bondkly 2022; El-Bondkly and El-Gendy 2022; Razzak et al. 2022). After being released into the environment, heavy metals have long been recognised to exert undesirable effects on human health, plants, and microbial communities. Thus, the need to develop an efficient technology for their successful elimination from contaminated sites is evident (Abd Elnabi et al. 2023; Ghuge et al. 2023; Kou et al. 2023).
Different approaches in the physical, chemical, and biological categories have been demonstrated to significantly remove heavy metals from contaminated sites (Topare and Wadgaonkar 2023). Physical methods of removal involve the application of processes like flotation, membrane filtration, and sedimentation (Xiang et al. 2022; Peyravi and Rezaei 2023; Schlebusch et al. 2023), whereas chemical methods employ the process of ion exchange, solvent extraction, precipitation, coagulation, and adsorption (Lee et al. 2023; Lin et al. 2023; Skotta et al. 2023). Since physico-chemical methods produce large amounts of secondary sludge, require strict operating conditions, are expensive, not ecologically sound, and suffer from the limitations of safe disposal, biological agent-based removal of heavy metal is regarded as a promising approach (Rastegari et al. 2019). The biological method of heavy metal removal is based on the application of live as well as dead plants, bacteria, algae, and fungi (Ahmad et al. 2023; Chen et al. 2023; Maity et al. 2023; Paranjape and Sadgir 2023; Sharma et al. 2023). In contrast to other microorganisms, fungi (especially white rot fungi) are considerably remarkable agents for the removal of heavy metals because of the synthesis of chitin in their cell walls (Tamjidi et al. 2023), degradation of lignin and other chemically similar organic contaminants, dependence on copper and manganese during biodegradation (Baldrian 2003), easy growth on lignocellulose-based substrates (Sharma et al. 2020), suggesting potential role in waste valorisation (Dhiman et al. 2024), and characteristic nature of membrane containing phospholipid, sterol, and protein (Ayele et al. 2021). Also, different functional groups such as PO43– (phosphate), –NH2 (amino), and –OH (hydroxyl) in these fungal cell walls contribute substantially to heavy metal sequestration (Racić et al. 2023).
Recent years have witnessed the attention of researchers towards the application of white rot fungi for the removal of heavy metals with considerable success due to ease in cultivation and high biomass produced on a wide range of substrates, enhanced surface area-to-volume ratio, and most strikingly the presence of polymers supporting the binding of metals (Baldrian 2003). The white rot fungi consisting of basidiomycetes members are represented by genera including Ganoderma lucidum, Irpex lacteus, Phanerochaete chrysosporium, Phlebia brevispora, Pleurotus ostreatus, Polyporus versicolor, Stropharia rugosoannulata, and Trametes versicolor (Yetis et al. 1998; Sharma et al. 2020; Alshiekheid et al. 2023; Tan et al. 2023). The essential mechanisms of heavy metal removal by fungi include biosorption, synthesis of surfactants, mineralisation, and precipitate formation, in addition to varied extracellular and intracellular enzymatic processes (Figure 1; Ghosh et al. 2023). The removal of heavy metals by white rot fungi is influenced by many factors including pH, time, fungal strains, nutrient composition, abundance of oxygen, temperature, biomass, initial concentration of heavy metals in a given environment, organic compounds, and availability of competing ions in the medium (Sing and Yu 1998; Bayramoğlu et al. 2003; Hanif and Bhatti 2015; Noormohamadi et al. 2019; Latif et al. 2023). However, the microorganisms face several challenges that hamper their bioremediation potential of heavy metals. Therefore, there is a need to collate the information on white rot fungi-mediated remediation of heavy metals from contaminated aqueous and terrestrial ecosystems.
Figure 1.
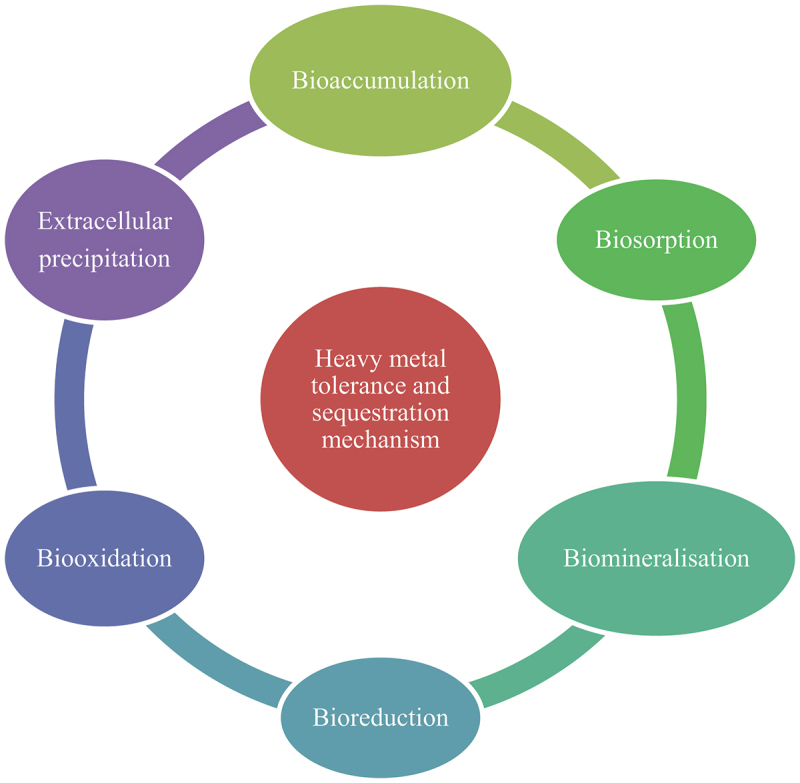
An overview of important mechanisms responsible for the heavy metal removal by white rot fungi.
A systematic review covering the information related to white rot fungi use in sustainable remediation of heavy metals in the knowledge of authors is lacking. Therefore, the objective of the present review was to collate the information and discuss the emerging potential of using different white rot fungi to remove heavy metals and other relevant contaminants. Moreover, emphasis has been given to exploring the mechanisms involved in the remediation of heavy metals, such as sorption, precipitation, accumulation, and the effect of environmental factors on heavy metal-induced changes, apart from the possibilities and associated limitations from the current perspective. For a systematic literature search and presentation of the research trends, bibliometric analysis tools were also used to carry out this review.
2. Methodology: bibliometric analysis
To observe the trends in research concerned with heavy metal removal using white rot fungi during the last three decades (1995–2024), we performed a bibliometric analysis using the search query “white rot fungi” AND “heavy metal” in the “Web of Science Core Collection” and “Scopus” databases by following Singh et al. (2023). This analysis helps overview the research trends and find possible gaps for future research in particular areas. The “bibliometrix” package in R (ver. 4.1.0) was used for the bibliometric analysis (Aria and Cuccurullo 2017). The search query yielded 128 and 129 documents for the Web of Science and Scopus databases, respectively, published until the first quarter of 2024. Annual scientific production for the selected search string is presented in Figure 2 which depicts the growth of the research field over the last three decades. To further present the research trend, we preferred the keyword-plus (ID) data from the Web of Science search results, as it covers a wide range of publications indexed after a rigorous process. Word cloud diagram, keyword co-occurrence network plot, strategic thematic map, multiple-correspondence analysis (MCA), and thematic evolution plots have been used for presenting the research trend on the selected topic using the bibliometrix package (Aria and Cuccurullo 2017). The main information of the bibliometric analysis is presented in Table S1. In brief, a total of 128 documents (108 articles, 19 reviews, and 1 conference abstract) from 83 sources with 550 keywords plus (ID) and 426 authors’ keywords were obtained for the search query “(TITLE-ABS-KEY (‘white rot fungi’) AND (‘heavy metal’) PUBYEAR > 1995)”. The annual growth rate and citations per document for the current search were 5.08% and 35.48, respectively.
Figure 2.
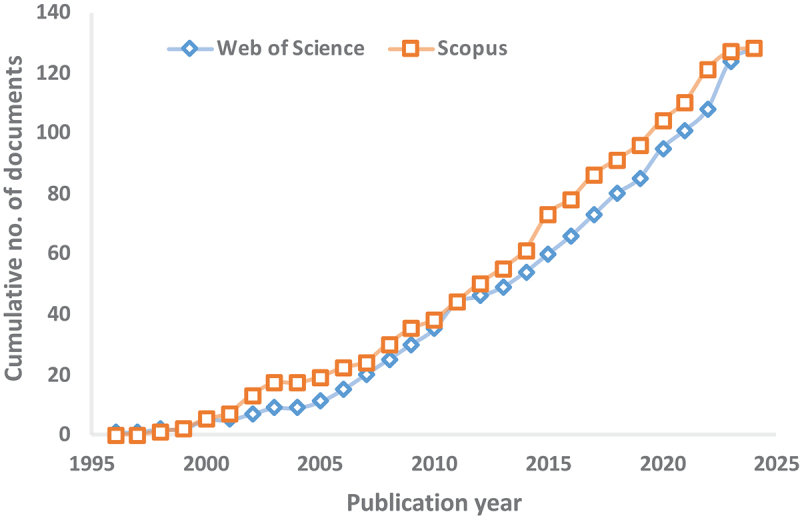
Cumulative number of documents related to use of white rot fungi for heavy metal remediation published during the last three decades (1995 to 2024) in web of science core collection and Scopus databases (data source: Scopus 2024; Web of Science Core Collection 2024).
3. Research trends in heavy metal remediation using white rot fungi
The first document on heavy metal remediation using white rot fungi was published in 1996. However, the field started getting increased attention from researchers in 2011 (9 documents published) onwards (Figure 2). In 2023, a total of 16 papers were published related to the use of white rot fungi for heavy metal remediation. Moreover, four documents have already been published in the first quarter of 2024, which reveals an increasing attention of researchers. Out of the total publications, 50%–60% of the documents have been published during the last 10 years. This reflects the increased attention this research field has received in the last few years. Table S2 lists the top 10 countries, institutions, authors, and journals/sources that publish research on using white rot fungi for heavy metal remediation from contaminated sites. Among the top countries publishing research on this topic, China stands first with a frequency of 100 documents published till the first quarter of 2024, followed by India (48), Turkey (41), Italy (28), and Pakistan (27) (Table S2). Interestingly, the Turkish researchers had initiated pioneer work on this research topic. However, the field has recently received wider attention among the research communities in South Asian countries. These findings are in coherence with the top researchers and institutions focusing on different dimensions of utilising white rot fungi for heavy metal remediation (Table S2). Among the top sources (journals) publishing research on white rot fungi as heavy metal bioremediation agents, Bioresource Technology ranks first by publishing seven documents up till the first quarter of 2024, followed by Chemosphere (6), Journal of Hazardous Materials (5), Environmental Science and Pollution Research (5), and Ecotoxicology and Environmental Safety (3).
