ABSTRACT
Escherichia coli has been a vital model organism for studying chromosomal structure, thanks, in part, to its small and circular genome (4.6 million base pairs) and well-characterized biochemical pathways. Over the last several decades, we have made considerable progress in understanding the intricacies of the structure and subsequent function of the E. coli nucleoid. At the smallest scale, DNA, with no physical constraints, takes on a shape reminiscent of a randomly twisted cable, forming mostly random coils but partly affected by its stiffness. This ball-of-spaghetti-like shape forms a structure several times too large to fit into the cell. Once the physiological constraints of the cell are added, the DNA takes on overtwisted (negatively supercoiled) structures, which are shaped by an intricate interplay of many proteins carrying out essential biological processes. At shorter length scales (up to about 1 kb), nucleoid-associated proteins organize and condense the chromosome by inducing loops, bends, and forming bridges. Zooming out further and including cellular processes, topological domains are formed, which are flanked by supercoiling barriers. At the megabase-scale both large, highly self-interacting regions (macrodomains) and strong contacts between distant but co-regulated genes have been observed. At the largest scale, the nucleoid forms a helical ellipsoid. In this review, we will explore the history and recent advances that pave the way for a better understanding of E. coli chromosome organization and structure, discussing the cellular processes that drive changes in DNA shape, and what contributes to compaction and formation of dynamic structures, and in turn how bacterial chromatin affects key processes such as transcription and replication.
KEYWORDS: chromosomal structure, nucleoid-associated proteins, macrodomains, supercoiling, chromosome conformation capture, transcription, replication
INTRODUCTION
Bacterial chromatin is shaped by an intricate interplay of the genetic material itself, with the cellular milieu of water, ions, and small molecules, plus a massive array of proteins that bind, shape, and often alter the DNA. These processes are interconnected, and there are no one-sided relationships; rather, a constant reciprocal relationship exists between the structure of the DNA itself, the effects of that structure on biological processes such as replication, transcription, and protein binding, and then the corresponding impact that those biological processes have on the chromosome. To provide an integrative understanding of the structure of E. coli chromatin, we will follow a pattern working from the shortest length scales (e.g., a single turn of DNA) up to the entire chromosome (Fig. 1), considering at each point along the way the key processes influencing, and influenced by, the chromosomal structure at that length scale.
Fig 1.
Overview of length scales and organizational principles governing the E. coli chromosome. Shown are key length scales and corresponding features that will be considered, from smallest to largest, over the course of the present review.
SMALL-SCALE ARCHITECTURE
At its most fundamental level, the chromosomal DNA of Escherichia coli (and virtually all chromosomal DNA of cellular organisms) can be considered largely as consisting of double-stranded, Watson-Crick base paired DNA in a B-form or nearly B-form helix. This is the commonly pictured right-handed double helix, with ~10.5 bp helical turns, containing a major and minor groove (1). Key physical properties, which affect chromosomal structure at a wide range of scales, include a highly negative charge (−e for each phosphate unit along the backbone, under physiological conditions), and a fairly long persistence length (the distance over which the DNA remains approximately rigid), on the order of 150 bp (2). Thus, to a first approximation, DNA can be considered a fairly rigid, highly anionic polymer. Both of these characteristics, the highly negative charge of DNA and its high rigidity, pose substantial challenges for the overall architecture of the cell, as they tend to expand the overall volume required for the chromosome and counteract packing. To provide a clear understanding of the physical forces driving the behavior of the chromosome, and how various cellular mechanisms for chromosomal organization affect the layout of the genome in the cell, we can make use of a simplified computational model. When treating the chromosome as a polymer consisting of rigid blocks of length equal to the DNA’s persistence length [50 nm, approximated following 2], and unconstrained by a cell envelope or DNA binding proteins, it would be roughly 4.4 micrometers long, several times the length of the cell (1–2 micrometers). By making use of a simple polymer model to represent the chromosome (see the supplemental material for methodological details and some commentary on other simulation approaches taken to modeling the E. coli chromosome), we provide an example conformation of an unconstrained chromosome in Fig. 2A, demonstrating the extent to which the chromosome would be expected to exceed the size of the cell. We will update this picture in lockstep with our discussion below as additional physical constraints on the chromosomal DNA are added. We also note that many of the considerations below have also been considered in a recent review by Verma et al. (3) and Dame et al. (4); we add here additional perspectives arising from several recent advances such as new methods for profiling chromosomal structure and an increasing awareness of the role of biophysical processes such as phase separation in shaping the E. coli genome, as well as the insights provided by our integrative polymer modeling.
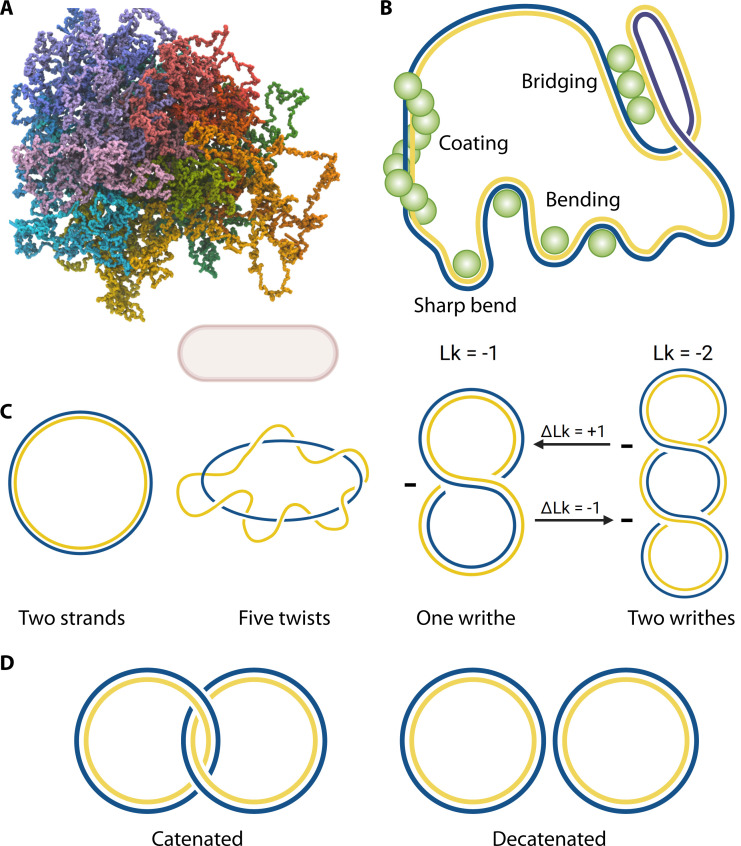
Fig 2.
DNA biophysics and supercoiling shape the fundamental structure of the chromosome. (A) Sample conformation for a polymer model of the unconstrained E. coli chromosome, modeled as described in the text. Colors run across a rainbow spectrum (red → purple → red) around the length of the chromosome; for scale, a 1 micron long cell is shown. (B) DNA can be constrained by DNA-binding proteins, and despite its rigidity, it will form bends and bridges, as well as long inflexible filaments. (C) DNA can take on two forms of superhelicity, twists and writhes, and both contribute to the Lk number. Starting from the left, the two DNA strands have a Lk = 0, they do not interact. Twists are calculated by determining how many times the two strands of DNA cross each other; in the depicted figure, this occurs five times, thus, Lk = 5. A writhe is formed when both strands cross themselves. Supercoiling alters Lk, by adding or removing twists and writhes. Negative supercoiling occurs when the DNA is underwound, or a left-handed twist, while positive supercoiling occurs when the DNA is overwound, or a right-handed twist. Here, we show a supercoiled strand with two points of crossover in a left-handed coil, and therefore, Lk = −2. Removing a writhe (delta Lk = +1) brings the value of Lk to −1. The supercoil takes on a plectonemic shape. While a change in writhe is shown here, the change in linking number could be manifested either in twist or writhe. (D) Physically breaking the strands apart to link the DNA strands together, like links of a chain, is called catenating, and this introduces catenanes. Unlinking DNA by breaking the strands is termed decatenation.
Despite its typical rigidity, DNA has some intrinsic curvature that occurs even in the absence of any physical constraints. This curvature occurs as a result of sequence-dependent bending. A short segment of adenines followed by virtually any other sequence will take on a bent shape (5); thus, there are sequence-specific changes to the effective persistence length of DNA that can reduce the overall size of the nucleoid (Fig. 2B). In addition, neutralization of the DNA backbone charge by changes to backbone phosphate ionization states, counterion condensation (e.g., Mg2 + binding), or positively charged regions of DNA-binding proteins can substantially reduce the rigidity of the double helix (6–8). DNA-binding proteins in particular can induce strong changes to DNA conformations (Fig. 2B) both due to charge-charge interactions and through the removal of a screening cloud of ions through the interaction of a relatively apolar protein – if the usual condensed cloud of cations is displaced on one side of the DNA by a less positively charged protein, the DNA will bend in the opposite direction due to internal electrostatic repulsion (9).
In addition to the bending of DNA, winding of the DNA about its axis can introduce twists and/or writhe (collectively referred to as supercoiling) into the system, without breaking the strands (10). The twist is the number of helical turns in a section of DNA, caused by the twisting of the double helices around each other, resulting in torsional strain (Fig. 2C). The writhe is a measure of the coiling of the axis, i.e., how many times the circular DNA is twisted in half or more, which results in bending (11) (Fig. 2C). Over a given segment of DNA, the sum of the twist and writhe is defined as the linking number (Lk), which is the number of times DNA winds around a helical axis, or supercoils (Fig. 2C). Lk cannot change in a closed circle unless broken (12)—it is a topological property of the DNA that is unaffected by changes in the shape or size of the DNA. Processes that alter supercoiling typically require breakage of at least one DNA strand. Processes resulting in a change in Lk (denoted ΔLk) involve increases or decreases in writhe and twist, which add strain into the system, introducing supercoils (Fig. 2C). A supercoil is a measure of winds and, therefore, strain on the DNA, which can be more tight (positive) or less tight (negative). Energetically, the interwound superhelix is favored over the solenoid superhelix, and multiple experiments have demonstrated that the small and circular chromosomes of prokaryotes generally take on the interwound, plectonemic, shape (13–18) (Fig. 2C, right).
A measure of DNA supercoiling is calculated by determining the specific linking difference or supercoiling density, σ. This is the number of turns, or level of supercoiling, the DNA takes on (Lk) relative to its relaxed state (Lk0). The supercoiling density is defined numerically as σ = (Lk − Lk0)/Lk0. The average supercoil density of DNA in exponentially growing E. coli is −0.06, i.e., for every hundred turns the DNA makes, the DNA is underwound by six turns (19–21). Superhelicity is altered throughout the cell cycle, and while DNA prefers a “relaxed” state, reactions that require the unwinding of DNA, such as replication and transcription, are favored over the rewinding of the DNA once the replication fork passes (22). Here, and throughout the remainder of this review, the term “relaxed” will refer to DNA which twists once around the helical axis approximately every 10.5 bp; this neutral, un-supercoiled state has a linking number defined as Lk0.
The main purposes of supercoiling are to compact DNA into a manageable size to fit into the cell, maintain DNA organization, and assist in some cellular functions. In E. coli K12 specifically, the genome contains 4.6 Mbp of DNA which stretches out to 1.6 mm if linearly extended and is compacted into a 1.5 micron long cell (23). This is possible due to global supercoiling, specifically negative supercoiling, the main mechanism by which prokaryotes and archaea are able to fit the entirety of their DNA into cells (20). Prokaryotes generally have circular and small chromosomes, unlike eukaryotes, with their supercoils taking on a plectonemic shape (18, 24).
There are four elements that are instrumental in generating and maintaining supercoils: transcription, replication, topoisomerases, and nucleoid-associated proteins (NAPs). The combined efforts of all four contribute to the higher-order structure of the chromosome, particularly at the meso scale, and maintain supercoiling homeostasis (25), which affects gene expression globally. Of the processes noted here, topoisomerases require special attention as they represent the primary process in cells through which linking numbers are actually changed (Fig. 2C and D). However, as we will describe in the next section, other processes, such as transcription and replication, can lead to local supercoiling accumulation due to the presence of topological barriers, and even with a fixed linking number, twist and writhe can be interconverted freely. We will discuss the effects of each of these factors throughout this review.
MESO SCALE ARCHITECTURE
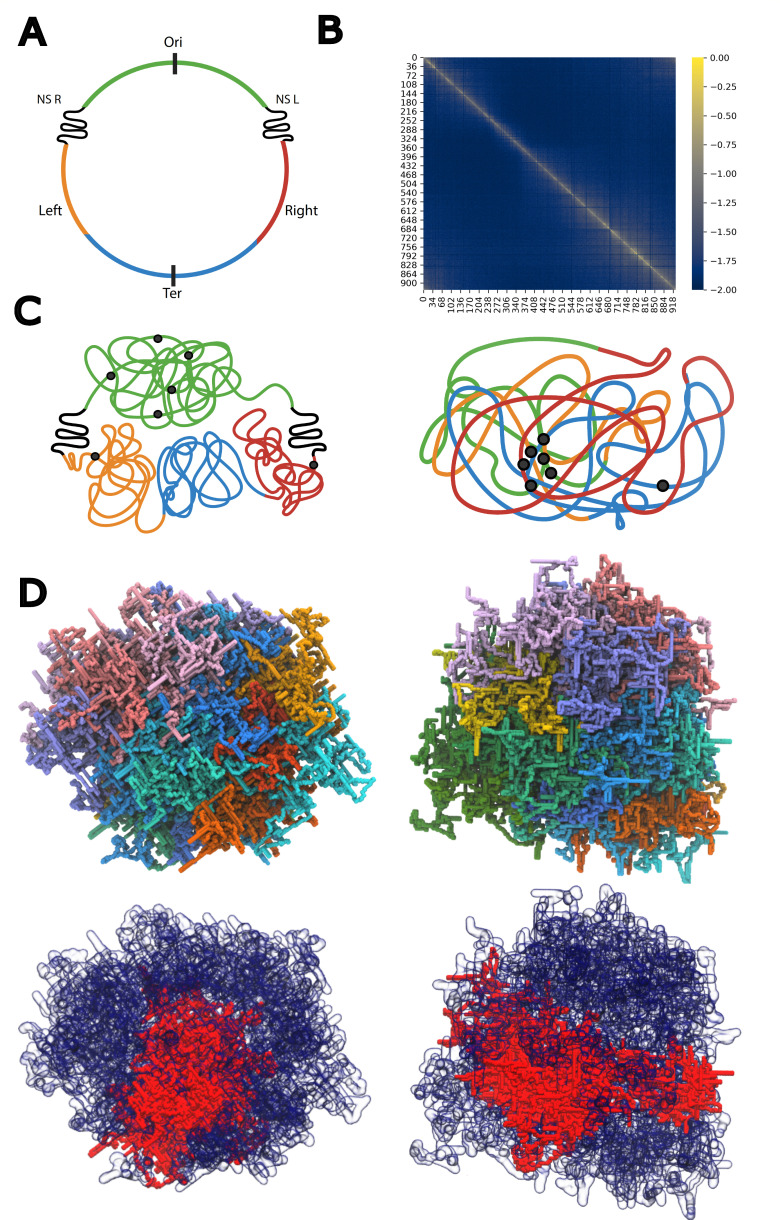
Our next length scale classification, the meso scale, is characterized by DNA structures on the order of 10–200 kb, forming plectonemic loops and supercoils. The formation of these structures, which generally keep the chromosome in a negatively supercoiled bundle, is primarily driven by transcription and replication, topoisomerases, and NAPs. The extent of both global and local supercoiling is influenced by environmental conditions and the growth phase. Early experiments using site-specific recombination found that 40% of the E. coli genome is unbound by proteins and is able to form unconstrained supercoils and plectonemes (18). At a local level, supercoiling influences the shape of topological domains. These are fluid and small sections of DNA that do not permit propagation of supercoiling between domains (i.e., changes in Lk of one topological domain do not impact another). Topological domains allow for continuous replication and repair while also still enabling compaction of the genome into the small cell, without forcing a rigid structure (15). They are transient in nature and need not be compact and need not be physically isolated from other topological domains. The formation of topological domains is due to the presence of barriers which prevent the diffusion of supercoiling across the entire chromosome. These barriers can arise due to the binding of a NAP or topoisomerase, membrane anchoring, or simply the activity of transcription and replication (26–29). Thus, each topological domain effectively has its own linking number (30).
First recognized based on careful analysis of electron micrographs of lysed E. coli, topological domain boundaries are dynamic and randomly distributed, with each domain ranging from 2 to 66 kb in size (15). At this length scale, topological domains form from many plectonemic loops (21, 31) (Fig. 3). The boundaries of these looped domains can change in accordance with environmental state (32–34), which, in turn, influences gene expression (35, 36) due to a lack of propagation of supercoiling changes across domain boundaries. Importantly, some of these works were completed in Salmonella typhimurium, which exhibits a significant likeness in their genome contents and metabolic networks to Escherichia coli.
Fig 3.
Topological domains. Topological domains are dynamic and transient regions of the chromosome flanked by active transcription sites, NAPs, and replication which act as temporary domain boundaries (3, 15). As is shown in the nucleoid depiction, topological domains need not be compact nor physically isolated from other topological domains. Chromosome compaction by MukB is achieved by organizing the DNA into loops around a series of MukBEF complexes forming an axial core (see left panel), and extruding plectonemic domains (see bottom of panel).
Topological domains also protect the chromosome from unraveling when there is DNA damage, as DNA relaxation does not spread throughout the chromosome following the introduction of double-stranded breaks (DSBs) (15, 37). Therefore, DSBs in one topological domain do not influence supercoiling in adjacent domains. More recently, the development of supercoiling detection methods has enabled the field to generate maps that reveal nuances in the supercoiling landscape and further expand the resolution of known E. coli topological domains. One such method, GapR-seq, has been used to map the distribution of positive supercoils in the chromosome. With the use of GapR, a protein known to associate with overtwisted DNA, GapR-seq detects positive supercoils based on immunoprecipitation and sequencing of DNA bound by the exogenously introduced protein (38). Conversely, psoralen, a compound that binds to negatively twisted DNA, has been used to map negative supercoils by crosslinking it to DNA (39, 40). The ongoing development and application of these and similar methods will be necessary in order to fully understand the landscape of supercoiling domains across the chromosome, their relative states across different physiological conditions, and how these supercoiling states affect (and are affected by) other processes such as transcription and replication.
Supercoiling induced by transcription, replication, topoisomerases, and NAPs influence the dynamics, size, and structure of topological domains (41); each will be discussed in detail in the subsequent sections.
Transcription effects at the meso scale
All genetic material within the cell, both DNA and RNA, as well as proteins, impact transcription, which, in turn, affects genomic DNA architecture. While we will cover the effect of transcription on the chromosome conformation at the meso scale in this review, a more in depth look into the relationship between transcription and bacterial chromatin can be found in reference (42).
For transcription to initiate, unwinding the DNA is necessary in order to allow access to the Watson-Crick face of the template strand to the RNA polymerase. The unwinding action of the double strands induces overwinding (positive supercoiling) ahead of RNA polymerase and underwinding (negative supercoiling) behind it (26, 43). Due to the topological domain barriers constraining the DNA (see Fig. 4A and B), DNA unwinding is isolated to the topological domain in which transcription is occuring (44). It is important to note that the net linking number stays constant through this process, but positive and negative supercoils accumulate downstream and upstream of RNA polymerase, respectively. Generally, positive supercoils induced by transcription will diffuse downstream of the transcribing region, until they hit a boundary, such as a flanking topological barrier or another RNA polymerase. Interestingly, at a kilobase level, sites of DNA gyrase catalysis have also been found to act as barriers to supercoiling diffusion (42, 45). As barriers prevent the diffusion of supercoils, tension begins to build in the domain due to an accumulation of torque. Angular optical trap (AOT) studies have shown that RNA polymerase will stall and even backtrack when there is an excess in torque buildup caused by overwound supercoils (46). To prevent the DNA from damage caused by a buildup of positive supercoils ahead of elongating RNA polymerase, and of negative supercoils in its wake, two topoisomerases are involved: DNA gyrase, which removes the positive supercoils, and Topo I, which removes the negative (26) (Fig. 4D).
Fig 4.
Effects of transcription on DNA topology. (A) RNA polymerase (RNA pol) travels along the DNA, unwinding as it moves along, overwinding ahead. Domain boundaries may take on the form of DNA-bound proteins or other regions of transcription. (B) RNA pol acts as a barrier, preventing the diffusion of coils it generates, which are also constrained by flanking domain barriers, forcing the DNA to take on positive supercoils ahead of the polymerase and negative supercoils behind it. (C) R-loops and the resulting RNA:DNA hybrids are transcription intermediates that, when accumulated, will lead to genome instability. (D) DNA gyrase works ahead of RNA polymerase to resolve positive supercoils it generates, while Topo I works behind it to prevent R-loop formation and assist in resolving negative supercoils.
In principle, negatively supercoiled DNA should be easier to melt (as it is already under-wound), whereas positively supercoiled DNA is more difficult to open. Indeed, the rate at which these supercoils are built up is faster than they can be resolved by topoisomerase activity. Consistently, it has been shown that the accumulation of positive supercoils ahead of RNA polymerase can slow down and even block the transcription of highly expressed genes, as measured by OsO4 in situ assays and RT-PCR (44, 47). The buildup of positive supercoils can lead to a phenomenon known as transcriptional bursting and results, at least in large part, from inhibition of transcription due to an accumulation of positive supercoils ahead of RNA polymerase. The result is a sharp increase in transcription once DNA gyrase (which is rate-limiting) stochastically resolves the excess supercoils at a particular locus and allows transcription to continue (47). Importantly, environmental stimuli, such as osmolarity and temperature changes, have effects on DNA supercoiling (48–50), which can manifest as changes in gene expression. Additional examples are reviewed in reference (51).
Not every transcription reaction is perfect, and many events can result in natural intermediates. One such example is an R-loop, which may form when the RNA of a transcription bubble stably anneals with the template DNA, yielding a three-stranded structure with a displaced DNA strand (Fig. 4C) (43). The hybridization of the nascent RNA strand with the template DNA can be detrimental to normal gene expression and cell growth (52, 53). In particular, due to their stability, R-loops can disrupt DNA replication (41, 54–57), with particularly severe problems observed in reporter systems when highly expressed genes are oriented head-on with respect to replication (58, 59). Indeed, the effects of transcription-replication conflicts are apparently severe enough to have applied selective pressure biasing essential genes toward co-directional orientations (60). Negative supercoiling has been shown to promote and stabilize R-loops, and every turn of an R-loop that forms effectively removes a single negative supercoil (that is, the effective ΔLk is +1, made possible by the free end of the RNA) (61, 62). The consequences of this additional layer of feedback between transcription, R-loop formation, and supercoiling have not yet been fully explored. R-loops and other types of RNA-DNA hybrids are cleared from cells through the activities of RNase H enzymes, while R-loops are prevented by Topo I activity (as will be discussed shortly) (57, 63–65).
Topoisomerases and their effects on meso scale
Topoisomerases are a group of enzymes that maintain the topology of DNA by resolving extra supercoils and maintaining supercoiling levels (66, 67). By breaking and then re-sealing at least one DNA strand of the double helix, topoisomerases can change Lk of DNA (68). One of the ways this is achieved is by forming and resolving catenanes, or links between strands (see Fig. 2D). Catenanes are made during replication, becoming more apparent as the replication fork nears the end. As the two newly synthesized DNA strands need to be separated, the catenanes that hold them together need to be unlinked, or decatenated. Topoisomerases act both in front of and behind the replication fork, during and after replication, to iron out any extra supercoils, by resolving catenanes and precatenanes. When replication begins, DNA helicase will act as a simultaneous topological barrier and strand melter, moving through the genome. As in the case of transcription, this action generates positive supercoils in front of the replisome which can spread throughout the topological domain. Thus, the nascent daughter strands intertwine to form replication intermediates behind the replisome, termed precatenanes. Precatenanes differ from catenanes only in that they form behind the replication fork and are resolved by topoisomerases before replication is completed (69–71) (Fig. 1E). If left unresolved, precatenanes will lead to improperly segregated chromosomes and anucleate cells (71–73). Importantly, replicative catenated intermediates have only been detected in plasmids (41, 71). Plasmids extensively rely on the replication machinery of the host for their own replication; hence, studies on plasmid replication provide valuable insights into the mechanisms underlying chromosome replication (41). Both Topo III and Topo IV can contribute to the formation and resolution of catenanes in E. coli. The importance of topoisomerase activity to cells is clearly apparent both from the essentiality of Topo I, IV and DNA gyrase (74), and the fact that the highly successful quinolone class of antibiotics, which have rapid bactericidal activity, specifically targets Type II topoisomerases (75).
E. coli has four topoisomerases (see Table 1), and each of these is able to raise or lower the Lk in ds- or ssDNA (76). All topoisomerases in E. coli use strand passage to regulate topology. Topo I and Topo III are Type I topoisomerases, which act by forming a single-strand break and then guiding the other strand (or a double-stranded region of DNA) through the break, creating a ΔLk of +1 (77). Topo IV and DNA gyrase are Type II topoisomerases and form double-strand breaks as an intermediate in their cycle of action, resulting in a ΔLk of −2.
TABLE 1.
Topoisomerase classification
| Topo type | Enzymes | Properties |
|---|---|---|
| Type I | Topo I, Topo III | Single-stranded cleavage, ΔLk of +1, not ATP-dependent, relax negatively supercoiled DNA |
| Type II | Topo IV, DNA gyrase | Double-stranded cleavages, ΔLk of −2, ATP-dependent |
Topo I
Topoisomerase I (Topo I), encoded by topA, is a Type I topoisomerase that removes negative supercoils (78, 79) in both DNA and RNA (80). It works via strand passage activity: binding dsDNA, cutting a single strand, passing the second intact strand through, ligating the cut strand, then performing the same set of steps to the other strand (81, 82). In this way, although Topo I is unable to break dsDNA, it is able to achieve an equivalent outcome in a few extra steps. TopA mutant experiments typically generate suppressors, or compensatory mutations arising in DNA gyrase (78, 83, 84), suggesting a complete loss of the role played by Topo I is lethal (85). Interestingly, topA transcription increases with an increase in negative superhelicity of the DNA (86), suggesting there may be some feedback loop present to regulate local superhelicity. Additionally, lysine acetylation of Topo I was shown to decrease its catalytic activity, which demonstrates how Topo I can be regulated at a post-translational level (87).
Recent ChIP-seq experiments have demonstrated that Topo I colocalizes with RNA polymerase (RNAP) throughout the cell cycle (65). Decoupling the colocalization between Topo I and RNAP leads to R-loop formation and accumulation, suggesting this interaction, and Topo I specifically, are required to eliminate (or at least discourage) R-loops, as demonstrated by qPCR, next-generation sequencing, and Topo-seq (a ChIP-seq-based protocol) (65, 88). Thus, Topo I has been consequently attributed to preventing constitutive stable DNA replication (cSDR) by preventing R-loop formation (89, 90). These topics are discussed further in the subsection “The Influence of DNA Structure on Replication Initiation” below.
Topo III
Topo III is the second Type I topoisomerase in E. coli (91) and also has RNA strand passage activity (92). It removes positive precatenanes from the nascent strand following replication (93) and decatenates dsDNA molecules with the assistance of RecQ (94). As Topo III is a type I topoisomerase, and thus only forms a single strand break at a time, its role in resolving multi-catenanated dimers was initially surprising, but it has since been demonstrated that two separate and sequential strand passage reactions occur in order to enable the needed double-strand passage (i.e., changing Lk by +2, in two separate reactions) (95, 96). In addition to its role in separating sister chromatids, Topo III can also decatenate RNA (92), as well as resolve recombination intermediates (97) and prevent R-loops (88). Topo III deletion mutants remain viable (98) except in temperature-sensitive Topo IV mutants, suggesting that other topoisomerases may take over its role in decatenation in its absence.
DNA gyrase
DNA gyrase is a Type II topoisomerase, with subunits encoded by gyrA and gyrB, that relieves topological stress on DNA by removing positive supercoils and introducing negative supercoils (99, 100). As it is the only topoisomerase that can introduce negative supercoils in E. coli, it plays an essential role in maintaining the overall negatively supercoiled state of the chromosome (18). DNA gyrase expression is regulated by supercoiling levels, with expression increasing when the DNA is relaxed, or supercoiling is decreased (25).