Based on the Web of Science Keyword-plus datasets, Figure 3 represents the focus areas of white rot fungi for heavy metal remediation. It can be seen that degradation/biodegradation, removal, biosorption, wastewater, and polyaromatic hydrocarbons are the top five most frequent words (>15 frequencies), which have been considerably explored in recent years (Figure 3). Heavy metals-degradation-wastewater (green), white rot fungi-Phanerochaete chrysosporium-Trametes versicolor (red), removal-biosorption-cadmium (blue), and mechanism-accumulation (magenta) form four different but interrelated clusters for overall research on white rot fungi-mediated metal remediation (Figure 4). Further, phytoremediation of organic pollutants and heavy metals by using bacteria and fungi constituted the motor and niche themes whereas agricultural wastes, contaminated soil, microbial biomass, maturity and sludge stability, identification, and protection were observed in the emerging/declining themes in the thematic map (Figure 5). It reflects the research focus on the identification of new species of white rot fungi and sludge management for the sustainable remediation of heavy metals.
Figure 3.
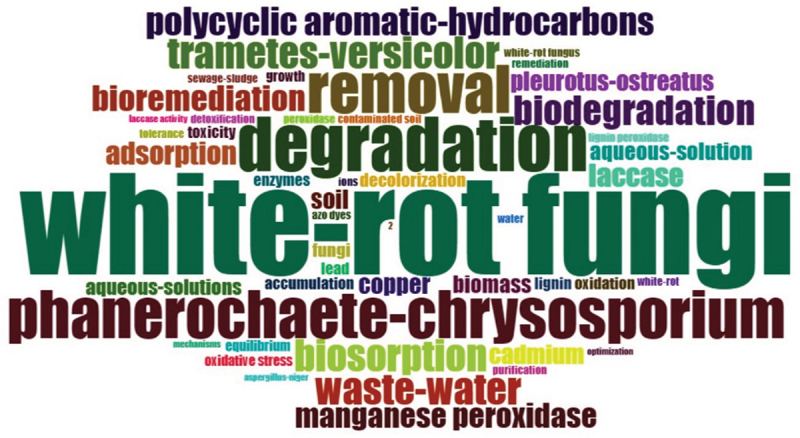
Word cloud diagram based on keyword plus database depicting the focus areas of research related to white rot fungi for heavy metal remediation. The size of words represents the frequency of occurrence in the literature search (data source: Web of Science Core Collection 2024).
Figure 4.
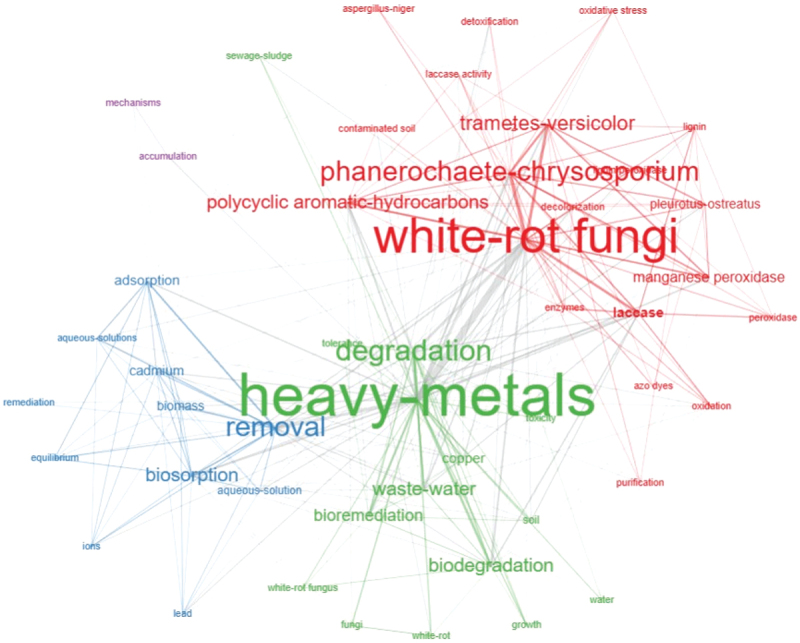
Keyword co-occurrence network map of research on heavy metal remediation using white rot fungi. The texts represent the nodes, whereas threads/edges represent the interconnections of different keywords. The size of texts and strength of threads/edges are based on the frequency and interconnectedness of the keywords. Assemblages of similar nodes are represented by similar colors and the cluster is named based on the larger node with maximum interconnecting threads/edges (data source: Web of Science Core Collection 2024).
Figure 5.
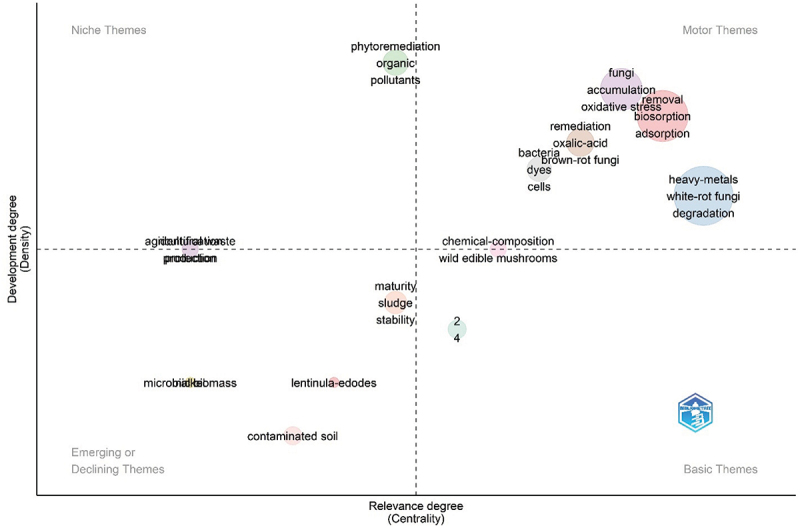
Strategic thematic map of research on heavy metal remediation using white rot fungi. It represents the conceptual evolution of the topic by distributing the keywords in different themes/quadrants based on the centrality (horizontal x-axis) and density (vertical y-axis). Here, centrality represents the frequency of linkages between different clusters (themes), whereas density represents the frequency of internal links within a cluster (theme). The quadrants of the thematic map have intuitive importance. They are named motor themes (1st upper right), niche themes (2nd upper left), emerging or disappearing themes (3rd lower left), and basic themes (4th lower right) in an anti-clockwise manner (data source: Web of Science Core Collection 2024).
A conceptual structure map based on the multiple correspondence analysis revealed the formation of two distinct clusters related to the application of white rot fungi in the remediation of heavy metals from aqueous solution (blue cluster) and soil (red) ecosystems (Figure 6). These observations corroborate with the co-occurrence network plot. The two dimensions of these clusters cumulatively cover ~48% of the contribution of the research on this topic. The thematic evolution plot (Figure 7) reveals that during the initial study period (1996–2011), the focus was mainly on using white and brown-rot fungi to remove heavy metals from wastewater via accumulation and biosorption. These fields further diversify towards the sorption/adsorption of heavy metals and dyes from aqueous solutions and contaminated soils using different fungal species (e.g. Lentinula edodes, Phanerochaete chrysosporium, Trametes versicolor) and enzymes (e.g. laccases) for their sustainable remediation during the recent period i.e. 2020–onwards (Figure 7). Convergence of wastewater, microbial biomass, and heavy metal nodes (1996–2011) towards heavy metals (2012–2020) and then its divergence towards the soil, heavy metals, bioremediation, and laccases further revealed the emergence of research on the enzymatic remediation of heavy metals in the recent years (Figure 7), which is also depicted in conceptual structure plots (Figure 6).
Figure 6.
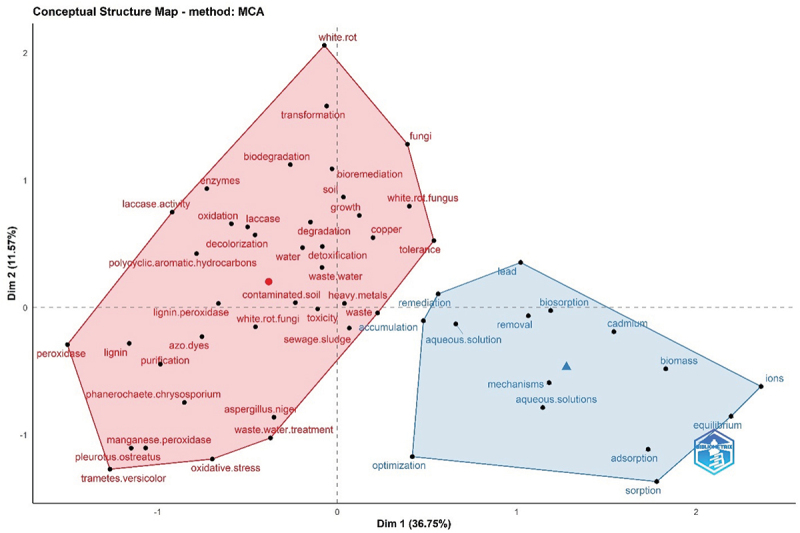
Conceptual structure plot using multiple correspondence analysis (MCA) based on keyword plus database related to the topic of heavy metal remediation using white rot fungi (data source: Web of Science Core Collection 2024).
Figure 7.
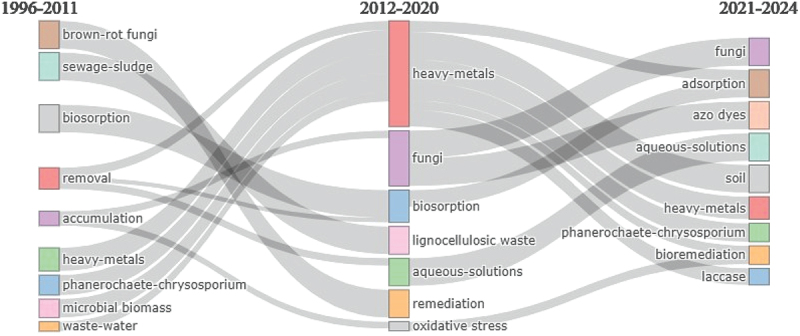
Thematic evolution map of the research topic “heavy metal remediation using white rot fungi” based on keyword plus database of web of science core collection for the time period 1995 to 2024. The evolution map is presented in three time periods, viz., 1995 to 2011 representing the initial 15 years of research, followed by 2012 to 2020 highlighting the last 10 years, and 2021 to 2024 representing the focus of research in the last four to five years. The map showed the convergence and divergence of different research areas within the given time periods (data source: Web of Science Core Collection 2024).