The mechanism of DNA gyrase relies on ATP hydrolysis to catalyze the cutting of DNA, making it sensitive to cellular ATP levels (101). The enzyme binds DNA to form a nucleoprotein complex (102), then cuts the dsDNA, twists the strands around each other, and re-ligates, thus forming supercoils (29, 103, 104). Single-molecule imaging found that during replication, DNA gyrase enzymes are highly localized and active around the replication fork to remove positive supercoils but are also still present around the non-replicating parts of the chromosome to maintain a steady-state of negative supercoils (99). In addition, this action is processive, in that DNA gyrase can perform several catalytic actions without needing to dissociate from the DNA strand (105). Recent Topo-seq experiments have demonstrated that areas downstream of highly transcribed operons are enriched for DNA gyrase cleavage sites (103). These data also identified a DNA gyrase-binding motif, which suggests that the bendability of the DNA region may promote gyrase binding (103).
Topo IV
Topo IV is the second type II topoisomerase in E. coli and is its primary decatenase. Similar to DNA gyrase, it relies on ATP to relax both positive and negative supercoils. Unlike gyrase, however, Topo IV cannot introduce negative supercoils. It can be found at the ter region of chromosomes, decatenating the sister chromatids and removing knots (106), which is made possible, in part, by its interaction with MukBEF, the SMC complex (107). MukBEF orients TopoIV to its decatenation site, and the interaction between the two enzymes allows for the timely segregation of sister chromatids by separating the oris (107). Pulse labeling experiments found that in Topo IV deletion mutants, most newly replicated DNA was still catenated (108), suggesting the decatenation ability of DNA gyrase is insufficient (108), making Topo IV the primary decatenator. However, in DNA gyrase mutants, the sister chromatids still retain some catenanes, suggesting the condensing activity of DNA gyrase on sister chromatids is necessary for Topo IV binding and decatenation (108–110).
While Topo IV activity is highest around the ter, it is also scattered throughout the genome removing precatenanes following the replisome and colocalizes with MukBEF at the ori to assist in separating the sister chromatids in preparation for cytokinesis (107, 111). More recent ChIP-seq experiments have found active DNA replication, and therefore DNA structure, controls where Topo IV interacts with the chromosome during replication, and there are several binding modes Topo IV achieves throughout the cell cycles (111).
NAPs and meso scale
If the E. coli chromosome were constrained by only the walls of the cell, it would form a coil although this is not sufficient in compacting it down to the size it achieves in the nucleoid. Further condensation of chromosomal DNA past that induced by supercoiling is achieved by DNA-binding proteins, which have functions in more than just chromosomal architecture. The E. coli chromosome is associated with approximately 10 DNA-binding proteins that are highly abundant and have substantive and interconnected roles in maintaining chromosomal structure and influencing gene expression under various biological conditions (112, 113). Proteins in this set are often referred to as “nucleoid-associated proteins” (NAPs), although the term is perhaps not entirely appropriate as many other, lower abundance proteins also associate with the nucleoid (including a multitude of lower-abundance transcriptional regulators). Below, we provide a current synthesis of the understanding of the most abundant and heavily studied NAPs and when and how NAPs bind DNA and shape local chromosomal structure.
HU
HU, encoded by hupA and hupB, is a highly conserved, non-sequence-specific NAP and IHF homolog, whose function is to coordinate the E. coli transcriptome and DNA repair by compacting down the chromosome into loops and modifying levels of supercoiling (Fig. 5A) (114–118). HU can be active as a dimer of any combination of HU-α and HU-β; strains with mutations in both genes encoding HU show defects in cell division and cell growth (119, 120) and had reduced viability in response to both cold and heat shock, implying their importance in the stress response to temperature variations (120). For the majority of its functions, HU requires binding DNA with high affinity (121–123); however, it has no sequence-specificity, relying on distorted structures such as single-strand breaks or DNA gaps and junctions to bind (121, 124, 125). Interestingly, HU’s role in chromosome condensation relies on its low-affinity binding to linear DNA. Researchers determined via small-angle X-ray scattering (SAXS) experiments that by binding non-specifically at low affinity, HU changes in expression allowed for the condensation of the chromosome via negative supercoiling (117). When multiple HU dimers bind DNA, they form filaments which straighten DNA then, due to the multimerization of HU, form a bunched DNA network, which causes significant condensation of the chromosome (117). Others have found that HU-α mutants significantly condensed the chromosome by increasing positive supercoils, resulting in the repression and derepression of a number of genes (126). These effects were the result of changes in promoter accessibility and changes in expression patterns of global regulators and distinctly show the significance of HU in nucleoid condensation and gene regulation.
Fig 5.
(A) Nucleoid-associated proteins (NAPs) shape regions of the chromosome by binding and inducing bends, bridging, and looping. Each NAP has its own mechanism of binding and DNA shaping, depending on sequence or environmental conditions. (B) Hfq may phase separate into droplets when bound to DNA in a polyphosphate-dependent manner. (C) MukBEF will load DNA and extrude DNA which twists into loops and plectonemes as the SMC moves further down toward the DNA base. MukBEF can condense DNA by bridging and generating loops. (D) H-NS forms filaments which may generate DNA bridges, where both sides of H-NS bind to two DNA strands, bringing them together. In stiff DNA sections, H-NS binds only one strand of DNA, on one side of the H-NS filament, creating stiffer sections. Taken as a whole, panel D would be an example of the hemi-sequestration model, which regions of the H-NS filament showing both bridging and stiffening conformations. (E) Randomly sampled chromosome conformation accounting for the DNA binding effects of NAPs; in particular, the DNA bending effects of Fis and IHF are modeled alongside supercoiling, overcoming the normally long persistence length of the DNA (see the supplemental material for details). Colors run across a rainbow spectrum (red → purple → red) around the length of the chromosome; for scale, a 1 micron long cell is shown; c.f. Fig. 2A. (F) As in panel E, but with the additional effects of MukB-based looping (see Materials and Methods for details); the same length scale as in panel E is used.
HU also plays a prominent role in long-distance spatial organization of the nucleoid, not only in the formation of DNA loops and condensation (115, 116) but also in forming DNA-DNA contacts between regions kilobases apart (Fig. 5A). High-resolution microscopy experiments have implicated HU in global genomic structuring via the formation of contained supercoiled structures around highly transcribed regions, such as the ribosomal operons (rrns) (127). In addition, studies using psoralen mapping to determine changes in supercoiling between stationary and exponential growth phase found that there is a gradient in negative supercoiling from the origin to the terminus, and HU is necessary to form that gradient. It is important to note, however, that DNA-bound proteins and unwound DNA may inhibit psoralen intercalation and make interpreting these data more difficult (128). Recent 3C experiments (described below) have found hupAB mutants demonstrated the formation of filaments and made fewer long-range contacts (129), compared to WT. Similarly, an orthogonal phage Mu-based method for measuring chromosomal conformations showed that a hupA knockout showed decreased interaction rates between loci distant along the linear genome (130). The mechanism by which these distant contacts are brought together is not well understood, but HU filament formation may be responsible (117).
The full range of the DNA-binding properties of HU are extensive and out of the scope of this review; more information can be found in reference (131).
IHF
IHF is the second most abundant NAP in growing E. coli cells, playing a role in nucleoid structure during the phase transition (112, 132), acting as a transcription factor (133), and serving as a cell cycle regulator by activating the oriC to initiate replication (134). IHF binding to DNA induces the sharpest DNA bend of all NAPs, >160 degrees (Fig. 5A) (135), and which can make the DNA inaccessible to transcription. Chromosome regions containing promoters tend to have less looping, which is resolved when IHF binds, demonstrating its function in loop formation (136). IHF has both specific and nonspecific binding sites, and several binding modes (137), not just the sharp bend found by early crystallography. Depending on the IHF-binding site number, there may be DNA bridging between two DNA strands (137). In stationary phase, IHF abundance significantly increases, along with Dps, and it binds genome-wide with less specificity (132, 138), suggesting IHF plays a role in helping Dps compact the nucleoid during cell starvation (139).
Fis (factor for inversion stimulation)
Fis, encoded by the fis gene, is the most abundant DNA-binding protein in exponentially growing E. coli cells (112, 140). Initially thought only to be involved in site-specific recombination for inversion stimulation, it is now known to have a hand in much more, including transcriptional activation of ribosomal RNA operons (141), regulation of both housekeeping and virulence genes (142), and initiation of replication at oriC (143). Fis has many modes of site-specific binding to DNA and plays an important role in organizing the nucleoid into topological domains by organizing loops in a growth phase-dependent manner (23). It binds as a homodimer to a degenerate 15 base pair sequence motif (144) via a C-terminal helix-turn-helix motif (145). The affinity of Fis to DNA is variable, depending on flanking DNA sequence and “bendability” (146, 147). Because the two DNA recognition sites are spaced 2.5 nm apart, Fis can only bind bent, not straight, DNA (145, 147). The DNA is further bent when Fis binds, by approximately 90° (148). Independent of the bending ability of Fis, it can also change DNA structure by forming branches (149), which keep the chromosome region in a state open to transcription.
In this way, it acts as a topological DNA protein that stabilizes DNA loops by trapping them, which increases DNA branching and compacts DNA via bending (Fig. 5A) (23, 149).
The intracellular concentration of Fis is highly dynamic over the course of the cell cycle, reaching a peak during exponential growth but being virtually undetectable in stationary phase (112). Binding to hundreds of sites along the length of the genome, Fis exerts varying regulatory activities over the course of cellular growth, including a crucial role in activating transcription of ribosomal RNAs and tRNAs during exponential growth (141, 150, 151) and an increasingly expansive role in repressing expression of genes involved in metabolism and amino acid biosynthesis (among others) as Fis accumulates in late exponential cultures (152). When it binds to DNA, it also acts as a barrier to supercoil diffusion and, therefore a topological domain barrier (28).
As the cell transitions to exponential growth phase, the effects of Fis on DNA topology increase, by modulating supercoiling levels (153). This is achieved via its activating effect on topA during oxidative stress (36), and repression of gyrA and gyrB promoters which decreases intracellular levels of DNA gyrase (154). Interestingly, Fis activity is highest at high levels of negative supercoiling, which then prevents DNA gyrase from generating more negative supercoils, and then Fis activity is decreased at low levels of negative supercoiling, a negative feedback loop demonstrating how superhelical density is responsible for fis activity (155).
H-NS (histone-like nucleoid structuring protein)
E. coli H-NS is a highly abundant DNA-binding protein involved in organizing, compacting, and regulating chromosomal DNA. It acts as a xenogeneic silencer, binding highly degenerate sequences with GC contents lower than that of the resident genome (156). In this way, by binding and repressing transcription of high AT content DNA, it acts as a bacterial defense against foreign DNA, which is often of high AT content relative to the host genome (157–159). Loss of H-NS causes de-repression of many horizontally acquired regions of the genome, including virulence genes (133, 157).
At low concentrations, H-NS exists as a dimer, while at higher concentrations, H-NS will oligomerize (160). This allows it to coat the DNA and spread in a linear fashion after it has bound to its AT-rich high-affinity binding sites (161) (Fig. 5B). DNase I footprinting and ChIP-on-chip experiments have found 422 specific binding H-NS binding sites in the E. coli K12 genome, suggesting a model of H-NS binding to regulatory regions, such as promoters, to directly repress gene expression at different levels (158, 159, 162, 163). The bridging of H-NS filaments along the DNA interferes with transcription by creating a loop that restricts RNA polymerase escape, therefore acting as a silencer (164). The H-NS filaments cause RNA polymerase to pause and stop, leading to shortened mRNA strands (165). Scanning force microscopy (SFM) showed that DNA adjacent to RNA polymerase in the initiation complex is bridged by H-NS, suggesting H-NS must repress transcription initiation at rrnBP1, the ribosomal operon promoter (166). More recently, it has been inferred that spurious transcription may occur, as ChIP-seq mapping experiments have shown shortened mRNA transcripts in H-NS-occupied regions (167), suggesting that H-NS occupied DNA may not be completely impervious to RNA polymerase. This was also found to occur in Salmonella (168), and in fact, RNA polymerase activity continued to elongate the new transcript from spurious promoters, and eject bound H-NS from the genome (168, 169).
The topic of H-NS binding to DNA has undergone rigorous and thorough examination since the discovery of H-NS, yet, there remains an ongoing discourse within the field. NMR and optical tweezers have been used to study H-NS-DNA binding, finding that the C-terminal domain of H-NS binds DNA while the N-terminal domain is involved in dimerization and DNA recognition (170–172). Two models have been presented for the precise nature of the contacts and binding sites formed by oligomerized H-NS: the bridging model and the linear stiffening model, in which H-NS interacts with two segments or one segment of DNA, respectively (Fig. 5B). Previously considered to be in conflict, more recent experiments have demonstrated that different physiological conditions can facilitate the switching of H-NS between DNA bridges and the more rigid linear filaments, demonstrating that the models are not mutually exclusive, both aiding in gene silencing under different circumstances (173). Helper proteins such as Hha and YdgT interact directly with H-NS to modify its structure, enabling it to form stiffening or bridging conformations (174).
In the bridging model, H-NS binds DNA as a dimer, the binding domains in the dimer interact near the DNA strands, thus facilitating H-NS bridging across different DNA strands (171) (Fig. 5B), and even allowing the formation of “bridged filaments” in which two or more H-NS filaments, separated in 1-D primary structure space, interact over long distances to form robust complexes (174). When H-NS takes on the stiff conformation, only one side will bind DNA (175). Experiments using magnetic tweezers (MT) stretched tethered DNA in a range of protein and DNA concentrations, and found compared to naked DNA, H-NS binding made the DNA stiffer and more extended (176). Additionally, MT have shown that the stiff mode will prevent the formation of DNA plectonemes, but H-NS bound to DNA as a bridged filament will promote plectoneme formation (177). Combined TEN-map, OH footprinting, and MD simulations plus modeling found DNA-binding domains in H-NS filament contact DNA with irregularity, but at the same location regardless of whether H-NS forms a bridged or linear filament. These data support a new theory, the hemi-sequestration model of linear to bridged H-NS switching (178) (Fig. 5B). Under this model, one H-NS DNA-binding domain binds to DNA, while the second is sequestered by interactions with the N- terminal domains (178), thus closing off one side to DNA binding. In this way, H-NS filaments tend to prevent transcription initiation and slow elongation by RNAP (164, 166) . StpA, an H-NS paralog, and Hha both modulate H-NS activity, and aid in H-NS filament formation, and in fact, may form mixed filaments to further impact gene expression (179). These works showcase a nuanced interplay between DNA supercoiling and the physical organization of DNA by H-NS, while also providing fresh insights into how H-NS carries out its in vivo functions. More on these perspectives can be found in reference (180).