4. Management of heavy metal contaminated sites
4.1. Physico-chemical methods
Many physico-chemical methods have been used to remove heavy metals from a contaminated environment. Among physico-chemical methods, adsorption is the most commonly employed technique because of easy handling, ease of access, cost-effective nature, efficacy, and good efficiency (Mudhoo et al. 2012). The phenomenon of adsorption is based on the adherence of a substance to different phases. It has been found to be effective for organic contaminants and inorganic contaminants, including heavy metals (Jadoun et al. 2023). Recently, the retention of different heavy metals on zeolites employing the phenomenon of adsorption has been reviewed by Velarde et al. (2023). Membrane filtration is another technique widely utilised to separate heavy metals like arsenic, mercury, lead, and cadmium from contaminated water. Different forms of membrane filtration currently in use are nanofiltration, ultrafiltration, microfiltration, and reverse osmosis, with a quite success in the area of water decontamination and salinity removal (Monachan et al. 2022; Samavati et al. 2023). Chemical precipitation is classified as hydroxide, sulphide, and chelating agent, one of which is the conventional heavy metal removal during wastewater purification. The process effectively removed heavy metals, including zinc, lead, cobalt, copper, chromium, nickel, and iron (Fei and Hu 2023). The process encompasses the generation of insoluble precipitate of target heavy metal by amendment of precipitants and pH modifications followed by separation using sedimentation and filtration depending on the conditions (Chen et al. 2018; Benalia et al. 2022; Liu et al. 2023). However, as mentioned earlier, the physico-chemical approaches have several challenges related to optimised working conditions, environmental factors, cost-effectiveness, etc., limiting their broader uses. It paves the way to explore the biological approaches for the sustainable remediation of heavy metals from contaminated sites.
4.2. Biological methods based on white rot fungi
Researchers have continuously searched for new biological agents to eliminate hazardous heavy metals negatively affecting diverse environmental complexes. Biological methods of heavy metals are considered quite appealing because of their non-toxicity, inexpensive in nature, effective for low concentration, ease of handling and renewable property of biosorbent, high volume contaminant treatment in short duration, increased surface binding and accumulation as well as the most characteristically the generation of substantial amount of active biomass (Lo et al. 2014; Sharma and Malaviya 2016; Yin et al. 2019; Kumar and Dwivedi 2021) to be employed further for sequestration of contaminants other than heavy metals in native as well as chemically modified form. For example, Phanerochaete chrysosporium, a white rot fungus, has been suggested for the removal of more than 90% lead from contaminated environments having 50 mg/L concentration through the mechanisms involving both extracellular surface retention as well as intracellular accrual (Huang et al. 2017). Phanerochaete chrysosporium assisted removal of cadmium and nickel has shown adsorption efficiencies equivalent to 96.23% and 89.48%, whereas the adsorption capacities were registered as 71.43 mg/g and 46.50 mg/g, respectively, suggesting the suitability of white rot fungi (Noormohamadi et al. 2019).
The experimental investigations of Wollenberg et al. (2021) have documented the uranium sequestration capacities for Schizophyllum commune and Pleurotus ostreatus in the order of 463.2 ± 38.1 μmol/g and 441.8 ± 79.4 μmol/g indicating opportunities in cleaner production technology. Sharma et al. (2022) have explored the efficiency of white rot fungus identified as Phlebia floridensis for removing mercury in a batch culture system at a specified temperature. The fungus having a tolerance up to 100 μmol/L was able to remove 70%–84% of mercury depending upon the initial concentration encompassing both intracellular accumulation and surface adsorption, thereby advocating the employment for the treatment of wastewater laden with mercury. The contribution of Lentinus crinitus for removing heavy metals from tannery, galvanic effluent, and synthetic medium has been elucidated by Osório da Rosa et al. (2022). The fungus was able to reduce the concentration of lead to 85.29% from synthetic medium, whereas removal of higher than 98% was recorded for iron, chromium, and aluminium present in tannery wastewater, hence verifying an important strategy for the bio-removal.
The investigations of Sharma et al. (2023) have validated the applicability of white rot fungi, namely Phlebia brevispora, Phlebia floridensis, and Phanerochaete chrysosporium for the removal of nickel, cadmium, and lead from industrial wastewater maximally up to 99% as confirmed through atomic absorption spectrophotometer (AAS) and energy-dispersive X-ray spectroscopy, hence pointing towards the application as reasonable biosorbent. Further, the study revealed the induction of deformities and uneven development of fungal mycelia exposed to heavy metals in wastewater. The suitability of two white rot fungi, including Pleurotus ostreatus and Agaricus bisporus, for successful biosorption of heavy metals such as lead, mercury, and cadmium has recently been suggested (Sarwar et al. 2023). Pleurotus ostreatus proved to be a better mycofiltration candidate with the sorption potential 9–189 mg/g and 1–21.4 mg/g for lead and cadmium, respectively, compared to A. bisporus. In contrast, considerable mercury removal, ranging from 0.6 to 10 mg/g was noticed for A. bisporus. Additionally, the bio-removal by candidate white rot fungi reflected enhancement in mycofiltration with the temperature rise.
4.3. Advantages of white rot fungi over other biological and physico-chemical methods
Some of the well-known characteristics of white rot fungi include their growth on lignin-rich substrates, high biomass production, tolerance to heavy metals, ease in cultivation, ability to degrade a wide range of agro-wastes (Dhiman et al. 2024) and organic contaminants using enzymatic systems such as laccase, lignin peroxidase, and manganese peroxidase. White rot fungi grow profusely on lignin-rich substrate and, therefore, can be employed not only for the management of the massive amount of lignocellulosic waste generated globally, through the improved biosynthesis of lignocellulolytic enzymes (Huang et al. 2024), but also for the synthesis of biocomposites (Saini et al. 2024). Further, white rot fungi play an important role in the cycling of heavy metals through the binding and release process. White rot fungi are considered as efficient degraders of lignin-based substrates and hence would be envisaged to induce a negligible effect on agroecosystems. In addition, because of a large amount of biomass production, white rot fungi would remove metal and other contaminants more effectively in comparison to other biological and physico-chemical treatment methods. White rot fungi can be quickly grown on lignin-based substrates for heavy metal removal, in contrast to the requirement for high chemical doses in chemical treatment processes. The used biomass can be regenerated multiple times for further use as a biosorbent. The white rot fungus Phanerochaete chrysosporium has been most widely utilised to remove heavy metals (Chen et al. 2022) because of ease of cultivation, availability, and increased biomass production. Recent studies have indicated a significant contribution of yeast (class ascomycetes) and other filamentous fungi in eliminating heavy metals (Kumar and Dwivedi 2021; Jamir et al. 2024).
The type of heavy metal removed by white rot fungi and other fungal groups is determined by the nature of the cell wall, the chemical composition of extracellular matrices, enzymes produced, surface charge, biomass, contact duration, and most strikingly the presence of competing cations and anions (Li et al. 2020; Zhao et al. 2020; Yildirim et al. 2022). The removal of heavy metals for valence and ionic states is determined by their interaction with cell wall constituents. Thus, the highly negatively charged cell wall will attract more positively charged metal ions. Overall, the higher the degree of ionisation and valence, the greater attractive forces would be expected with the resultant removal of heavy metals. Apart from the valence state, ionic state, and type of element, the concentration of metal ions, the dose of fungal biomass, and duration of contact also regulate the process of heavy metal removal. The property of single heavy metal removal and sorption by fungus in a mixture of heavy metals differs (Gola et al. 2016). A list of different white rot fungi showing heavy metal sequestration is presented in Table 1.
Table 1.
Application of white rot fungi for the remediation of heavy metals from the contaminated sites.
| S. No. | Name of fungi | Heavy metal | Remarks | References |
|---|---|---|---|---|
| 1 | Phlebia brevispora and Phlebia floridensis | Pb, Cd, and Ni | Nearly complete removal of Ni, and Cd in comparison to 12% to 98% removal of Pb. | Sharma et al. (2023) |
| 2 | Pleurotus ostreatus | Cu, As, Cd, and Pb | The content of metal accumulation increased with the rise in substrate and varied according to the strain used. | Atila and Kazankaya (2023) |
| 3 | Trametes pubescens | Zn | Zn removal increased with the elapse of time. The study revealed 67.1% removal in 120 hours with sorption capacity as 44.7 mg/g. | Farhadi et al. (2023) |
| 4 | Trametes pubescence | Ni and Pb | Nearly 100% removal of Pb and 9% removal of Ni at 1,000 mg/L concentration was observed. Both live and dead biomass accumulated metals. | Enayatizamir et al. (2020) |
| 5 | Phanerochaete chrysosporium | Cd2+ and Ni2+ | The response surface method was employed for optimization in terms of pH, temperature, contact time, and initial metal content. The Cd and Ni accumulation were found as 96.23% and 89.48% at a concentration of 25 mg/L and 16 mg/L, respectively, under defined conditions. | Noormohamadi et al. (2019) |
| 6 | Phanerochaete chrysosporium | Pd | The removal efficiency was determined in the range of 22–128 Pd mg/g of fungal biomass and involved the generation of Pd nanoparticles by the process of biomineralization. | Tarver et al. (2019) |
| 7 | Pleurotus ostreatus HAAS | Pb, Cd, and Cr | The order of metal removal was noted as Pb > Cd > Cr. Also, the oxalic acid secreted by the white rot fungus reduced the content of heavy metal by chelation. | Yang et al. (2017) |
| 8 | Pleurotus ostreatus | Cr(III), Cd(II), and Cu(II) | Optimum adsorption occurred in the pH range 4–5 with flow rate 2.5 mL/min. | Kocaoba and Arısoy (2011) |
| 9 | Immobilized Pycnoporus sanguineus | Cd | The uptake enhanced with the rise in pH, temperature, and initial concentration. The sorption by selected fungus was endothermic and spontaneous process. | Mashitah et al. (2008) |
| 10 | Phanerochaete chrysosporium and Funalia trogii | Cu | The pH 5.0 was optimum for adsorption and did not depend on temperature between 20–45 °C. Live biomass proved to be superior in comparison to dried one. Under optimized condition, the biosorption by both live and dead biomass ranged from 40%–60%. | Sibel et al. (2005) |
| 11 | Trametes versicolor | Cu2+, Pb2+, and Zn2+ | Maximum sorption was recorded at the pH range 4 to 6. Temperature variation between 15–45 °C did not influence sorption. | Bayramoğlu et al. (2003) |
| 12 | Trametes versicolor | Cd | Nearly complete Cd removal was achieved within first two hours involving energy independent sorption process with the rate equivalent to nearly 2 mg Cd per g biomass. | Jarosz-Wilkołazka et al. (2002) |
| 13 | Pycnoporus sanguineus | Pb, Cu, and Cd | Biosorption was suggested as complex phenomenon. The biosorbent developed can be used for multiple removal experiment after regeneration. | Zulfadhly et al. (2001) |
| 14 | Trametes versicolor | Cd | Sorption capacities for live and dead immobilized fungal biomass was determined in the order of 102.3 ± 3.2 mg Cd(II)/g and 120.6 ± 3.8 mg Cd(II)/g with the attainment of sorption equilibrium in one hour. | Arıca et al. (2001) |
| 15 | Phanerochaete chrysosporium | Cu | The fungus removed 3.9 mmol of Cu per gram dry weight. The maximum adsorption by fungal mycelia was observed at pH range near 6.0. The fungus proved better sorbent in comparison to resin. | Sing and Yu (1998) |
5. Effect of heavy metals on white rot fungi
Exposure to heavy metals induces different antioxidant defence enzymes, including catalase, superoxide dismutase, and glutathione transferase, in addition to crucial molecules of glutathione, ascorbate, oxalate, laccase, and phenolic constituents in white rot fungi (Jarosz-Wilkołazka et al. 2006; Chen et al. 2014). For instance, catalase directs the conversion of hydrogen peroxide to water and oxygen (Nandi et al. 2019). Superoxide dismutase defends the cell against reactive oxygen species (Fujii et al. 2022). In addition, different enzymes are known to facilitate the oxidation and reduction of heavy metals, thereby protecting against cellular damage. As a general rule, the elevation in stress response in the presence of rising heavy metal content results in the increment of antioxidant activities. Nevertheless, such modulations may vary regarding selected fungal strains, culture conditions, and metal content (Xu et al. 2021). Noteworthy, the effect of heavy metal dose and treatment duration on antioxidant defence responses as reflected by variations in the level of superoxide dismutase, catalase, peroxidase, glutathione, reactive oxygen species, and malondialdehyde was reported by Chen et al. (2014). Such responses, however, may be more complex for a given white rot fungus in the presence of more than one contaminant of either organic or inorganic nature (Feng et al. 2018; Guo et al. 2018). The induction of oxidative stress led by heavy metals promotes the increased expression of an array of genes encoding extracellular enzymes, facilitating the degradation of organic contaminants (Liu et al. 2020). Reduction in dry weight and rise in lipid peroxidation as measured through estimation of malondialdehyde content of white rot fungus Pleurotus ostreatus with the rise in soil cadmium content, thereby suggesting inhibitory action, has been well acknowledged recently (Dou et al. 2023). In addition, the elevation in the level of cadmium led to a substantial increment in superoxide dismutase and peroxidase in the fruiting body. The most notable enzymes in white rot fungi contributing to metal leaching are lignin peroxidase, manganese peroxidase, laccase, and CYP450. A list of enzymes released by white rot fungi that help remediate heavy metals is presented in Table 2.
Table 2.
A list of enzymes released by the white rot fungi that help leach and remove heavy metals from contaminated sites.
| S. No. | White rot fungi | Name of enzyme | Leaching/removal of heavy metal | References |
|---|---|---|---|---|
| 1 | Ganoderma multipileum | Laccase | More than 94% of 100 μg/mL hexavalent chromium was reduced to trivalent chromium. | Alshiekheid et al. (2023) |
| 2 | Phanerochaete chrysosporium | Lignin peroxidase, Mn peroxidase | Nearly 50% gold recovery was attained in the presence of white rot fungus. | Hein et al. (2023a) |
| 3 | Phanerochaete chrysosporium | Lignin peroxidase, Mn peroxidase | Approximately 45% gold was recovered after oxidation of ore. | Hein et al. (2023b) |
| 4 | Phanerochaete chrysosporium | Laccase, lignin peroxidase, Mn peroxidase | Copper leaching ascribed to fungal enzymes and organic acids was recorded as 54%. | Liu et al. (2022) |
| 5 | Pleurotus florida | Laccase | The biological activity of laccase was found to be responsible for biosorption and leaching of copper and iron from print circuit boards under submerged conditions. | Kaur et al. (2022) |
| 6 | Trametes pubescence | Laccase | Laccase released from white rot fungus assisted the biosorption of lead and nickel. | Khozani et al. (2021) |
| 7 | Phanerochaete chrysosporium | Lignin peroxidase, Mn peroxidase, laccase | Treatment with the white rot fungus for 14 days led to 60.96% removal of copper in comparison to the control. The leaching was also facilitated by release of different organic acids such as oxalic, citric, and gluconic acid. | Liu et al. (2020) |
| 8 | Trametes hirsuta | Laccase | Nearly absolute removal of hexavalent chromium was observed. | Liu et al. (2020) |
| 9 | Phanerochaete chrysosporium | Not specified | Arsenic, zinc, lead, chromium, cobalt, nickel etc. removal. | Park and Liang (2019) |
| 10 | Phanerochaete chrysosporium | Lignin peroxidase, Mn peroxidase, laccase | Fungal treatment was responsible for the leaching of 18% of iron from pyrite. The leaching was ascribed to the combined action of enzyme, hydrogen peroxide, and organic acids. | Yang et al. (2018) |
| 11 | Phanerochaete chrysosporium | CYP450 | The inhibitor-induced reduction in the removal of cadmium suggested the role of enzyme CYP450 in heavy metal sequestration. | Zhang et al. (2015) |
| 12 | Phanerochaete chrysosporium | Lignin peroxidase, Mn peroxidase | The presence of white rot fungus improved the recovery of gold. | Ofori-Sarpong et al. (2010) |
6. Mechanisms of heavy metal remediation
Although several mechanisms have been proposed for removing heavy metals (Figure 1), biosorption is the primary method (Sharma et al. 2021). The heavy metals interaction with constituents of the cell wall and plasma membrane helps organisms to cope with the negative consequences (Figure 8). The biosorption may involve three distinct steps, including rapid adherence, gradual mobilisation from the outer to the internal environment, and eventually, the attainment of the equilibrium stage (Lu et al. 2020). The interaction of heavy metals with different functional groups present in the fungal cell proteins, fatty molecules, and carbohydrates can be satisfactorily revealed by using Fourier transform infrared (FTIR) spectroscopy (Rudakiya et al. 2018). The release of organic compounds such as oxalic acid by Pleurotus ostreatus HAAS in response to lead and chromium stress has been considered to play an important role in the chelation of heavy metals and subsequent sequestration as evidenced by reduction in soluble fraction is elucidated (Yang et al. 2017). The induced biosynthesis of exopolysaccharide in white rot fungi harbouring diverse functional groups (e.g. amide, phosphoryl, and sulphhydryl) upon exposure to heavy metal stress is promising in the field of bioremediation (Wang et al. 2015). It mediates heavy metal removal through the phenomena of ion exchange, complex formation, and precipitate generation on its exterior cell components (Wang et al. 2015). Another strategy suggested for heavy metal remediation adopted by the white rot fungus Pleurotus ostreatus HAU-2 is intracellular bioaccumulation, indicating its role in the cleansing of the contaminated environments, including soil (Li et al. 2017).
Figure 8.
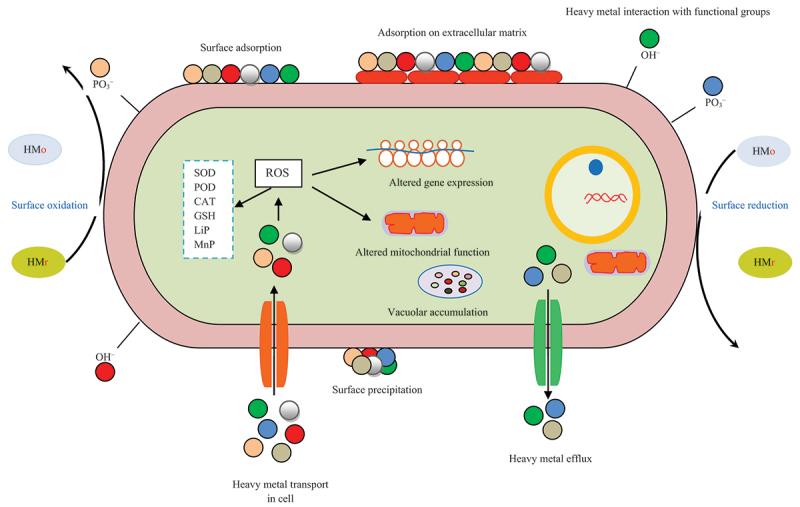
Mechanism of heavy metal removal by white rot fungi and effect on cellular processes. HMr: Heavy metal reduced; HMo: Heavy metal oxidized; SOD: Superoxide dismutase; POD: Peroxidase; CAT: Catalase; LiP: Lignin peroxidase; MnP: Manganese peroxidase; ROS: Reactive oxygen species. Variations in circle color depict changes in the type of heavy metal.
The intracellular accumulation may be linked to the association of short peptides, including metallothionein and glutathione, with heavy metals followed by mobilisation in the vacuole and subsequent vacuole transport facilitated by microtubules (Xu et al. 2014; Brunsch et al. 2015; Schlunk et al. 2015).
7. Factors influencing white rot fungi assisted remediation
Various factors including substrate, tolerance of test fungus, the toxicity and content of heavy metals, presence of chelating ions, and the amount of competing ions influence the removal of heavy metals by white rot fungus. In addition, several environmental factors, including pH, temperature, initial metal concentration, contact duration, media composition, competing ions, biomass, shaking versus non-shaking, redox status, whether immobilised or not, live or dead, and oxygen availability considerably govern the detoxification and remediation of heavy metal contaminated environment by white rot fungi (Figure 9; Bayramoğlu et al. 2003; Javaid et al. 2011; Priyanka and Dwivedi 2023). Changes in pH favour the alterations in charge on the cell exterior as well as the level of ionisation (Aksu 2005). The reduction in the sorption of heavy metals at higher pH has been attributed to precipitation and net charge modification (Dönmez and Aksu 2002; Bayramoglu et al. 2005). The rise in the percent removal of heavy metals with the increase in biomass and shaking up to a certain extent, followed by saturation, was reported by Javaid et al. (2011). The increase in biomass offers abundance in available binding sites rendering improved interaction with heavy metals. Similarly, the biomass agitation process exposes maximum-binding sites to associate with heavy metals of interest. Therefore, the optimisation of process parameters is one of the crucial factors for the sequestration of target heavy metals or complexes thereof from the contaminated sites.