Horizontal gene transfer is a crucial factor in the rapid evolution of bacterial species, and xenogeneic silencers like H-NS likely play a critical role in the regulation and eventual domestication of newly acquired genetic elements (181–184). At the same time, it is notable that the integration of new genes can result in novel effects on chromosome structure (185, 186). Additionally, variations in supercoiling levels between species can lead to imported genes adopting new expression patterns in their new host’s genome, potentially altering their contribution to the cell’s function and enabling them to adapt to new conditions (182, 185, 187). There are considerable differences in gene function, spatial organization, superhelical density between species, even closely related enterobacteria such as E. coli and S. typhimurium (48, 188–190). The full interplay between binding of H-NS (and related proteins), binding and regulation of recently acquired DNA, and the impact of local chromatin structure remain to be fully explored.
Hfq
Hfq (host factor for phage Qβ replication) is a highly abundant nucleic acid-binding protein playing a wide range of roles in the cell. While it was included in an influential early census of DNA-binding nucleoid-associated proteins (113), over the past few decades, Hfq has been much more intensely studied as an RNA-binding protein. Indeed, Hfq plays an instrumental role acting as an RNA chaperone to mediate the regulatory effects of small RNAs (191–193), assists in ribosome assembly (194, 195), and has more recently been found to act as an organizing center for stress granule-like ribonucleoprotein foci that appear in nitrogen-starved E. coli (196–198). Nevertheless, Hfq also shows fairly sequence-independent DNA binding especially to curved DNA (113), and 10%–20% of the Hfq in the cell appears to be nucleoid-associated under standard growth conditions (199). More recently, Hfq has been found to play an apparently RNA-independent role in transcriptional silencing of several horizontally acquired regions of the E. coli genome including lambdoid prophages (200); mechanistically, this repressive activity appears likely to arise from the ability of Hfq to form phase-separated condensates in the presence of high-molecular-weight polyphosphate that can also incorporate DNA and/or RNA (201) (Fig. 5C). An emerging model, based on a combination of biophysical data and tracking of the binding locations of Hfq to DNA in the presence vs absence of polyphosphate, suggests that Hfq may preferentially bind and silence AT-rich regions of the genome in a polyphosphate-dependent manner (201). Key remaining questions include the precise mechanisms through which Hfq is guided to form silencing complexes at specific genomic loci, what the exact nucleic acid composition of Hfq condensates is in vivo (both in terms of whether RNA and DNA co-occur or are parts of separate condensates, and in terms of what specific RNA molecules and genomic regions are included), and how condition-dependent changes in polyphosphate abundance and chain length alter Hfq behavior.
SMC
Structural maintenance of chromosome (SMC) proteins are the primary drivers of chromosome condensation in both prokaryotes and eukaryotes (202). They form complexes, called condensins, and play an active role in shaping nucleoid architecture by compacting the chromosome and aiding in the separation of sister chromatids during cell division (202, 203). The presence of SMC proteins is highly conserved, including the MukBEF complex in E. coli and other gamma-proteobacteria (204). However, MukBEF is not a true homolog of the classic SMC proteins found in most other domains of life (including most prokaryotic clades) (205); instead, it is a functionally and structurally similar analog that has emerged through convergent evolution (206, 207).
Components of the MukBEF complex were first discovered in studies screening for proteins associated with chromosome segregation. Mutations in any of the MukBEF genes in E. coli result in anucleate cells, temperature sensitivity, and structural defects (208–211). This is mostly due to the decondensation of the chromosome (211), demonstrating the importance of this SMC-like family for chromosome segregation and compaction.
MukB, the main condensin, is made up of two ATPase heads (the caps) which are held together by MukF, the kleisin. MukE is the kite protein; it wraps the kleisin and modulates MukB ATPase activity (209, 212–216). The two heads of MukB dimerize and interact with MukF at their base (Fig. 5D). MukF forms dimers that associate with MukE and result in the dimer of dimer MukBEF complexes (206, 213, 217–220). The MukB arm has an elbow that allows it to fold and could aid in DNA capture and condensing (206). Failure of MukF to dimerize and bind to MukE, as well as the failure of MukB to dimerize and interact with MukF, prevents MukBEF from stably binding to the chromosome, leading to anucleate cells and failed chromosome segregation (218). While MukBEF alone can condense DNA, MukB-Topo IV complexes achieve greater compaction (221, 222) although this has recently been disputed via in vitro experiments (223). The splitting of these complexes causes decondensation and irregularities in chromosome segregation (221, 222), indicating the requirement of MukB-Topo IV complex formation to achieve the greatest chromosome compaction.
In E. coli, chromosome compaction by MukB is achieved by organizing the DNA into loops around a series of MukBEF complexes forming an axial core and extruding plectonemic domains (Fig. 3 and 5D). This process requires ATP hydrolysis by MukB (212, 216) and allows for the opening and closing of the MukBEF complex and, therefore, the loading and unloading of DNA (224). Cryo-EM experiments showed how MukBEF will capture DNA loops inside of its ring, this entrapment called the “double-lock configuration” (225), and have provided further insight into some of the conformations of DNA-bound MukBEF.
While MukBEF can load on both positive and negative supercoils, in vitro AFM experiments have found there is a strong preference for positive supercoils (226). MukBEF will load onto a plectoneme, then subsequently extrude a growing loop of DNA (Fig. 5D), which with the assistance of Topo IV, generates supercoils (226). As there are only around 200–400 copies of MukB in a growing cell, it is clear the SMC does not load at every plectoneme and, thus, must act more as a DNA scaffold protein than strictly as a condensin (227).
The SMC system used by E. coli and other members of Enterobactericiae is unusual in several respects which are worth noting. The ParAB system used in most bacterial clades loads at origin-proximal parS sites, has a profound effect on the chromosomal architecture of the cell, and actively drives chromosomal segregation (228, 229). In contrast, in E. coli, there is no parS site for the SMC (MukBEF, in this case) to load specifically (230, 231); aside from Enterobactericiae, the complete absence of a chromosomal par system has only been observed in Pasteurellaceae and Mycoplasmataceae (228). Notably, while there is no par system involved in partitioning of the E. coli chromosome, several low-copy plasmids such as P1 and P7, indeed, depend on a par system for proper segregation (e.g., 232, 233), and those plasmids can partition properly even in the absence of a functional host MukBEF (234).
Given the absence of a parS site, there is no consensus yet on where MukBEF loads onto E. coli DNA. The ter and the ori regions have both been implicated via microscopy and modeling experiments (235–237), while others have suggested it loads randomly around the chromosome, except around ter (238). Using Hi-C to visualize chromosomal interactions, MukBEF deletions demonstrated a loss of long range contacts, further providing support for MukBEF as an organizer of DNA loops (129). The activity of MukB likely further condenses the chromosome; the effects of adding MukB-like looping to our polymer model are shown in Fig. 5F.
MatP, a ter region DNA-binding protein, is responsible for the shape of the MukBEF axial core in addition to displacing MukBEF from the ter region (216, 238). In matP knockout cells, circular axial cores were formed by MukBEF around the entire chromosome, which led to defects in chromosome segregation (216, 238). When MatP binds the ter, it inhibits the condensing activity of MukBEF by pushing it off the DNA, cutting off long-range contacts to regions outside the ter (236). The Ter region contains 23 repeats of the 13 bp palindromic matS sequence, to which MatP binds (235, 236), causing this displacement. Additionally, more recent experiments have found that MatP dimers will bind the MukB hinge, competitively with the ParC domain of Topo IV, which dislodges MukBEF from the DNA (236, 239). Once MukBEF leaves ter, it is led to colocalize with the ori, which is necessary for the proper orientation of the chromosome to prepare for cell division (214–216, 219, 236, 240, 241). Additional information on the role of MukB/MatP interplay in chromosomal replication can be found under the “Chromosome Replication and Cell Division” heading below.
Large extended protein occupancy domains as a function analog of heterochromatin
While the various E. coli nucleoid-associated proteins described above have been studied in isolation for decades, over the last ~10 years, the emergence and widespread application of high-throughput sequencing technologies has permitted a more global perspective to emerge. Indeed, a pioneering application of genome-wide mapping of protein-binding sites on the E. coli chromosome revealed the presence of hundreds of large (>1 kb) regions of the genome that were the sites of dense protein occupancy, referred to as extended protein occupancy domains (EPODs) (242). Based on comparison with gene expression microarrays, EPODs were initially separated into two categories: highly expressed EPODs (heEPODs) and transcriptionally silent EPODs (tsEPODs), which appeared to occupy opposing extremes on the spectrum of gene expression levels present in the cell (242). Subsequent mechanistic work demonstrated that the heEPODs, in fact, simply represented regions of high RNA polymerase occupancy (243), whereas tsEPODs appear to exist in several “flavors” characterized by binding of different combinations of NAPs (200). Notably, tsEPODs appear to also repress newly integrated genes which fall into them and, thus, repress truly repressive chromosomal contexts (244). The activity of xenogeneic silencers such as H-NS in repressing horizontally acquired genes, and thus facilitating the domestication of those genes within bacterial genomes, has long drawn analogies to heterochromatin, both in terms of the structural nature of the tightly packed nucleoprotein assembly present in both cases, and the regulatory roles in terms of silencing genomic parasites and regulating what might be termed developmental processes. More recent findings that EPODs in E. coli can, in fact, represent any of several classes of dense, transcriptionally silencing protein occupancy [including, but not limited to, H-NS/StpA filaments, and also likely encompassing mixed regions of IHF/H-NS occupancy and also Hfq condensates (200, 201, 243)] demonstrates an expanded set of regulatory options for cells in silencing potentially harmful chromosomal regions, or at least those requiring tight regulation. While the presence of highly curved and AT-rich DNA appears to be a common thread across the binding preferences of many proteins involved in forming the various classes of EPODs, we still lack a complete understanding of why silenced, heterochromatin-like regions form in some AT-rich regions or not others, the dynamics of disassembly/reassembly of these complexes upon passage of the replication fork, and the extent to which post-translational modifications on NAPs [which have been observed in several mass spectrometry studies (245–247)] may act as a bacterial analog to the eukaryotic histone code. All of these questions remain areas of active research.
LARGE-SCALE ARCHITECTURE (SPATIAL LEVEL)
At kilobase- and megabase-scale lengths, scales beyond those of topological domains, research over the past two decades has provided considerable insight into the layout of the E. coli chromosome in the cell. A central concept in the large-scale chromosomal architecture of E. coli is that of the macrodomain: a large region of the chromosome that is self-associated, but isolated from other chromosomal elements, in three-dimensional space. Macrodomains are large, at the level of megabase-length scale, and are hypothesized to be flanked, in part, by ribosomal operons, which act as topological and spatial barriers between macrodomains owing to their high transcription (39, 248). The organizational unit of a macrodomain is a chromosome interacting domain (CID), which is on the 100 kb length scale, and CIDs within a macrodomain will interact more frequently with each other than CIDs of other macrodomains. CIDs are spatially isolated domains and are considered to be flanked by plectoneme-free regions (PFRs) and ribosomal operons, which serve as domain barriers (39, 249). It is essential to note the distinction between spatial proximity—which defines macrodomains and CIDs and refers to locations in three-dimensional space—and topological connectivity, which defines the topological domains discussed above, and is defined solely by often-stochastic barriers that prevent the propagation of supercoiling. Formally, it is possible, but not required, that boundaries between topological domains coincide with boundaries between spatial domains. Given that regions of high RNA polymerase binding have been shown to act as supercoiling domain boundaries (250), and that highly expressed genes occur at CID boundaries in a wide range of species (249, 251, 252), it appears very likely that, in many cases, the two domain boundary types would, indeed, coincide although this need not always be the case.
Early fluorescence in situ hybridization (FISH) studies suggested that the E. coli chromosome compacts down into a ring containing two macrodomains, the Ori macrodomain and the Ter macrodomain, each about 1 Mb in size (253), containing the origin of replication (oriC) and terminus of replication (ter), respectively (Fig. 6). By tracking the motion of 23 evenly-spaced FISH targets, Niki et al. (253) found that the linear arrangement of loci within the E. coli genome approximately matched the relative arrangement of those loci within the compacted nucleoid, but that a large region around the origin underwent similar dynamics throughout the cell cycle, as did a large region around the terminus (253). The authors also found the origin and terminus to localize near the midcell, rather than opposite cell poles, before and during early replication (253). Similar results were found using a single-locus FISH survey: Wang et al. (254) determined that the Ori and Ter domains lie at the mid-cell, while the replichores, or chromosome arms of the circular E. coli chromosome, are located on opposite halves of the cell and split transversely during replication. Both publications suggest the E. coli chromosome has a highly ordered structure that is dynamic throughout chromosomal replication and set the stage for future studies investigating macrodomains and other higher-order structures (253, 254).
Fig 6.
Higher-order chromosome architecture techniques. Top to bottom, FISH experiments determined that the Ori and Ter regions will migrate toward the cell center near replication initiation, suggesting there may be large regions that will undergo similar dynamics within themselves throughout the cell cycle (253). λ Int-mediated recombination experiments found recombination frequencies were observed to be relatively high within each macrodomain (but still dependent upon linear distance), but to drop off sharply at macrodomain boundaries. The authors found six total regions within the chromosome: Ori, Ter, Left, Right, and two non-structured domains flanking the Ori macrodomain (255). Chromosome conformation capture experiments use crosslinking to fix contacts in place and then use downstream processing to and sequencing techniques to determine nearby locus contacts (129, 256). ParB/parS techniques use fluorescently-labeled loci to track chromosome contact interactions via microscopy, in real time (130, 257). Mu transposition experiments track the hopping of barcoded phage Mu to generate contact maps between bins (130).