Figure 9.
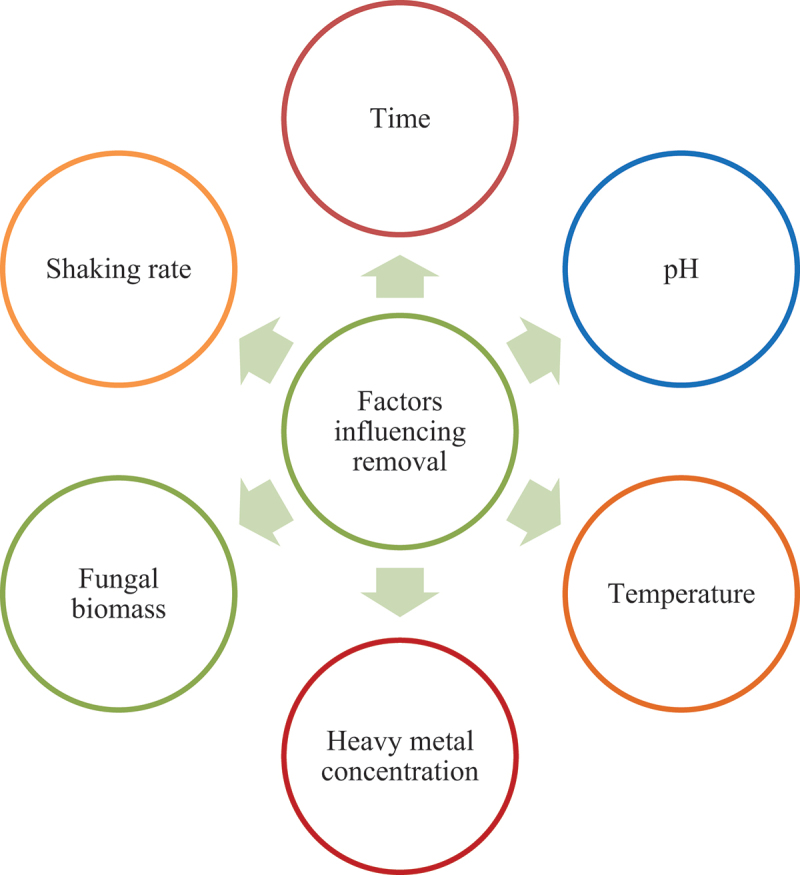
Factors influencing heavy metal removal by white rot fungi.
Most of the studies have reported the potential of white rot fungi in the removal of heavy metals from aquatic ecosystems (Arıca et al. 2001; Yang et al. 2017; Noormohamadi et al. 2019; Sharma et al. 2022) in comparison to soil ecosystems (Novotný et al. 2000). Most of the studies on soil decontamination are confined to laboratory studies. However, for treatment, the fixed amount of soil is liquefied with liquid culture and inoculated with spore to observe the removal of heavy metals in comparison to appropriate control conditions (He et al. 2022). The treatment of heavy metal contaminated aquatic environment involved fungal growth in a suitable medium followed by the adjustment of pH, temperature, shaking condition, and contact duration for the optimal removal (Sharma et al. 2020).
Overall, the challenges of bioremediation using white rot fungi can be envisaged as follows: a) slow biological activity, b) reduced activity of microbes under field conditions, c) requirement of specific substrates in some cases for efficient sequestration, and d) strain specificity for target heavy metals. Thus, a particular microbe cannot be equally effective for all types of heavy metals in a contaminated environment.
8. Conclusions and future perspectives
Heavy metal contamination around the globe has significantly affected the integrity of aquatic and terrestrial environments, posing immense risks to human health manifested in the form of myriads of diseases. So far, different physico-chemical strategies have been employed for the decontamination of sites affected by heavy metals. Nevertheless, those relying on biological methods have drawn significant interest because of process efficiency, low cost, chemical-free nature, and environment friendliness. Plethora of white rot fungi have successfully been demonstrated to sequester the hazardous heavy metals from contaminated aqueous and terrestrial environments due to easy growth on simple substrates and high biomass production. The important mechanism underlying heavy metal elimination involves surface binding through adsorption, intracellular accumulation, precipitation, mineralisation, and complexation with exopolysaccharides. Heavy metal removal is governed by several factors, such as pH, temperature, agitation, media composition, biomass, competing ions, etc. Therefore, clean technology is fundamental to optimising process conditions for maximising heavy metal removal. Most of the studies pertaining to the role of white rot fungi in heavy metal remediation have been conducted under laboratory conditions. Nevertheless, success at the laboratory level would certainly promote collaboration between industry and policymakers. Future research on heavy metal removal by white rot fungi needs to focus on the following aspects:
Each kind of contaminated site could not be remediated effectively with the same fungus. The efficiency of a given white rot fungus for heavy metal removal may differ for aquatic and terrestrial environments, thus, demanding exhaustive research work to translate the full potential.
A particular white rot fungus may not always be suitable for all heavy metals existing in the environment; therefore, searching for new fungi and detailed mechanisms involved should be prioritised to improve the effectiveness of the process. The research should be done on the isolation of white rot fungi showing tolerance to a wide range of organic contaminants such as pesticides, herbicides, and petroleum products; emerging contaminants such as endocrine disruptors, pharmaceuticals; and inorganic contaminants such as heavy metals as the environment is often enriched with complex pollutants.
Some white rot fungi may show better efficiency in heavy metal removal programmes while working in association with the bacteria. The application of a consortium of white rot fungi alone or with bacteria, another approach, may aid in the effectiveness of decontamination. The application of consortia could be a viable option for the sustainable management of inorganic residues and organic contaminants.
The pre-adaptation of a particular white rot fungus to a range of heavy metals could offer new directions in decontamination. Also, the isolation of fungus tolerant to multiple heavy metals could provide better opportunities in the field of bioremediation.
Growth optimisation for successful remediation of heavy metal contaminants under natural conditions must be studied extensively.
Despite large in vitro evidence of efficient remediation capabilities of white rot fungi, success at the industrial scale is still awaited. Modifying biomass using heat or chemical treatment to increase the surface-binding sites could be considered a vital remediation strategy.
Noteworthy, the development of genetically modified strains with improved enzyme expression and tolerance to multiple metals is another area of interest in translating the potential of ligninolytic fungi. Genetically engineered white rot fungi can be a better candidate for bioremediation of heavy metals; however, the study on genetically modified white rot fungi is still in its infancy. The major objective of harnessing the potential of white rot fungi lies in the efficient sequestration of heavy metal without compromising the metal detoxification phenomena. However, strict regulatory laws would be required for the application of genetically modified organisms to avoid plausible environmental and health risks.
Supplementary Material
Acknowledgments
The authors are thankful to their institution’s authorities for providing necessary infrastructure and support for writing this manuscript.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21501203.2024.2389290.
References
- Ab Rhaman SMS, Naher L, Siddiquee S.. 2021. Mushroom quality related with various substrates’ bioaccumulation and translocation of heavy metals. J Fungi. 8(1):42. doi: 10.3390/jof8010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd Elnabi MK, Elkaliny NE, Elyazied MM, Azab SH, Elkhalifa SA, Elmasry S, Mouhamed MS, Shalamesh EM, Alhorieny NA, ElatyAE A, et al. 2023. Toxicity of heavy metals and recent advances in their removal: a review. Toxics. 11(7):580. doi: 10.3390/toxics11070580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I, Gul I, Irum S, Manzoor M, Arshad M. 2023. Accumulation of heavy metals in wild plants collected from the industrial sites-potential for phytoremediation. Int J Environ Sci Te. 20(5):5441–5452. doi: 10.1007/s13762-022-04340-3. [DOI] [Google Scholar]
- Ahmed T, Noman M, Ijaz M, Ali S, Rizwan M, Ijaz U, Hameed A, Ahmad U, Wang Y, Sun G, et al. 2021. Current trends and future prospective in nanoremediation of heavy metals contaminated soils: a way forward towards sustainable agriculture. Ecotox Environ Safe. 227:112888. doi: 10.1016/j.ecoenv.2021.112888. [DOI] [PubMed] [Google Scholar]
- Aksu Z. 2005. Application of biosorption for the removal of organic pollutants: a review. Process Biochem. 40(3–4):997–1026. doi: 10.1016/j.procbio.2004.04.008. [DOI] [Google Scholar]
- Alshiekheid MA, Umar A, Ameen F, Alyahya SA, Dufossé L. 2023. Biodegradation of chromium by laccase action of Ganoderma multipileum. JKing Saud Uni-Sci. 35(10):102948. doi: 10.1016/j.jksus.2023.102948. [DOI] [Google Scholar]
- AL-Huqail AA, El-Bondkly AMA. 2022. Improvement of Zea mays L. growth parameters under chromium and arsenic stress by the heavy metal-resistant Streptomyces sp. NRC21696. Int J Environ Sci Te. 19(6):5301–5322. doi: 10.1007/s13762-021-03532-7. [DOI] [Google Scholar]
- Aria M, Cuccurullo C. 2017. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Inf. 11(4):959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- Arıca MY, Kacar Y, Genç Ö. 2001. Entrapment of white-rot fungus Trametes versicolor in Ca-alginate beads: preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution. Bioresource Technol. 80(2):121–129. doi: 10.1016/S0960-8524(01)00084-0. [DOI] [PubMed] [Google Scholar]
- Atila F, Kazankaya A. 2023. Evaluation of the yield and heavy metal bioaccumulation in the fruit body of Pleurotus ostreatus grown on sugar mill wastewaters. Biomass Conv Bioref. 1–10. doi: 10.1007/s13399-023-03913-7. [DOI] [Google Scholar]
- Ayele A, Haile S, Alemu D, Kamaraj M, Cameselle C. 2021. Comparative utilization of dead and live fungal biomass for the removal of heavy metal: a concise review. The Sci World J. 2021:1–10. doi: 10.1155/2021/5588111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian P. 2003. Interactions of heavy metals with white-rot fungi. Enzym Microb Technol. 32(1):78–91. doi: 10.1016/S0141-0229(02)00245-4. [DOI] [Google Scholar]
- Bayramoğlu G, Bektaş S, Arıca MY. 2003. Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater. 101(3):285–300. doi: 10.1016/S0304-3894(03)00178-X. [DOI] [PubMed] [Google Scholar]