To increase the three-dimensional resolution when determining E. coli chromosome structure, and to put an emphasis on the physical contacts between different sites, Boccard and colleagues adapted site-specific recombination systems to track chromosomal architecture (255). Their methods work based on the assumption that the recombination frequency is a good proxy for physical proximity between loci (255), building off of prior findings regarding the requirement for physical contact between DNA fragments to allow λ Int-mediated recombination events (258). In a seminal paper pioneering these recombination-based methods, Valens et al. (255) inserted pairs of λ att sites throughout the E. coli genome and tracked the rate of recombination events (upon expression of λ Int +Xis) between ~180 different site pairs. The resulting recombination frequencies supported previous findings that macrodomains do, indeed, exist within the E. coli genome (Fig. 6; see also Fig. 7A and C) (255). In particular, this study provided evidence for regions similar to the Ori and Ter found in Niki et al. (253), plus additional macrodomains on the left and right chromosomal arms referred to as “L” and “R,” and two less structured domains. Recombination frequencies were observed to be relatively high within each macrodomain (but still dependent upon linear distance), but to drop off sharply when crossing macrodomain boundaries. Subsequent papers from the same group found that the DNA within the four macrodomains is more restricted in its movement than the DNA found in the non-structured (NS) regions (259). While macrodomains are dynamic, they are restrained to isolated areas within the cell, as determined by time-lapse fluorescence microscopy (259) and undergo consistent patterns of movement over the course of the cell cycle, again consistent with the understanding of macrodomains arrived at by the FISH experiment outlined above. In addition, the Ter domain was found to be associated with the replication machinery at mid-cell, and the organization of Ter relied on the protein MatP, which assists in compaction and timely separation of the sister chromatids in E. coli (235, 260).
Fig 7.
Models for the overall layout of the E. coli chromosome. (A) Macrodomain regions in E. coli, from reference (255). (B) Hi-C contact map replotted using data from reference (129) (contact frequencies from the associated GEO entry are log10 scaled relative to the highest observed count, with a pseudocount of 0.01 added to all bins). The x and y axes show indices of 5 kb bins. (C) Suggested folding patterns of the chromosome if following either the macrodomain model (left) or the small world model (right) with ribosomal operon clustering as in reference (257). Ribosomal operons in black circles. (D) Example chromosomal conformations obtained by adding Hi-C contacts from reference (129) to the polymer model used in prior figures; see the supplemental material for details. Shown are side (left) and top (right) views of the entire chromosome, and (below each view) a replotted version highlighting the ter macrodomain region in red.
Advances in genomic techniques have allowed for even greater resolution in chromosome conformation studies. Chromosome conformation capture (3C) and related methods, especially when using a high-throughput sequencing readout, have much higher throughput than previously described methods and consequently provide a higher resolution model of DNA structure. 3C uses crosslinking of DNA, fragmentation with restriction enzymes, then ligation and junction detection to measure the relative contact frequencies of various positions of the genome (Fig. 6). The method involves fixation of the cells with formaldehyde to preserve the physical interactions of proximal loci during dilution and ligation. Following ligation of the loci, the junctions are analyzed, classically using agarose gels (3C), but in some variants using microarrays (e.g., 4C/5C) or even high-throughput sequencing readouts (see below). An interaction score is given to every locus relative to each other locus and mapped on an interaction graph. However, given that the sequencing primers of 3C are locus specific (and in particular, a given primer pair can only read out a single interaction pair), there is a bias when studying interactions between genes, as the investigator chooses which gene interactions to assay. The 4C and 5C methods provide increased flexibility by either providing a one-to-all (4C) or many-to-many (5C) map of interactions in a single experiment (261).
The advent of high-throughput sequencing has substantially facilitated the comprehensive analysis of bacterial chromosomal structure. Using 3C-like proximity cross-linking followed by high-throughput sequencing (referred to as “genome conformation capture,” or “GCC”), Cagliero et al. performed an analysis of the DNA collisions of E. coli in starved cells compared to a growing population (256). While the authors were able to identify some incidence of Ori and Ter macrodomains, they did not find any indication of L, R, or NS macrodomains, as previously described (255, 256), and the Ter macrodomain was defined in their data not as a mutually interacting region but as a region with uniformly low contact frequencies throughout the chromosome. Notably, however, the initial data analysis presented in reference (256) did not normalize the inferred interaction frequencies based on the frequency of appropriate restriction sites or overall abundances of different parts of the genome. Xie et al. (262) and Walker et al. (130) subsequently post-processed to renormalize the GCC data noted above and did not find the domains of the original GCC work to be replicable (262).
An even higher-throughput version of 3C, Hi-C, uses biotinylated nucleotides with next-generation sequencing, which allows for analyzing genome-wide interactions in an “all-vs-all” approach. This method was developed to identify long-range genomic locus interactions (263). Every locus is mapped against every other locus to determine their interaction frequencies. The original developed method was performed in mammalian cells, and they, indeed, found topologically associated domains, or TADs, which are the eukaryotic equivalent to CIDs. Interaction heatmaps are characterized by a strong diagonal line along the center showing enrichment of contacts between neighboring loci (263), with squares around the diagonal line indicating the presence of CIDs and macrodomains.
As the field continued to find the existence of higher-order structures, it was important to address what role CIDs and macrodomains play and what evolutionary advantages they give organisms across the bacterial kingdom. Using 3C and Hi-C, 23 CIDs were also found in Caulobacter crescentus, and it was determined supercoiling is responsible for these phenomena, defining boundaries of these regions as areas of high gene expression (252). CIDs in Caulobacter have been found inside Mb-scale macrodomains and have been demonstrated to have fixed barriers (249). Following the discovery of CIDs in Caulobacter, Wang 2015 (264) and Marbouty 2015 (265) found that Bacillus subtilis also contains 20–25 CIDs with boundaries occurring by highly expressed genes (264). Subsequent studies using 3C and Hi-C in C. crescentus found that the rate of transcription and the transcript length are responsible for the formation of the CID and macrodomain borders (249), and they confirmed domains are flanked by highly expressed genes which appear as PFRs. In B. subtilis, three global domains were confirmed, and it was determined that the folding conformation of the Ori domain has a regulatory function in the final chromosome arrangement (265). A model has emerged that the formation of CIDs is due to barriers created by highly transcribed regions where the unwound DNA generated by RNAP forms PFRs. The PFR acts as a domain barrier by preventing the spread of supercoiling which physically separates genomic loci and prevents or decreases neighboring interactions.
Following the advances on chromosome interaction domain mapping and function in B. subtilis and C. crescentus, similar progress was made in the study of the role of CIDs and macrodomains in E. coli. Using Hi-C, Lioy et al. were able to find 31 CIDs in exponentially-growing E. coli and observed the Ter macrodomain [which is approximately 800 kb in size (129)] and two loosely structured regions. The Ter macrodomain contains MatP, which prevents the folding and consolidation function of MukBEF, forcing the formation of a discrete structure (129). In addition, MatP, when bound to its binding site in the Ter, matS, will form a bridged tetramer by binding a distant MatP-matS complex (266). The looping of various MatP-matS complexes in Ter may help contribute to its insulation from the rest of the chromosome (266) alongside the MukBEF exclusion noted above. Performing Hi-C experiments on cells with any of several deletions of key chromosomal structuring factors yielded several additional insights: loss of MatP removed the special sequestered nature of the Ter domain and made it behave like the remainder of the genome (although, notably, contacts between matS sites were not apparent); loss of MukB and/or HU caused a general reduction in long-range contact frequencies; loss of Fis appears to cause the loss of long-range contacts (>100 kb); and loss of H-NS caused an increase in contact formation involving H-NS bound regions (presumably due to removal of the normally repressive and bulky H-NS filaments). A more recent Hi-C experiment explored the consequences on chromosome organization when removing each topoisomerase (267). The authors confirmed that supercoiling homeostasis is critical in CID organization in E. coli, and each topoisomerase plays a key role in maintaining either short- or long-range genomic contacts that facilitate that homeostasis (267). It is notable, however, that the aforementioned GCC study using similar methods but a lower concentration of formaldehyde for crosslinking showed qualitatively different results (256), specifically no macrodomains. At present, it is not clear whether the discrepancy arises due to the crosslinking procedure itself or some other differences between the methods used in the studies noted above. In addition, while the Hi-C experiments described here have provided substantial insight into the domain structure of the E. coli genome, primarily in the form of various-sized “boxes” around the diagonal in the contact map (Fig. 7B), and also revealed systematic changes in long-range contact frequencies upon the deletion of several NAPs, some previously observed contacts such as the spatial clustering of ribosomal RNA operons appear to be missing.
Microscopy and recent higher-throughput sequencing experiments found that spatial clustering can occur for genes that are not near each other in linear genomic space but are sometimes co-regulated (130, 257). FROS microscopy experiments show that six out of the seven rRNA operons colocalized in three-dimensional space despite being separated by >2 million bp on the linear genome (257). This was confirmed via parS/ParB microscopy, which showed not only the clustering of ribosomal operons but also the clustering of several genes involved in DNA replication and separately, the clustering of genes from the phenylalanine biosynthesis pathway (130) (Fig. 6), and was supported by Mu transposition experiments. This is consistent with previous re-analysis of an E. coli GCC study that had suggested genes which are coregulated may cluster (262). This could be the case as genes seem to cluster by pathway and co-express (244, 262), suggesting the existence of transcription factories, which may explain the impact of highly expressed regions on genome architecture found in previous work (249, 264, 265); it is notable, however, that clustering of the rRNA operons does not require active transcriptional elongation (based on treatment with rifampicin) (257), leaving open the question of what factors drive rrn co-localization. Interestingly, these long-range contacts were not seen in 3C studies (129). Likewise, using 3C and fluorescence microscopy, Weickert et al. have found the transcription factor GalR to form long-range simultaneous interactions with its binding sites located on opposite sides of the chromosome (268, 269), while others have found that GalR can form DNA bridges between distant sites by mediating the formation of loops (270). These studies have suggested that while the large-scale chromosome conformation is boundaryless and well-mixed, there are conserved pairwise interactions between linearly distant loci (257).
Recent work has provided alternative, and potentially complementary, methods to determine DNA contact frequency (130). The transposable phage Mu can integrate its DNA into E. coli at a high frequency with virtually no location bias (271, 272) and transposes via replicative transposition. Similar to the λint system described above, Mu will only transpose when it physically interacts with the target site (176, 272). By tracking transposition of Mu as a proxy for physical contacts between distant genomic loci without the need for formaldehyde crosslinking, these methods are opening the field to exploring bacterial genome organization at a higher rate with less potential for artificial genome conformations. Through utilization of Mu transposition, the authors found some co-regulated clusters of genes that are not near each other linearly but may co-localize in 3D space (130); however, their study only looked at 35 sites of Mu transposition, leaving thousands of base pairs in between transposed sites (Fig. 6). The overall picture that emerged, however, is that of a boundary-less E. coli genome that is mostly well-mixed, but with some specific long-range contacts (Fig. 7C). Key long-range contacts notably included clustering of six out of the seven ribosomal RNA operons [consistent with fluorescence microscopy data (257)], as well as clustering of functionally related genes such as those involved in aromatic amino acid metabolism (130). These findings are consistent with Hi-C experiments performed at low formaldehyde concentrations (256) but not at high formaldehyde concentrations (129), as noted in reference (3). At present, the apparent tension between the extremes of a genome highly structured by macrodomains/CIDs, and a mostly well-mixed chromosome maintaining specific long-range contacts, remains unresolved; it appears likely that the different experimental methodologies reviewed above probe different aspects of chromosomal structure, and may be more or less sensitive to factors such as timescales of contacts, progression of the cell cycle over the course of the measurements, and presence and nature of proteins bound at different loci. Additional experimental data and careful consideration of the assumptions underlying existing studies will likely be necessary to understand, and fully incorporate, the precise biological entities being measured by each method. Such an integrative approach will be necessary to synthesize a complete model of the ensemble of chromosomal conformations present in live cells.
Sequestration of the ter region from the remainder of the chromosome has been one of the most consistently observed phenomena across all of the chromosomal structure measurement experiments described above; it is plainly apparent, for example, in the Hi-C maps of reference (129) (Fig. 7B and D). Recent efforts to investigate the role of MatP in ter organization have demonstrated that the ter is much more dynamic than previously characterized (273). Indeed, MatP does constrain the ter, but this was only apparent after replication was complete but before decatenation of sister chromatids, as determined via in vivo microscopy foci tracking and in vitro high-throughput tethered particle motion (TPM) experiments (273). It is vital to consider, then, that while high-throughput sequencing experiments such as Hi-C provide a high-resolution data set, an ensemble average of DNA conformations is collected across a population, low-throughput experiments such as microscopy may capture a more nuanced image, but at a much lower throughput. An example of such behavior can be observed in Fig. 7D, which represents a plausible model for the overall structure of the nucleoid upon consideration of Hi-C derived contacts, but is only one of a huge number of possible conformations even subject to those constraints; thus, it should not be surprising that in any one cell, the entire ter macrodomain (for example) may still not form a single compact globule. Instead, the ter tends to self-associate, and averaged across a large population of cells, a strong pattern of internal contacts emerges (as in Fig. 7B). Compounding the issue, synchronization of E. coli is more technically challenging than it is for many other organisms, and thus while Hi-C experiments have been performed on synchronized cultures of organisms such as B. subtilis (274), equivalent data are not available in E. coli.
Moving forward, several driving questions remain for the field, including How are the organization of the E. coli nucleoid and gene expression correlated? Does gene function dictate folding patterns? Is the physical location of functionally similar genes in three-dimensional space somehow correlated with the gene regulation? To what extent do the boundaryless vs CID models of the genome hold, and to what extent does this behavior change across the cell cycle? A combination of improved experimental methodologies and careful re-analysis of existing data will likely provide answers.