- Bayramoglu G, Çelik G, Yalçin E, Yilmaz M, Arica M. 2005. Modification of surface properties of mycelia by physical and chemical methods: evaluation of their Cr removal efficiencies from aqueous medium. J Hazard Mater. 119(1–3):219–229. doi: 10.1016/j.jhazmat.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Benalia MC, Youcef L, Bouaziz MG, Achour S, Menasra H. 2022. Removal of heavy metals from industrial wastewater by chemical precipitation: mechanisms and sludge characterization. Arab J Sci Eng. 47(5):5587–5599. doi: 10.1007/s13369-021-05525-7. [DOI] [Google Scholar]
- Brunsch M, Schubert D, Gube M, Ring C, Hanisch L, Linde J, Krause K, Kothe E, Pöggeler S. 2015. Dynein heavy chain, encoded by two genes in Agaricomycetes, is required for nuclear migration in Schizophyllum commune. PLoS One. 10(8):e0135616. doi: 10.1371/journal.pone.0135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Zeng G, Chen G, Liu L, Shang C, Hu X, Lu L, Chen M, Zhou Y, Zhang Q. 2014. Plasma membrane behavior, oxidative damage, and defense mechanism in Phanerochaete chrysosporium under cadmium stress. Process Biochem. 49(4):589–598. doi: 10.1016/j.procbio.2014.01.014. [DOI] [Google Scholar]
- Chen L, Zhang X, Zhang M, Zhu Y, Zhuo R. 2022. Removal of heavy-metal pollutants by white rot fungi: mechanisms, achievements, and perspectives. J Clean Prod. 354:131681. doi: 10.1016/j.jclepro.2022.131681. [DOI] [Google Scholar]
- Chen Q, Yao Y, Li X, Lu J, Zhou J, Huang Z. 2018. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng. 26:289–300. doi: 10.1016/j.jwpe.2018.11.003. [DOI] [Google Scholar]
- Chen Z, Osman AI, Rooney DW, Oh WD, Yap PS. 2023. Remediation of heavy metals in polluted water by immobilized algae: current applications and future perspectives. Sustainability-Basel. 15(6):5128. doi: 10.3390/su15065128. [DOI] [Google Scholar]
- Dhiman S, Kaur P, Narang J, Mukherjee G, Thakur B, Kaur S, Tripathi M. 2024. Fungal bioprocessing for circular bioeconomy: exploring lignocellulosic waste valorization. Mycology. 1–26. doi: 10.1080/21501203.2024.2316824.38558835 [DOI] [Google Scholar]
- Dönmez G, Aksu Z. 2002. Removal of chromium (VI) from saline wastewaters by Dunaliella species. Process Biochem. 38(5):751–762. doi: 10.1016/S0032-9592(02)00204-2. [DOI] [Google Scholar]
- Dou R, Xie Y, Liu FX, Wang B, Xu F, Xiao K. 2023. In situ mycoremediation of acid rain and heavy metals co-contaminated soil through microbial inoculation with Pleurotus ostreatus. Sci Total Environ. 912:169020. doi: 10.1016/j.scitotenv.2023.169020. [DOI] [PubMed] [Google Scholar]
- El-Bondkly AMA, El-Gendy MMAA. 2022. Bioremoval of some heavy metals from aqueous solutions by two different indigenous fungi Aspergillus sp. AHM69 and Penicillium sp. AHM96 isolated from petroleum refining wastewater. Heliyon. 8(7):e09854. doi: 10.1016/j.heliyon.2022.e09854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayatizamir N, Liu J, Wang L, Lin X, Fu P. 2020. Coupling laccase production from Trametes pubescence with heavy metal removal for economic waste water treatment. J Water Proc Eng. 37:101357. doi: 10.1016/j.jwpe.2020.101357. [DOI] [Google Scholar]
- Farhadi A, Enayatizamir N, Moradi N, Taghavi M. 2023. Zinc’s impact on the growth and laccase activity of Trametes pubescens and an equilibrium study of zinc adsorption. Chem And Ecol. 39(9):991–1006. doi: 10.1080/02757540.2023.2269939. [DOI] [Google Scholar]
- Fei Y, Hu YH. 2023. Recent progress in removal of heavy metals from wastewater: a comprehensive review. Chemosphere. 335:139077. doi: 10.1016/j.chemosphere.2023.139077. [DOI] [PubMed] [Google Scholar]
- Feng M, Yin H, Cao Y, Peng H, Lu G, Liu Z, Dang Z. 2018. Cadmium-induced stress response of Phanerochaete chrysosporium during the biodegradation of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47). Ecotox Environ Safe. 154(154):45–51. doi: 10.1016/j.ecoenv.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Fujii J, Homma T, Osaki T. 2022. Superoxide radicals in the execution of cell death. Antioxidants. 11(3):501. doi: 10.3390/antiox11030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Rusyn I, Dmytruk OV, Dmytruk KV, Onyeaka H, Gryzenhout M, Gafforov Y. 2023. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front Bioeng Biotechnol. 11:1106973. doi: 10.3389/fbioe.2023.1106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuge SA, Nikalje GC, Kadam US, Suprasanna P, Hong JC. 2023. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J Hazard Mater. 450:131039. doi: 10.1016/j.jhazmat.2023.131039. [DOI] [PubMed] [Google Scholar]
- Gola D, Dey P, Bhattacharya A, Mishra A, Malik A, Namburath M, Ahammad SZ. 2016. Multiple heavy metal removal using an entomopathogenic fungi Beauveria bassiana. Bioresource Technol. 218:388–396. doi: 10.1016/j.biortech.2016.06.096. [DOI] [PubMed] [Google Scholar]
- Guo X, Peng Z, Huang D, Xu P, Zeng G, Zhou S, Gong X, Cheng M, Deng R, Yi H, et al. 2018. Biotransformation of cadmium-sulfamethazine combined pollutant in aqueous environments: Phanerochaete chrysosporium bring cautious optimism. Chem Eng J. 347:74–83. doi: 10.1016/j.cej.2018.04.089. [DOI] [Google Scholar]
- Hanif MA, Bhatti HN. 2015. Remediation of heavy metals using easily cultivable, fast growing, and highly accumulating white rot fungi from hazardous aqueous streams. Desalin Water Treat. 53(1):238–248. doi: 10.1080/19443994.2013.848413. [DOI] [Google Scholar]
- He N, Hu L, Jiang C, Li M. 2022. Remediation of chromium, zinc, arsenic, lead and antimony contaminated acidic mine soil based on Phanerochaete chrysosporium induced phosphate precipitation. Sci Total Environ. 850:157995. doi: 10.1016/j.scitotenv.2022.157995. [DOI] [PubMed] [Google Scholar]
- Hein G, Mahandra H, Ghahreman A. 2023a. Application of Phanerochaete chrysosporium for enhancing gold recovery via bio-oxidation of refractory sulfidic ores in a circumneutral environment. Process Saf Environ. 178:135–146. doi: 10.1016/j.psep.2023.07.095. [DOI] [Google Scholar]
- Hein G, Mahandra H, Ghahreman A. 2023b. Assessment of modified culture conditions for fungal bio-oxidation of sulfidic gold ores performed at circumneutral pH. Sustainability-Basel. 15(21):15559. doi: 10.3390/su152115559. [DOI] [Google Scholar]
- Huang C, Lai C, Xu P, Zeng G, Huang D, Zhang J, Zhang C, Cheng M, Wan J, Wang R. 2017. Lead-induced oxidative stress and antioxidant response provide insight into the tolerance of Phanerochaete chrysosporium to lead exposure. Chemosphere. 187:70–77. doi: 10.1016/j.chemosphere.2017.08.104. [DOI] [PubMed] [Google Scholar]
- Huang J, Wang J, Liu S. 2024. Advances in the production of fungi-derived lignocellulolytic enzymes using agricultural wastes. Mycology. 15(4):523–537. doi: 10.1080/21501203.2023.2253827. [DOI] [Google Scholar]
- Jadoun S, Fuentes JP, Urbano BF, Yáñez J. 2023. A review on adsorption of heavy metals from wastewater using conducting polymer-based materials. J Environ Chem Eng. 11(1):109226. doi: 10.1016/j.jece.2022.109226. [DOI] [Google Scholar]
- Jamir I, Ezung LY, Merry L, Tikendra L, Devi RS, Nongdam P. 2024. Heavy metals clean up: the application of fungi for biosorption. Geomicrobiol J. 41(3):201–212. doi: 10.1080/01490451.2024.2307899. [DOI] [Google Scholar]
- Jarosz-Wilkołazka A, Grąz M, Braha B, Menge S, Schlosser D, Krauss GJ. 2006. Species-specific Cd-stress response in the white rot basidiomycetes Abortiporus biennis and Cerrena unicolor. Biometals. 19:39–49. doi: 10.1007/s10534-005-4599-4. [DOI] [PubMed] [Google Scholar]
- Jarosz-Wilkołazka A, Malarczyk E, Pirszel J, Skowroński T, Leonowicz A. 2002. Uptake of cadmium ions in white-rot fungus Trametes versicolor: effect of Cd (II) ions on the activity of laccase. Cell Biol Int. 26(7):605–613. doi: 10.1006/cbir.2002.0887. [DOI] [PubMed] [Google Scholar]
- Javaid A, Bajwa R, Shafique U, Anwar J. 2011. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy. 35(5):1675–1682. doi: 10.1016/j.biombioe.2010.12.035. [DOI] [Google Scholar]
- Kaur P, Sharma S, Albarakaty FM, Kalia A, Hassan MM, Abd-Elsalam KA. 2022. Biosorption and bioleaching of heavy metals from electronic waste varied with microbial genera. Sustainability-Basel. 14(2):935. doi: 10.3390/su14020935. [DOI] [Google Scholar]
- Khozani MA, Emtiazi G, Aghaei SS, Ghasemi SM, Zolfaghari MR. 2021. Application of fungal laccase for heavy metals precipitation using tannin as a natural mediator. Int J Environ Sci Te. 18(8):2335–2344. doi: 10.1007/s13762-020-02992-7. [DOI] [Google Scholar]
- Kocaoba S, Arısoy M. 2011. The use of a white rot fungi (Pleurotus ostreatus) immobilized on amberlite XAD-4 as a new biosorbent in trace metal determination. Bioresource Technol. 102(17):8035–8039. doi: 10.1016/j.biortech.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Kou B, He Y, Wang Y, Qu C, Tang J, Wu Y, Tan W, Yuan Y, Yu T. 2023. The relationships between heavy metals and bacterial communities in a coal gangue site. Environ Pollut. 322:121136. doi: 10.1016/j.envpol.2023.121136. [DOI] [PubMed] [Google Scholar]
- Kumar V, Dwivedi SK. 2021. Mycoremediation of heavy metals: processes, mechanisms, and affecting factors. Environ Sci Pollut R. 28(9):10375–10412. doi: 10.1007/s11356-020-11491-8. [DOI] [PubMed] [Google Scholar]
- Latif W, Ciniglia C, Iovinella M, Shafiq M, Papa S. 2023. Role of white rot fungi in industrial wastewater treatment: a review. Appl Sci-Basel. 13(14):8318. doi: 10.3390/app13148318. [DOI] [Google Scholar]
- Lee JC, Kurniawan K, Kim S, Nguyen VT, Pandey BD. 2023. Ionic liquids-assisted solvent extraction of precious metals from chloride solutions. Sep Purif Rev. 52(3):242–261. doi: 10.1080/15422119.2022.2091458. [DOI] [Google Scholar]
- Li N, Liu J, Yang R, Wu L. 2020. Distribution, characteristics of extracellular polymeric substances of Phanerochaete chrysosporium under lead ion stress and the influence on Pb removal. Sci Rep-UK. 10(1):17633. doi: 10.1038/s41598-020-74983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Y, Pan Y, Yu H, Zhang X, Shen Y, Jiao S, Wu K, La G, Yuan Y, et al. 2017. Mechanisms of Cd and Cr removal and tolerance by macrofungus Pleurotus ostreatus HAU-2. J Hazard Mater. 330:1–8. doi: 10.1016/j.jhazmat.2017.01.047. [DOI] [PubMed] [Google Scholar]
- Lin G, Zeng B, Li J, Wang Z, Wang S, Hu T, Zhang L. 2023. A systematic review of metal organic frameworks materials for heavy metal removal: synthesis, applications and mechanism. Chem Eng J. 460:141710. doi: 10.1016/j.cej.2023.141710. [DOI] [Google Scholar]
- Liu J, Liu F, Ding C, Ma F, Yu H, Shi Y, Zhang X. 2020. Response of Trametes hirsuta to hexavalent chromium promotes laccase-mediated decolorization of reactive black 5. Ecotoxicol Environ Safe. 205:111134. doi: 10.1016/j.ecoenv.2020.111134. [DOI] [PubMed] [Google Scholar]
- Liu Q, Bai J, Li R, Gu W, Peng S, Wang J, Tang Z, Yu C. 2022. Electrochemical oxidation of copper-clad laminate for manufacturing printed circuit boards via bioleaching by the fungus Phanerochaete chrysosporium. Bioelectrochemistry. 144:108002. doi: 10.1016/j.bioelechem.2021.108002. [DOI] [PubMed] [Google Scholar]
- Liu Q, Bai JF, Gu WH, Peng SJ, Wang LC, Wang JW, Li HX. 2020. Leaching of copper from waste printed circuit boards using Phanerochaete chrysosporium fungi. Hydrometallurgy. 196:105427. doi: 10.1016/j.hydromet.2020.105427. [DOI] [Google Scholar]
- Liu Y, Wang H, Cui Y, Chen N. 2023. Removal of copper ions from wastewater: a review. Int J Env Res Pub He. 20(5):3885. doi: 10.3390/ijerph20053885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Hu T, Zhai Y, Qin H, Aliyeva J, Zhang H. 2020. Fungal cell with artificial metal container for heavy metals biosorption: equilibrium, kinetics study and mechanisms analysis. Environ Res. 182:109061. doi: 10.1016/j.envres.2019.109061. [DOI] [PubMed] [Google Scholar]
- Lo YC, Cheng CL, Han YL, Chen BY, Chang JS. 2014. Recovery of high-value metals from geothermal sites by biosorption and bioaccumulation. Bioresource Technol. 160:182–190. doi: 10.1016/j.biortech.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Maity S, Sarkar D, Poddar K, Patil P, Sarkar A. 2023. Biofilm-mediated heavy metal removal from aqueous system by multi-metal-resistant bacterial strain Bacillus sp. gh-s29. Appl Biochem Biotech. 195(8):4832–4850. doi: 10.1007/s12010-022-04288-7. [DOI] [PubMed] [Google Scholar]
- Mashitah MD, Azila YY, Bhatia S. 2008. Biosorption of cadmium (II) ions by immobilized cells of Pycnoporus sanguineus from aqueous solution. Bioresource Technol. 99(11):4742–4748. doi: 10.1016/j.biortech.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Monachan M, Dixit N, Maliyekkal SM, Singh S. 2022. Reverse osmosis (RO) and nanofiltration (NF) membranes for emerging contaminants (ECs) removal. In: P. Singh S, Agarwal AK, Gupta T, Maliyekkal SM (eds). New Trends in Emerging Environmental Contaminants. Energy, Environment, and Sustainability. Springer, Singapore. p. 407–425. doi: 10.1007/978-981-16-8367-1_17. [DOI] [Google Scholar]
- Mudhoo A, Garg VK, Wang S. 2012. Removal of heavy metals by biosorption. Environ Chem Lett. 10(2):109–117. doi: 10.1007/s10311-011-0342-2. [DOI] [Google Scholar]
- Nandi A, Yan LJ, Jana CK, Das N. 2019. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med Cell Longev. 2019:1–19. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noormohamadi HR, Fat’hi MR, Ghaedi M, Ghezelbash GR. 2019. Potentiality of white-rot fungi in biosorption of nickel and cadmium: modeling optimization and kinetics study. Chemosphere. 216:124–130. doi: 10.1016/j.chemosphere.2018.10.113. [DOI] [PubMed] [Google Scholar]
- Novotný Č, Erbanova P, Cajthaml T, Rothschild N, Dosoretz C, Šašek V. 2000. Irpexlacteus, a white rot fungus applicable to water and soil bioremediation. Appl Microbiol Biot. 54(6):850–853. doi: 10.1007/s002530000432. [DOI] [PubMed] [Google Scholar]
- Ofori-Sarpong G, Tien M, Osseo-Asare K. 2010. Myco-hydrometallurgy: coal model for potential reduction of preg-robbing capacity of carbonaceous gold ores using the fungus, Phanerochaete chrysosporium. Hydrometallurgy. 102(1–4):66–72. doi: 10.1016/j.hydromet.2010.02.007. [DOI] [Google Scholar]
- Osório da Rosa L, da Rosa Lo L, Rodrigues LF, Fontana RC, Moser LI, Lanzer RM, Campos CS, Camassola M. 2022. Mycotechnology to remove of metals from tannery and galvanic effluents-fungal species from the Amazon and Atlantic Forest show high efficiency. J Environ Manage. 319:115677. doi: 10.1016/j.jenvman.2022.115677. [DOI] [PubMed] [Google Scholar]
- Paranjape P, Sadgir P. 2023. Heavy metal removal using plant origin biomass and agricultural waste-derived biomass from aqueous media: a review. Water Cons Sci Eng. 8(1):9. doi: 10.1007/s41101-023-00177-0. [DOI] [Google Scholar]
- Park S, Liang Y. 2019. Bioleaching of trace elements and rare earth elements from coal fly ash. Int J Coal Sci Technol. 6(1):74–83. doi: 10.1007/s40789-019-0238-5. [DOI] [Google Scholar]
- Peyravi M, Rezaei H. 2023. Metals removal by membrane filtration. Met In Water. 331–351. doi: 10.1016/B978-0-323-95919-3.00014-8. [DOI] [Google Scholar]
- Priyanka DS, Dwivedi SK. 2023. Fungi mediated detoxification of heavy metals: insights on mechanisms, influencing factors and recent developments. J Water Proc Eng. 53:103800. doi: 10.1016/j.jwpe.2023.103800. [DOI] [Google Scholar]
- Racić G, Vukelić I, Kordić B, Radić D, Lazović M, Nešić L, Panković D. 2023. Screening of native Trichoderma species for nickel and copper bioremediation potential determined by FTIR and XRF. Microorganisms. 11(3):815. doi: 10.3390/microorganisms11030815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Nandimandalam JR. 2024. Environmental health risk assessment and source apportion of heavy metals using chemometrics and pollution indices in the upper Yamuna river basin, India. Chemosphere. 346:140570. doi: 10.1016/j.chemosphere.2023.140570. [DOI] [PubMed] [Google Scholar]
- Rastegari AA, Yadav AN, Gupta A. 2019. Prospects of renewable bioprocessing in future energy systems 518. Cham: Springer International Publishing. doi: 10.1007/978-3-030-14463-0. [DOI] [Google Scholar]
- Razzak SA, Faruque MO, Alsheikh Z, Alsheikhmohamad L, Alkuroud D, Alfayez A, Hossain SZ, Hossain MM. 2022. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ Adv. 7:100168. doi: 10.1016/j.envadv.2022.100168. [DOI] [Google Scholar]
- Rudakiya DM, Iyer V, Shah D, Gupte A, Nath K. 2018. Biosorption potential of Phanerochaete chrysosporium for arsenic, cadmium, and chromium removal from aqueous solutions. Glob Chall. 2(12):1800064. doi: 10.1002/gch2.201800064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini R, Kaur G, Brar SK. 2024. Textile residue-based mycelium biocomposites from Pleurotus ostreatus. Mycology. 15(4):683–689. doi: 10.1080/21501203.2023.2278308. [DOI] [Google Scholar]
- Samavati Z, Samavati A, Goh PS, Ismail AF, Abdullah MS. 2023. A comprehensive review of recent advances in nanofiltration membranes for heavy metal removal from wastewater. Chem Eng Res Des. 189:530–571. doi: 10.1016/j.cherd.2022.11.042. [DOI] [Google Scholar]
- Santos AF, Almeida PV, Alvarenga P, Gando-Ferreira LM, Quina MJ. 2021. From wastewater to fertilizer products: alternative paths to mitigate phosphorus demand in European countries. Chemosphere. 284:131258. doi: 10.1016/j.chemosphere.2021.131258. [DOI] [PubMed] [Google Scholar]
- Sarwar A, Nayyar BG, Irshad H, Anwar P, Olihk N, Ajmal M. 2023. Mycofiltration of heavy metals (Pb, Cd, Hg) from aqueous solution by living biomass of two mushrooms Pleurotus ostreatus and Agaricus bisporus as biosorbents. J Water Chem Technol. 45(6):599–606. doi: 10.3103/S1063455X23060097. [DOI] [Google Scholar]
- Schlebusch I, Pott RWM, Tadie M. 2023. The ion flotation of copper, nickel, and cobalt using the biosurfactant surfactin. Discov Chem Eng. 3(1):7. doi: 10.1007/s43938-023-00023-8. [DOI] [Google Scholar]
- Schlunk I, Krause K, Wirth S, Kothe E. 2015. A transporter for abiotic stress and plant metabolite resistance in the ectomycorrhizal fungus Tricholoma vaccinum. Environ Sci Pollut Res. 22:19384–19393. doi: 10.1007/s11356-014-4044-8. [DOI] [PubMed] [Google Scholar]
- Scopus . 2024. [accessed 2024 Mar 30]. https://www.scopus.com/results/results.uri?sort.