CHROMOSOME REPLICATION AND CELL DIVISION
The proper replication and segregation of chromosomes are integral for all life. To ensure that these processes are accurate and timed appropriately, a variety of mechanisms exist that oversee their completion. The situation is further complicated in many bacteria (including E. coli) due to the fact that multiple nested rounds of replication may be co-occurring with cell division. Thus, segregation of completed chromosomal copies must proceed even while both of those copies are undergoing further replication (275) (Fig. 8A and B). In this section, we will be reviewing the pathways involved in the replication of an entire chromosome into two sister chromatids, the geometry of the replicating chromosome in the cell and the process involved in ensuring that after cytokinesis, each daughter cell receives at least one copy.
Fig 8.
Replication and its effects on chromosomal structure. (A) Replication in E. coli occurs bidirectionally, with the replisomes colocalizing while the DNA moves through. The two newly synthesized strands form precatenanes and those are later resolved by topoisomerases. (B) In fast growth conditions where cell division occurs at a slower rate than chromosome replication, initiation may occur on an actively replicating chromosome. This generates a second set of replisomes per newly synthesized origin of replication, resulting in two chromosomes per decided cell. (C) DNA replication dynamics. DNA Gyrase moves ahead of the helicase, decatenating and generating negative supercoils for the helicase to melt. The leading strand is synthesized in a continuous manner while the lagging strand replication is discontinuous, by generation of Okazaki fragments that are ligated together after the DNA polymerase has passed. Topo III and Topo IV resolve any precatenanes and rewind the newly synthesized strands to maintain domain Lk.
Replication
The timing of replication initiation in E. coli is influenced more by ATP availability than by cell size. In eukaryotic cells, chromosome replication and cell division occur sequentially and in separate compartments. This is unlike prokaryotes, especially rapidly growing bacteria such as E. coli, where these processes are continually transpiring, and occur in parallel in rapidly growing cells. Notably, cell division does not start after a fixed amount of time, but rather, initiation and cell division are decoupled in fast growth conditions (276–278). Several models exist that outline the link between replication and cell division, the regulation of replication, and the stages therein, as reviewed in references (279–281); here, we briefly outline key points at which replication and chromosomal structure interact.
Replication begins when the ATPase initiator protein, DnaA, binds at sites near and at the oriC and recruits replisome elements to begin initiation. The oligomerization of DnaA initiates unwinding at the DNA Unwinding Element (DUE), a set of AT-rich regions in the origin (282), and with the help of HU and IHF (283), an open pocket of two ssDNA strands is generated. This is termed the open complex. IHF will create a bend in the DNA which is necessary for the formation of the open complex, as well as redistributing DnaA bound at high-affinity sites to its low affinity binding sites (134, 284, 285). The helicase will begin to unwind the DNA, and with the help of other enzymes, recruit DNA Polymerase III (286). Importantly, two replisomes are formed for bidirectional DNA synthesis, each containing a DnaB helicase, which melts the DNA double helix, DNA Pol III, which synthesizes the new strand, and one beta clamp, as determined by radiolabeling and replication assays (287). Toposiomerases act in front of and behind the replication fork to decatenate any changes in Lk caused by over- and under-twisting (Fig. 8C) (71).
In an ATP-driven manner, the helicase moves down the dsDNA and mechanically separates the strands, each of which is then bound by single strand binding proteins (SSBs). These proteins function to protect the ssDNA from nucleases and to prevent the two strands from reannealing (288, 289). As the helicase proceeds along the chromosome, the leading strand is synthesized continuously in the 5′ to 3′ direction, while the lagging strand is discontinuously synthesized in a manner that requires small fragments of newly synthesized DNA to be integrated that are later ligated together. Once the replication fork has passed through, the parent strand is able to be re-coiled; however, the nascent strand contains nicks and incompletely ligated Okazaki fragments that prevent the chromosome from supercoiling to its pre-replication state. DNA Pol I and DNA Ligase move in and resolve any gaps in the lagging strand, while Topo III and Topo IV follow behind the replication fork and remove any positive precatenanes in their support of nascent strand elongation (93, 290) (Fig. 8C).
Once a region of the chromosome has been replicated and rewound, the two nascent strands continue to interact. This cohesion can last between 7 and 15 min and is modulated by SeqA (291–294), a fork-tracking protein and Topo IV. SeqA maintains the catenated state of the newly replicated DNA, preventing Topo IV from acting, until the DNA is re-methylated by Dam, and subsequently decatenation activity by Topo IV may progress (294). Sister chromatid exchange happens in approximately 15% of cell division events in WT E. coli and is the result of a recombination event between the two newly replicated sister chromatids (295). The resulting dimers are resolved by the Xer recombination system via Holliday junctions at the dif site (296, 297).
As the replication factory nears the terminus of replication, the supercoiling and resolving action of topoisomerases, which have generated catenanes and knots, must be resolved to allow sister chromatids to separate. Topo IV, which contains a strong cleavage site near the dif, is loaded there with the help of XerC and MatP (111). It then interacts with XerC, which allows it to bind and cut within this region, yielding two decatenated chromosomes (111, 298, 299). The physical interaction of MukB and the ParC subunit of Topo IV stimulates Topo IV into relaxing negative supercoils (236, 300), which is achieved by its strand passage activity: breaking and re-ligating dsDNA (22, 301). Concomitantly, MatP binds to matS sites, displacing MukBEF, which begins its migration to the ori (Fig. 9B), and as Topo IV associates with MukBEF at the ter, the migration of the SMC complex toward the ori brings Topo IV along with it (236). This action begins the process of the separation of sister ori sites to opposite poles (described in more detail below).
Fig 9.
Nucleosome dynamics. (A) Stationary track model. During replication, the replisomes colocalize near the midcell while the chromosome is pulled through, synthesizing two identical sister chromatids. (B) The ori and ter of the chromosome are pulled to the cell center for replication initiation. As the newly synthesized strands are decatenated, the ori of each chromatid is pulled toward the opposite cell poles as the remainder of the chromosome is replicated. This is directed by a MukBEF gradient, with higher MukB concentrations at the poles. MatP concentrations are highest at the midcell, to anchor the ter to the Z-ring until septation. (C) When the cell begins to divide, the Z-ring will dissociate and the ter will be unlinked from the midcell and migrate toward poles to avoid guillotining.
Replication is complete when both replication forks meet at the Ter and Topo IV has decatenated links between newly replicated chromosomes. The XerCD/dif system then resolves dimers between chromosomes, which is achieved by tyrosine recombinases, XerC and XerD. They bind at the dif and catalyze strand exchange between sister chromatids that results in two chromosome monomers (302, 303), providing full resolution of replication. For a full characterization of replication termination and chromosome structure, more information can be found in reference (304).
Replisome dynamics
The dynamics of DNA as it is replicated is a topic under major scrutiny, in part, due to the challenges in studying this system. First, replication occurs very quickly [the replisome elongates at an average speed of 3,000 nucleotides/second (305)] and, thus, traverses a linear distance on DNA corresponding to a full cell length in less than a second (305). Second, DNA is highly compacted, even during replication, making it difficult to identify how the replisomes act in relation to each other (306). Two models were detailed to describe replisome (relative to the frame of reference of the cell membrane) localization during replication: the Replication Factory Model and the Stationary Track Model. In the Replication Factory Model, a conglomeration of proteins make up a stationary complex, the replisomes, through which DNA is pulled (Fig. 9A); both replicating arms move through the same factory, which is made up of 8–12 polymerases (307–311). In the Stationary Track model, DNA remains stationary, while the replisome moves along each arm, as if on a set of train tracks (312, 313), resulting in the separation of the replication forks as they move around the chromosome arms, then reconvene at the terminus of replication. More recently, the field has reached a tentative consensus mostly surrounding the Replication Factory Model, following time-lapse imaging used to observe DnaN complexes. These complexes localize to the replisomes and allow the movement of the complex to be watched in real time (311). The authors found that at the beginning of replication, there is a single replisome focus located near or at the origin which others have found to contain the two colocalizing sister replisomes (314). As initiation occurs, multiple foci appear and the replisomes will then stay colocalized in the quarter-cell position, only separating occasionally. The colocalization of the sister replisomes is important in establishing a successful and complete replication cycle: inhibition of one replisome will result in the decrease in progress by the other (314). Just before replication is complete, the foci will localize at the midcell. In total, the replisomes are near each other for about 80% of the replication cycle. During the remaining 20% of the replication cycle, the replisomes are separated enough to be seen as distinct puncta by fluorescence microscopy, suggesting there is still some aspect of replication that supports the track model (311), for a limited portion of the overall replication process.
The influence of DNA structure on replication initiation
The structure of the chromosome is another factor that may influence replication initiation. As the binding of the DnaA-ATP complex is required for the origin to unwind, any topological changes that would block or aid that binding will have effects on replication initiation, as will DNA-protein interaction or changes in supercoiling density that affect origin melting. More recently, several studies have demonstrated how activity near the oriC that changes the organization of the chromosome impacts replication initiation. Although the exact mechanism is still unknown, a tether between the chromosome and the cell membrane may block replication initiation, up to 1 Mb away from the origin (315). This is hypothesized to be due to constraints in supercoiling that block access to the oriC or a supercoiling-defecting transcription response (315). Others have suggested that the transcription of gidA and mioC, two genes adjacent to the oriC, may change local topology during their transcription although there is conflicting evidence on the mechanisms by which this is achieved (316).
There are also several NAPs that bind at the origin and bend the DNA, which prevents initiation. Both Fis and IHF knockouts disrupted synchronous replication initiation in fast-growing cells (283). Fis has several binding sites in the oriC, and will bend the DNA when bound, preventing the binding of Dna-ATP complexes to their low-affinity binding sites (317). As DnaA levels increase, it displaces Fis, which dissipates from the oriC, and IHF quickly distributes DnaA-ATP complexes to all binding sites (318) by inducing a sharp bend (284), stimulating the unwinding of the oriC. These observations are made in fast growing cells, and it is important to note that in slow-growth conditions, Fis was not seen to have the same regulatory effect on initiation (319).
Additionally, the E. coli chromosome may replicate the entire chromosome from aberrant sites in the absence of the oriC or dnaA. This aberrant replication has been observed to use R-loops as the primer for initiation (57, 320) and takes the form of persistent replication even with the addition of replication inhibitors. oriC/dnaA-independent replication has been observed in rnhA mutants, recG mutants, and dnaA mutants and is termed constitutive stable DNA replication (cSDR) (55). cSDR likely requires some interplay between the RecA proteins and R-loops for initiation and has been speculated to represent a potential ancestral form of replication, which may be constitutively active in a subpopulation of E. coli even under normal growth conditions [reviewed in references (320, 321)].
Influence of replication on DNA structure
As the replisome moves along the chromosome, the DNA is unwound and melted by the replicative helicase. The stress of this unwinding at the replication fork forces the overwinding of the region in front of the approaching replication fork, resulting in a less relaxed state of the DNA, i.e., less bp per twist around the helical axis (26), similar to the differential supercoiling shown in Fig. 4 in the vicinity of RNA polymerase. In this state, the Lk has not changed as compensatory twists are introduced; however, the DNA is overtwisted ahead of the fork, adding stress. Failure to address the overwound DNA would prevent the replisome from progressing (26, 322). Thus, the DNA will generate positive supercoils to maintain its relaxed state (Fig. 8C). Adopting positive supercoils in front of the replisome induces changes behind the replisome: the DNA is rewound and compensatory negative supercoils are induced, essentially pushing the replication fork forward (Fig. 8C) (43). As more supercoils are induced by the helicase and polymerase, stress is added to the DNA molecule, which would cause it to break without the help of topoisomerases (323).
Chromosome migration to cell center
In order for each daughter cell to receive a sister chromatid, or an identical copy of the chromosome, the nucleoid must be positioned accurately in the cell center, and then the chromatids must segregate before the cell fully divides. This is accomplished throughout the cell cycle via several cellular mechanisms that ensure the Ter region of the chromosome moves toward the divisome, at the midcell. The divisome is a complex of proteins that drive cell division (324), composed of over 30 proteins bound to the Z-ring (Fig. 9B). This ring is composed of FtsZ-protofilaments that act as the scaffold for the divisome (325), and its formation is the first step in cell division. There are four main mechanisms that ensure the precise positioning of the Z-ring at the midcell along with the chromosome and make sure the chromatids are split before the cell is split: nucleoid occlusion (NO), Ter linkage, the Min system, and FtsK. We will cover these briefly below, but more detailed reviews can be found in (326, 327).
Nucleoid occlusion
Nucleoid occlusion (NO) is the negative regulation of Z-ring formation at the cell center and works by preventing cell division during active transcription and translation around the nucleoid by various mechanisms. Early fluorescence microscopy observations of cell constriction locations determined that chromosomal DNA is absent from the area of constriction (328), suggesting that the presence of the nucleoid prevents cytokinesis from occurring where chromosomal DNA is located (329). This occurs partly because of SlmA, a NAP that binds to FtsZ and acts as an antagonist by depolymerizing FtsZ polymers (330–332). SlmA has binding sites all over the chromosome except at the Ter, where the Z-ring is directed to form (330, 331). Additionally, transcription has been found to prevent nucleoid occlusion, as cells treated with rifampicin had Z-rings form all over the nucleoid, resulting in anucleate daughter cells (333). Thus, by negative regulation, various mechanisms direct the Z-ring to form near the Ter via NO, as the ter migrates toward the midcell.
Min system
In addition to nucleoid occlusion, the Min system acts as a negative regulator for cell division proteins by blocking Z-ring formation at the cell poles. Three Min proteins, MinC, MinD, and MinE, are responsible for positioning the Z-ring and defining the midcell. Similar to SlmA, MinC is a FtsZ antagonist, while MinD and E properly position and stimulate MinC. MinC will bind to the C-terminal domain of FtsZ, which results in a depolymerization of the polymer (334, 335). The details of this mechanism have been studied extensively, e.g., as recently reviewed in reference (336). Importantly, the oscillations of the Min proteins concentrate the antagonizing effects of MinC on FtsZ at the cell poles (335, 337–339), preventing the Z-ring from forming there, and thus adding another mechanism of localization of the Z-ring at the midcell.
While the Min system and nucleoid occlusion both negatively regulate the positioning of the chromosome and Z-ring, cells without functional Min or nucleoid occlusion systems were still able to divide at the midcell although at a much lower success rate (340). This suggests that there are other systems that directly drive the chromosome localization to the midcell.
Ter linkage
Near the end of the E. coli chromosome replication cycle, as the replication machinery is replicating the ter region, divisome assembly starts, initiating the process of cell division (341). Ter linkage is a positive regulatory mechanism that directs the formation of the Z-ring to the midcell. It acts to coordinate the Ter region of the chromosome with the Z-ring, by first signaling the Z-ring to form near the terminus and then by forming a physical link between the Z-ring and the ter region. Several proteins contribute to the Ter linkage mechanism, including MatP, ZapA, and ZapB which, when deleted, significantly alter the accuracy of Z-ring positioning at the midcell (340). The colocalization of the Ter region, MatP, ZapB, and ZapA at the midcell acts as a landmark for FtsZ and drives the formation of the Z-ring there (340) (Fig. 9B). MatP interacts in vivo with matS, a 13 bp motif repeated 23 times in the ter. This interaction compacts the DNA upon binding (235) and allows the C-terminal end of MatP to interact with ZapB, a Z-ring-associated protein (241, 342). Using strains with a linearized chromosome split at Ter, Espeli and colleagues observed MatP holding the chromosome ends at midcell while anchored to ZapB (241). Concurrently, ZapB is also interacting with ZapA, a FtsZ interacting protein. Thus, the makeup of the Ter linkage complex is in the order of DNA-bound MatP, which interacts with ZapB that binds ZapA, ZapA is bound to FtsZ, which is making up the Z-ring. In this way, the Ter region can be directly moved to the midcell, where the Z-ring is located and, thus, be perfectly positioned during cytokinesis.
FtsK
FtsK is an ATP-dependent translocase component of the Z-ring that brings DNA across the cell to the divisome, enabling the divisome to rearrange the chromosome. FtsK binds to the DNA in a sequence-specific manner at KOPS sites (FtsK orienting/polarizing sequence) (343), located on either side of the ter, and then pumps the DNA toward the divisome. The pumping of FtsK releases proteins from the translocated region of chromosome, including MatP, leading to the dissociation of Ter linkage (344, 345). The release of MatP from the ter allows MukB binding, which helps facilitate the separation of the sister chromatids (273, 345, 346). At this point, FtsK also acts as a signal for the Xer recombination system to resolve intercatenation links and chromosome dimers between the sister chromatids (347, 348).
Sister chromatid separation and cytokinesis
The partitioning of sister chromatids occurs almost concurrently with the constriction of the cell membrane. Separation is necessary to ensure that both daughter cells receive a complete chromosome. In many species of bacteria, the parABS partitioning system assists in chromosomal segregation. As E. coli represents one of the rare exceptions to this pattern (229), a combination of several factors instead facilitates the successful separation of sister chromatids. More on bacterial chromosome segregation systems can be found here (229).
In E. coli, MukBEF forms a self-organized gradient in the cell to which the ori is attracted to, and in fact, the colocalization of MukB with the oriC is required for the separation of the newly replicated sister chromatids (Fig. 9B) (211, 214, 237, 238, 240, 349–351). This was validated by polymer simulations (237) and fluorescence microscopy experiments where the ori was seen to migrate toward MukBEF foci at quarter cell positions (214, 237, 240, 349). Topo IV has also been found to colocalize with MukBEF as it migrates to the ori, forming foci around the region (107, 349). Interestingly, the depletion of MukBEF has been shown to disrupt the formation of these foci, while depleting Topo IV does not have the same effect, suggesting that MukBEF is responsible for recruiting Topo IV to the ori (107). In addition, live cell imaging showed the failure of Topo IV to reach the origin region causes a lag in the segregation of the two sister oris (107), further demonstrating the significance of MukBEF and Topo IV interactions in the segregation of chromosomes. MukBEF mutants result in anucleate cells, as newly replicated chromosomes are unsuccessful in properly separating (238). Interestingly, the old template strand is directed toward the old cell pole, while the nascent template strand is directed to the new cell pole, from the previous round of division (238). This suggests there is a non-random segregation of sister chromatids in E. coli. Polymer simulations have suggested that the separation of chromatids is entropy-driven and that following the release of replication constraints, the chromosomes repel each other to opposite poles (352, 353) (Fig. 9C).
The final steps to cell division are the formation of a mature divisome and ultimately the recruitment of FtsN, which triggers peptidoglycan synthesis and the beginning of septation (354–357). Up to this point, the still replicating chromosome had blocked the start of septation, and the termination of replication and subsequent initiation of separation of daughter chromatids initiates constriction (277). Several models exist that outline the processes that regulate cell division and replication and are out of the scope of this review, more can be found here (229, 278, 358, 359).
We have carefully considered here the typical steps involved in chromosomal replication and division, including an intricate choreography in which the ori chromosomal regions are pulled to the cell poles and ter regions are aligned near the center at the time of division. Whereas at other times, the relative locations of the various chromosomal regions are likely not fixed (and, indeed, given the Replication Factory model, the precise locations of different genomic loci within the reference frame of the cell is likely highly dynamic over the course of DNA replication). One of the key challenges in the near future for our understanding of E. coli chromosomal structure is harmonizing the high-resolution contact maps obtained using methods such as Hi-C (which are highly accurate but average across an asynchronous population of cells) with microscopy experiments (which allow high temporal resolution and targeting of one cell, at the expense of clarity and throughput). We expect that with additional effort a fully dynamic map of the E. coli chromosome over the course of replication can be obtained, which we expect may resolve the discrepancies between different experimental methods and reveal the overall ground truth from which each experimental method samples its snapshots.
ENVIRONMENTAL CONDITIONS EFFECTS ON CHROMOSOME ORGANIZATION
The chromosomal dynamics described above provide an essential example of a process in which the spatial layout of the E. coli chromosome must be dynamically adjusted in connection with other cellular processes. Despite the decades of study that we have summarized, several additional complexities linger regarding chromosomal dynamics over the course of replication. For example, because E. coli undergoes multifork replication during rapid growth conditions (Fig. 8B), several copies of chromosomal regions near the origin are present even prior to completely resolving catenanes near the terminus of a previous round of replication (41). At present, it is not yet clear to what extent the excess replicated portions of the chromosome interact with each other or stay isolated from each other, and how any such interactions might affect the contact maps obtained through bulk measurements such as Hi-C and Mu-based contact mapping. Indeed, even at a finer scale, the interplay between passing replication forks and the assembly of stable nucleoprotein complexes (such as H-NS filaments) and dynamic machinery (such as SMC complexes) is largely unstudied but likely plays a profound role in the structure and gene regulation of rapidly growing cells.
Chromosomal replication is only one of several processes known to trigger (and, indeed, require) substantial rearrangements of the E. coli chromosome. Even at short length scales, DNA supercoiling states are strongly affected by the ATP-dependent activity of DNA gyrase, and indirectly by a range of stresses such as temperature changes, osmotic shock, and nutrient availability, thus leading to the potential for rapid supercoiling shifts that alter gene expression (18, 35, 48, 360). Notably, the effects of environmental stresses on supercoiling can even differ between such closely related bacteria as E. coli and Salmonella enterica (189). Moving to slightly longer length scales, many environmental stresses substantially perturb key factors contributing to mesoscale structure; for example, H-NS filaments can be disrupted by rising temperature, changes in pH, and osmotic shock (18, 173, 361–366). At the largest scale, physical stresses such as osmotic shock can cause substantial changes to the overall level of compaction of the entire nucleoid (367). Thus, physico-chemical changes, and altered metabolic states, can substantially alter chromosomal architecture of E. coli, and along with it, exert major impacts on downstream processes such as gene regulation. The following two sections will discuss examples of stress-specific conditions that cause major rearrangements of the nucleoid.
RecA filaments
Several specific stress conditions have also been shown to trigger major rearrangements in the structure of the nucleoid. An example of such a rearrangement is when double-stranded breaks (DSBs) occur on DNA, homologous recombination is part of the crucial SOS response in E. coli and will accurately restore the proper sequence (368, 369). A RecBCD complex will excise the ends of the broken DNA to form ssDNA (370, 371), and then a RecA filament wraps around each strand of DNA and begins a homology search around the nucleoid (372). From an initially diffuse localization throughout the cell, RecA will form a cluster and then grow into a bundle-like structure when it encounters DSBs (373) (Fig. 10A). This occurs after replication of the loci, with the filament moving a cut sister locus across the cell, forming long-range contacts as it moves to find homology in the sister chromosome. These filaments form a variety of conformations and can span the entire length of the cell (Fig. 10A), forming on the DSBs and then finding sequence homology in incredibly short timeframes, as short as 6 min (374) when grown in minimal media in normal growth conditions (37°C). It is initially counter-intuitive, but notable, that the RecA filament can easily span the entire length of the cell; since ssDNA has a length of 0.64 nm per base (375) and thus only a few kilobases of ssDNA would be needed to span E. coli from end to end. A more extensive review of DNA repair can be found in reference (376).
Fig 10.
Chromosomal rearrangements in response to specific stress conditions. (A) The RecA filament wraps around a double-stranded break in the DNA and then grows into a bundle-like structure, growing and searching for sequence homology to the site of the break. The homology search will pull the ssDNA across the cell, where the homologous strand is located, facilitating long-range interactions. Such a homology search is only possible when there are two newly synthesized strands as another replicated copy exists of the broken DNA. (B) The crystalline lattice structure of the Dps-coated DNA compacts the nucleoid significantly during starvation and forms phase-separated complexes that allow transcription to occur while protecting the DNA. (C) Side-by-side comparison of Hi-C contact maps (replotted data) from Lioy 2018 (129) during exponential growth in minimal media at 30C (left) vs in stationary phase (right).
Dps activity during starvation
While expression levels of most NAPs decrease in times of stress and starvation, to conserve resources, DNA-binding protein from starved cells (Dps) increases in abundance several folds (112). During the stationary phase, Dps will coat DNA in a crystalline lattice (377) forming super condensed Dps-DNA complexes that significantly compact the entire nucleoid (378, 379) (Fig. 10B). These complexes will phase separate, protecting the DNA from degradation but still allowing transcription within them (380, 381). In this way, E. coli is able to safeguard its genetic material, significantly compact the genome to save space and resources, and be able to quickly recover once conditions improve (378). IHF may also play a role in the compaction of the chromosome during starvation, as the concentration of free IHF significantly increases in the stationary phase (132), and it binds DNA non-specifically at low affinity (132, 138). In fact, Dps and IHF may bind and condense DNA competitively, in various stressful conditions (139).
While the nucleoid takes on an extremely compacted structure during periods of starvation (Fig. 10B), its structure during slow growth and stationary phase is incredibly dynamic. Hi-C maps show it to have an increase in long-range interactions, especially in the Ter region (129). Interestingly, CID boundaries changed, although not to the extent that we would expect, considering the significant compaction of the nucleoid in stationary cells (382), based on a side-by-side comparison of the log phase and stationary phase contact maps (Fig. 10C). One likely interpretation is that for a complete crystalline lattice to form, the cells need to remain in stationary phase for extended periods of time.
OUTLOOK
The spatiotemporal rearrangements highlighted above represent only a sampling of the large-scale rearrangements which must be accounted for in considering the dynamics of the E. coli chromosome. In order to truly understand the full extent of structural changes in the chromosome, and their interplay with gene expression, DNA replication, and both local and large-scale DNA conformations, a combination of new techniques will need to be developed and applied. Ideally, these will improve the ability to capture large amounts of single-cell data under well-defined conditions with high temporal resolution. In addition, consideration of a larger number of biological stresses, including additional temperature and pH stresses, various forms of nutrient limitation, and osmotic stresses, as well as composite stimuli better reflecting the complex combinations of environmental cues that bacteria have evolved to respond to reference (383), will be required, as also recently suggested by Verma et al. (3). While all of these various stimuli have been considered in isolation by at least some of the methods highlighted in the present review, comprehensive characterization of all aspects of structure across a range of conditions, using a unified set of methods probing aspects of chromosomal structure ranging from supercoiling states and NAP-binding locations, up through the overall megabase-layer contact map, will likely be necessary to truly identify (and subsequently, develop predictive models for) the complete interplay of structure and function in the E. coli chromosome.
ACKNOWLEDGMENTS
Special thanks to Dr. Jeremy Schroeder, Dr. Rebecca Hurto, and Dr. Tejas Navaratna for proofreading and providing edits and suggestions.
L.F. and S.K.R. were supported by NIH R35 GM128637. S.K.R. was additionally supported by the NIH Cellular and Molecular Biology Training Grant T32-GM145470.
Biographies

Sonya K. Royzenblat graduated with a degree in Biology from Wayne State University having worked on cave beetle circadian rhythm biology. Her studies transitioned to studying osteoclasts at the University of Michigan in the lab of Dr. Megan Weivoda. Sonya is currently a PhD student at the University of Michigan in Cellular and Molecular Biology within the Program in Biomedical Sciences under the mentorship of Lydia Freddolino. She is a Rackham Merit Fellow. Sonya’s PhD topic is the elucidation of E. coli chromosome conformations.

Lydia Freddolino graduated with a Bachelor’s Degree in Biology from the California Institute of Technology, and subsequently received her Ph.D. in Biophysics and Computational Biology (2009) at the University of Illinois under the mentorship of Professor Klaus Schulten. As a postdoctoral fellow, she pivoted from a focus on biomolecular simulation to bacterial genetics and systems biology, with a particular emphasis on the effects of bacterial chromatin on gene regulation. Since joining the faculty at the University of Michigan in 2015, her research program has evolved to span the areas covered in her doctoral and postdoctoral work, with major foci in bacterial chromosomal structure and transcriptional regulation (particularly emphasizing the roles of nucleoid-associated proteins), and in using molecular modeling as a gateway to predict the structures and functions of poorly annotated bacterial proteins.
Contributor Information
Lydia Freddolino, Email: lydsf@umich.edu.
Olivier Espeli, Collège de France, Paris, France.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/ecosalplus.esp-0001-2022.