- Sharma KR, Giri R, Sharma RK. 2020. Lead, cadmium and nickel removal efficiency of white-rot fungus Phlebia brevispora. Lett Appl Microbiol. 71(6):637–644. doi: 10.1111/lam.13372. [DOI] [PubMed] [Google Scholar]
- Sharma KR, Giri R, Sharma RK. 2023. Efficient bioremediation of metal containing industrial wastewater using white rot fungi. Int J Environ Sci Technol. 20(1):943–950. doi: 10.1007/s13762-022-03914-5. [DOI] [Google Scholar]
- Sharma KR, Naruka A, Raja M, Sharma RK. 2022. White rot fungus mediated removal of mercury from wastewater. Water Environ Res. 94(7):e10769. doi: 10.1002/wer.10769. [DOI] [PubMed] [Google Scholar]
- Sharma P, Pandey AK, Kim SH, Singh SP, Chaturvedi P, Varjani S. 2021. Critical review on microbial community during in-situ bioremediation of heavy metals from industrial wastewater. Environ Technol Innov. 24:101826. doi: 10.1016/j.eti.2021.101826. [DOI] [Google Scholar]
- Sharma S, Malaviya P. 2016. Bioremediation of tannery wastewater by chromium resistant novel fungal consortium. Ecol Eng. 91:419–425. doi: 10.1016/j.ecoleng.2016.03.005. [DOI] [Google Scholar]
- Sing C, Yu J. 1998. Copper adsorption and removal from water by living mycelium of white-rot fungus Phanerochaete chrysosporium. Water Res. 32(9):2746–2752. doi: 10.1016/S0043-1354(98)00024-4. [DOI] [Google Scholar]
- Singh AL, Singh VK. 2018. Assessment of groundwater quality of Ballia district, Uttar Pradesh, India, with reference to arsenic contamination using multivariate statistical analysis. Appl Water Sci. 8:1–18. doi: 10.1007/s13201-018-0737-3. [DOI] [Google Scholar]
- Singh VK, Singh R, Rajput VD, Singh VK. 2023. Halophytes for the sustainable remediation of heavy metal-contaminated sites: recent developments and future perspectives. Chemosphere. 313:137524. doi: 10.1016/j.chemosphere.2022.137524. [DOI] [PubMed] [Google Scholar]
- Sibel K, Dilek A, Sema E, Yesilada O. 2005. Biosorption of copper (II) by live and dried biomass of the white rot fungi Phanerochaete chrysosporium and funalia trogii. Eng Life Sci. 5(1):72–77. doi: 10.1002/elsc.200420057. [DOI] [Google Scholar]
- Skotta A, Jmiai A, Elhayaoui W, El-Asri A, Tamimi M, Assabbane A, El Issami S. 2023. Suspended matter and heavy metals (Cu and Zn) removal from water by coagulation/flocculation process using a new bio-flocculant: Lepidium sativum. J Taiwan Inst Chem Eng. 145:104792. doi: 10.1016/j.jtice.2023.104792. [DOI] [Google Scholar]
- Tamjidi S, Ameri A, Esmaeili H. 2023. A review of the application of fungi as an effective and attractive bio-adsorbent for biosorption of heavy metals from wastewater. Environ Monit Assess. 195(1):91. doi: 10.1007/s10661-022-10687-4. [DOI] [PubMed] [Google Scholar]
- Tan Z, Losantos D, Li Y, Sarrà M. 2023. Biotransformation of chloramphenicol by white-rot-fungi Trametes versicolor under cadmium stress. Bioresource Technol. 369:128508. doi: 10.1016/j.biortech.2022.128508. [DOI] [PubMed] [Google Scholar]
- Tarver S, Gray D, Loponov K, Das DB, Sun T, Sotenko M. 2019. Biomineralization of Pd nanoparticles using Phanerochaete chrysosporium as a sustainable approach to turn platinum group metals (PGMs) wastes into catalysts. Int Biodeter Biodegr. 143:104724. doi: 10.1016/j.ibiod.2019.104724. [DOI] [Google Scholar]
- Topare NS, Wadgaonkar VS. 2023. A review on application of low-cost adsorbents for heavy metals removal from wastewater. Mater Today-Proc. 77:8–18. doi: 10.1016/j.matpr.2022.08.450. [DOI] [Google Scholar]
- Velarde L, Nabavi MS, Escalera E, Antti ML, Akhtar F. 2023. Adsorption of heavy metals on natural zeolites: a review. Chemosphere. 328:138508. 10.1016/j.chemosphere.2023.138508. [DOI] [PubMed] [Google Scholar]
- Wang Y, Qin J, Zhou S, Lin X, Ye L, Song C, Yan Y. 2015. Identification of the function of extracellular polymeric substances (EPS) in denitrifying phosphorus removal sludge in the presence of copper ion. Water Res. 73:252–264. doi: 10.1016/j.watres.2015.01.034. [DOI] [PubMed] [Google Scholar]
- Waqas M, Ahmad H. 2024. Trapping of heavy metal ions from electroplating wastewater with phosphorylated double-shelled hollow spheres. Chemosphere. 350:140968. doi: 10.1016/j.chemosphere.2023.140968. [DOI] [PubMed] [Google Scholar]
- Web of Science Core Collection . 2024. [accessed 2024 Mar 30]. https://www.webofscience.com/wos/woscc/summary/7fea3284-0124-4b09-9b13-3b5ffc37f50d-da6e99ee/relevance/1.
- Wollenberg A, Kretzschmar J, Drobot B, Hübner R, Freitag L, Lehmann F, Günther A, Stumpf T, Raff J. 2021. Uranium (VI) bioassociation by different fungi–a comparative study into molecular processes. J Hazard Mater. 411:125068. doi: 10.1016/j.jhazmat.2021.125068. [DOI] [PubMed] [Google Scholar]
- Xiang H, Min X, Tang CJ, Sillanpää M, Zhao F. 2022. Recent advances in membrane filtration for heavy metal removal from wastewater: a mini review. J Water Proc Eng. 49:103023. doi: 10.1016/j.jwpe.2022.103023. [DOI] [Google Scholar]
- Xiong W, Yang M, Wang J, Wang H, Zhao P, Li Z, Liu B, Kong X, Duan H, Zhao Y. 2023. Removal, recycle and reutilization of multiple heavy metal ions from electroplating wastewater using super-stable mineralizer Ca-based layered double hydroxides. Chem Eng Sci. 279:118928. doi: 10.1016/j.ces.2023.118928. [DOI] [Google Scholar]
- Xu F, Chen P, Li H, Qiao S, Wang J, Wang Y, Wang X, Wu B, Liu H, Wang C, et al. 2021. Comparative transcriptome analysis reveals the differential response to cadmium stress of two pleurotus fungi: Pleurotus cornucopiae and Pleurotus ostreatus. J Hazard Mater. 416:125814. doi: 10.1016/j.jhazmat.2021.125814. [DOI] [PubMed] [Google Scholar]
- Xu P, Liu L, Zeng G, Huang D, Lai C, Zhao M, Huang C, Li N, Wei Z, Wu H, et al. 2014. Heavy metal-induced glutathione accumulation and its role in heavy metal detoxification in Phanerochaete chrysosporium. Appl Microbiol Biotechnol. 98(14):6409–6418. doi: 10.1007/s00253-014-5667-x. [DOI] [PubMed] [Google Scholar]
- Yang HY, Liu Q, Chen GB, Tong LL, Ali A. 2018. Bio-dissolution of pyrite by Phanerochaete chrysosporium. T Nonferr Metal Soc. 28(4):766–774. doi: 10.1016/S1003-6326(18)64709-0. [DOI] [Google Scholar]
- Yang S, Sun X, Shen Y, Chang C, Guo E, La G, Zhao Y, Li X. 2017. Tolerance and removal mechanisms of heavy metals by fungus Pleurotus ostreatus Haas. Water Air Soil Poll. 228(4):1–9. doi: 10.1007/s11270-016-3170-y. [DOI] [Google Scholar]
- Yetis Ü, Özcengiz G, Dilek FB, Ergen N, Erbay A, Dölek A. 1998. Heavy metal biosorption by white-rot fungi. Water Sci Technol. 38(4–5):323–330. doi: 10.2166/wst.1998.0656. [DOI] [Google Scholar]
- Yildirim N, Kardas E, Ince M. 2022. The use of Phanerochaete chrysosporium as an alternative bioremediation tool of heavy metal, chemical oxygen demand and phosphate from landfill leachate. Desalin Water Treat. 247:156–160. doi: 10.5004/dwt.2022.28042. [DOI] [Google Scholar]
- Yin K, Wang Q, Lv M, Chen L. 2019. Microorganism remediation strategies towards heavy metals. Chem Eng J. 360:1553–1563. doi: 10.1016/j.cej.2018.10.226. [DOI] [Google Scholar]
- Zhang Q, Zeng G, Chen G, Yan M, Chen A, Du J, Huang J, Yi B, Zhou Y, He X, et al. 2015. The effect of heavy metal-induced oxidative stress on the enzymes in white rot fungus Phanerochaete chrysosporium. Appl Biochem Biotech. 175(3):1281–1293. doi: 10.1007/s12010-014-1298-z. [DOI] [PubMed] [Google Scholar]
- Zhao WW, Zhu G, Daugulis AJ, Chen Q, Ma HY, Zheng P, Liang J, Ma XK. 2020. Removal and biomineralization of Pb2+ in water by fungus Phanerochaete chrysoporium. J Clean Prod. 260:120980. doi: 10.1016/j.jclepro.2020.120980. [DOI] [Google Scholar]
- Zulfadhly Z, Mashitah MD, Bhatia S. 2001. Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ Pollut. 112(3):463–470. doi: 10.1016/s0269-7491(00)00136-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