Methods for chromosome conformation calculation.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Dickerson RE, Drew HR, Conner BN, Wing RM, Fratini AV, Kopka ML. 1982. The anatomy of A-, B-, and Z-DNA. Science 216:475–485. doi: 10.1126/science.7071593 [DOI] [PubMed] [Google Scholar]
- 2. Manning GS. 2006. The persistence length of DNA is reached from the persistence length of its null isomer through an internal electrostatic stretching force. Biophys J 91:3607–3616. doi: 10.1529/biophysj.106.089029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verma SC, Qian Z, Adhya SL. 2019. Architecture of the Escherichia coli nucleoid. PLoS Genet 15:e1008456. doi: 10.1371/journal.pgen.1008456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dame RT, Rashid F-Z, Grainger DC. 2020. Chromosome organization in bacteria: mechanistic insights into genome structure and function. Nat Rev Genet 21:227–242. doi: 10.1038/s41576-019-0185-4 [DOI] [PubMed] [Google Scholar]
- 5. Barbič A, Zimmer DP, Crothers DM. 2003. Structural origins of adenine-tract bending. Proc Natl Acad Sci U S A 100:2369–2373. doi: 10.1073/pnas.0437877100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manning GS. 2003. Is a small number of charge neutralizations sufficient to bend nucleosome core DNA onto its superhelical ramp? J Am Chem Soc 125:15087–15092. doi: 10.1021/ja030320t [DOI] [PubMed] [Google Scholar]
- 7. Williams LD, Maher III LJ. 2000. Electrostatic mechanisms of DNA deformation. Annu Rev Biophys Biomol Struct 29:497–521. doi: 10.1146/annurev.biophys.29.1.497 [DOI] [PubMed] [Google Scholar]
- 8. Manning GS. 1978. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys 11:179–246. doi: 10.1017/s0033583500002031 [DOI] [PubMed] [Google Scholar]
- 9. Elcock AH, McCammon JA. 1996. The low dielectric interior of proteins is sufficient to cause major structural changes in DNA on association. J Am Chem Soc 118:3787–3788. doi: 10.1021/ja954061m [DOI] [Google Scholar]
- 10. Bates AD, Maxwell A. 2005. DNA topology. Oxford University Press. [Google Scholar]
- 11. Irobalieva RN, Fogg JM, Catanese DJ, Sutthibutpong T, Chen M, Barker AK, Ludtke SJ, Harris SA, Schmid MF, Chiu W, Zechiedrich L. 2015. Structural diversity of supercoiled DNA. Nat Commun 6:8440. doi: 10.1038/ncomms9440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Espeli O, Marians KJ. 2004. Untangling intracellular DNA topology. Mol Microbiol 52:925–931. doi: 10.1111/j.1365-2958.2004.04047.x [DOI] [PubMed] [Google Scholar]
- 13. Vologodskii AV, Cozzarelli NR. 1994. Conformational and thermodynamic properties of supercoiled DNA. Annu Rev Biophys Biomol Struct 23:609–643. doi: 10.1146/annurev.bb.23.060194.003141 [DOI] [PubMed] [Google Scholar]
- 14. Fuller FB. 1971. The writhing number of a space curve. Proc Natl Acad Sci U S A 68:815–819. doi: 10.1073/pnas.68.4.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Postow L, Hardy CD, Arsuaga J, Cozzarelli NR. 2004. Topological domain structure of the Escherichia coli chromosome. Genes Dev 18:1766–1779. doi: 10.1101/gad.1207504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kavenoff R, Ryder OA. 1976. Electron microscopy of membrane-associated folded chromosomes of Escherichia coli. Chromosoma 55:13–25. doi: 10.1007/BF00288323 [DOI] [PubMed] [Google Scholar]
- 17. Kavenoff R, Bowen BC. 1976. Electron microscopy of membrane-free folded chromosomes from Escherichia coli. Chromosoma 59:89–101. doi: 10.1007/BF00328479 [DOI] [PubMed] [Google Scholar]
- 18. Bliska JB, Cozzarelli NR. 1987. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J Mol Biol 194:205–218. doi: 10.1016/0022-2836(87)90369-x [DOI] [PubMed] [Google Scholar]
- 19. Muskhelishvili G, Travers A. 2016. The regulatory role of DNA supercoiling in nucleoprotein complex assembly and genetic activity. Biophys Rev 8:5–22. doi: 10.1007/s12551-016-0237-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duprey A, Groisman EA. 2021. The regulation of DNA supercoiling across evolution. Protein Sci 30:2042–2056. doi: 10.1002/pro.4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinden RR, Carlson JO, Pettijohn DE. 1980. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell 21:773–783. doi: 10.1016/0092-8674(80)90440-7 [DOI] [PubMed] [Google Scholar]
- 22. Crisona NJ, Strick TR, Bensimon D, Croquette V, Cozzarelli NR. 2000. Preferential relaxation of positively supercoiled DNA by E. coli topoisomerase IV in single-molecule and ensemble measurements. Genes Dev 14:2881–2892. doi: 10.1101/gad.838900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skoko D, Yoo D, Bai H, Schnurr B, Yan J, McLeod SM, Marko JF, Johnson RC. 2006. Mechanism of chromosome compaction and looping by the Escherichia coli nucleoid protein Fis. J Mol Biol 364:777–798. doi: 10.1016/j.jmb.2006.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SH, Ganji M, Kim E, van der Torre J, Abbondanzieri E, Dekker C. 2018. DNA sequence encodes the position of DNA supercoils. Elife 7:e36557. doi: 10.7554/eLife.36557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menzel R, Gellert M. 1983. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell 34:105–113. doi: 10.1016/0092-8674(83)90140-x [DOI] [PubMed] [Google Scholar]
- 26. Liu LF, Wang JC. 1987. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A 84:7024–7027. doi: 10.1073/pnas.84.20.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynch AS, Wang JC. 1993. Anchoring of DNA to the bacterial cytoplasmic membrane through cotranscriptional synthesis of polypeptides encoding membrane proteins or proteins for export: a mechanism of plasmid hypernegative supercoiling in mutants deficient in DNA topoisomerase I. J Bacteriol 175:1645–1655. doi: 10.1128/jb.175.6.1645-1655.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dages S, Zhi X, Leng F. 2020. Fis protein forms DNA topological barriers to confine transcription-coupled DNA supercoiling in Escherichia coli. FEBS Lett 594:791–798. doi: 10.1002/1873-3468.13643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown PO, Cozzarelli NR. 1979. A sign inversion mechanism for enzymatic supercoiling of DNA. Science 206:1081–1083. doi: 10.1126/science.227059 [DOI] [PubMed] [Google Scholar]
- 30. Prunell A. 1998. A topological approach to nucleosome structure and dynamics: the linking number paradox and other issues. Biophys J 74:2531–2544. doi: 10.1016/S0006-3495(98)77961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boles TC, White JH, Cozzarelli NR. 1990. Structure of plectonemically supercoiled DNA. J Mol Biol 213:931–951. doi: 10.1016/S0022-2836(05)80272-4 [DOI] [PubMed] [Google Scholar]
- 32. Hinnebusch BJ, Bendich AJ. 1997. The bacterial nucleoid visualized by fluorescence microscopy of cells lysed within agarose: comparison of Escherichia coli and spirochetes of the genus Borrelia. J Bacteriol 179:2228–2237. doi: 10.1128/jb.179.7.2228-2237.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Staczek P, Higgins NP. 1998. Gyrase and Topo IV modulate chromosome domain size in vivo. Mol Microbiol 29:1435–1448. doi: 10.1046/j.1365-2958.1998.01025.x [DOI] [PubMed] [Google Scholar]
- 34. Higgins NP, Yang X, Fu Q, Roth JR. 1996. Surveying a supercoil domain by using the γδ resolution system in Salmonella typhimurium. J Bacteriol 178:2825–2835. doi: 10.1128/jb.178.10.2825-2835.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peter BJ, Arsuaga J, Breier AM, Khodursky AB, Brown PO, Cozzarelli NR. 2004. Genomic transcriptional response to loss of chromosomal supercoiling in Escherichia coli. Genome Biol 5:R87. doi: 10.1186/gb-2004-5-11-r87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinstein-Fischer D, Elgrably-Weiss M, Altuvia S. 2000. Escherichia coli response to hydrogen peroxide: a role for DNA supercoiling, topoisomerase I and Fis. Mol Microbiol 35:1413–1420. doi: 10.1046/j.1365-2958.2000.01805.x [DOI] [PubMed] [Google Scholar]
- 37. Vologodskii AV, Cozzarelli NR. 1993. Monte Carlo analysis of the conformation of DNA catenanes. J Mol Biol 232:1130–1140. doi: 10.1006/jmbi.1993.1465 [DOI] [PubMed] [Google Scholar]
- 38. Guo MS, Kawamura R, Littlehale ML, Marko JF, Laub MT. 2021. High-resolution, genome-wide mapping of positive supercoiling in chromosomes. Elife 10:e67236. doi: 10.7554/eLife.67236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Visser BJ, Sharma S, Chen PJ, McMullin AB, Bates ML, Bates D. 2022. Psoralen mapping reveals a bacterial genome supercoiling landscape dominated by transcription. Nucleic Acids Res 50:4436–4449. doi: 10.1093/nar/gkac244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lal A, Dhar A, Trostel A, Kouzine F, Seshasayee ASN, Adhya S. 2016. Genome scale patterns of supercoiling in a bacterial chromosome. Nat Commun 7:11055. doi: 10.1038/ncomms11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Higgins NP, Vologodskii AV. 2015. Topological behavior of plasmid DNA. Microbiol Spectr 3. doi: 10.1128/microbiolspec.PLAS-0036-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shen BA, Landick R. 2019. Transcription of bacterial chromatin. J Mol Biol 431:4040–4066. doi: 10.1016/j.jmb.2019.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dorman CJ. 2019. DNA supercoiling and transcription in bacteria: a two-way street. BMC Mol Cell Biol 20:26. doi: 10.1186/s12860-019-0211-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rahmouni AR, Wells RD. 1992. Direct evidence for the effect of transcription on local DNA supercoiling in vivo. J Mol Biol 223:131–144. doi: 10.1016/0022-2836(92)90721-u [DOI] [PubMed] [Google Scholar]
- 45. Moulin L, Rahmouni AR, Boccard F. 2005. Topological insulators inhibit diffusion of transcription-induced positive supercoils in the chromosome of Escherichia coli. Mol Microbiol 55:601–610. doi: 10.1111/j.1365-2958.2004.04411.x [DOI] [PubMed] [Google Scholar]
- 46. Ma J, Bai L, Wang MD. 2013. Transcription under torsion. Science 340:1580–1583. doi: 10.1126/science.1235441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chong S, Chen C, Ge H, Xie XS. 2014. Mechanism of transcriptional bursting in bacteria. Cell 158:314–326. doi: 10.1016/j.cell.2014.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Champion K, Higgins NP. 2007. Growth rate toxicity phenotypes and homeostatic supercoil control differentiate Escherichia coli from Salmonella enterica serovar Typhimurium. J Bacteriol 189:5839–5849. doi: 10.1128/JB.00083-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldstein E, Drlica K. 1984. Regulation of bacterial DNA supercoiling: plasmid linking numbers vary with growth temperature. Proc Natl Acad Sci U S A 81:4046–4050. doi: 10.1073/pnas.81.13.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Higgins CF, Dorman CJ, Stirling DA, Waddell L, Booth IR, May G, Bremer E. 1988. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell 52:569–584. doi: 10.1016/0092-8674(88)90470-9 [DOI] [PubMed] [Google Scholar]
- 51. Martis B S, Forquet R, Reverchon S, Nasser W, Meyer S. 2019. DNA supercoiling: an ancestral regulator of gene expression in pathogenic bacteria? Comput Struct Biotechnol J 17:1047–1055. doi: 10.1016/j.csbj.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Massé E, Drolet M. 1999. R-loop-dependent hypernegative supercoiling in Escherichia coli topA mutants preferentially occurs at low temperatures and correlates with growth inhibition. J Mol Biol 294:321–332. doi: 10.1006/jmbi.1999.3264 [DOI] [PubMed] [Google Scholar]
- 53. Baaklini I, Hraiky C, Rallu F, Tse-Dinh Y-C, Drolet M. 2004. RNase HI overproduction is required for efficient full-length RNA synthesis in the absence of topoisomerase I in Escherichia coli. Mol Microbiol 54:198–211. doi: 10.1111/j.1365-2958.2004.04258.x [DOI] [PubMed] [Google Scholar]
- 54. Santos-Pereira JM, Aguilera A. 2015. R loops: new modulators of genome dynamics and function. Nat Rev Genet 16:583–597. doi: 10.1038/nrg3961 [DOI] [PubMed] [Google Scholar]
- 55. Wimberly H, Shee C, Thornton PC, Sivaramakrishnan P, Rosenberg SM, Hastings PJ. 2013. R-loops and nicks initiate DNA breakage and genome instability in non-growing Escherichia coli. Nat Commun 4:2115. doi: 10.1038/ncomms3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roberts RW, Crothers DM. 1992. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science 258:1463–1466. doi: 10.1126/science.1279808 [DOI] [PubMed] [Google Scholar]
- 57. Drolet M, Brochu J. 2019. R-loop-dependent replication and genomic instability in bacteria. DNA Repair (Amst) 84:102693. doi: 10.1016/j.dnarep.2019.102693 [DOI] [PubMed] [Google Scholar]
- 58. Hamperl S, Bocek MJ, Saldivar JC, Swigut T, Cimprich KA. 2017. Transcription-replication conflict orientation modulates R-loop levels and activates distinct DNA damage responses. Cell 170:774–786. doi: 10.1016/j.cell.2017.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lang KS, Hall AN, Merrikh CN, Ragheb M, Tabakh H, Pollock AJ, Woodward JJ, Dreifus JE, Merrikh H. 2017. Replication-transcription conflicts generate R-loops that orchestrate bacterial stress survival and pathogenesis. Cell 170:787–799. doi: 10.1016/j.cell.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rocha EPC, Danchin A. 2003. Essentiality, not expressiveness, drives gene-strand bias in bacteria. Nat Genet 34:377–378. doi: 10.1038/ng1209 [DOI] [PubMed] [Google Scholar]
- 61. Chedin F, Benham CJ. 2020. Emerging roles for R-loop structures in the management of topological stress. J Biol Chem 295:4684–4695. doi: 10.1074/jbc.REV119.006364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stolz R, Sulthana S, Hartono SR, Malig M, Benham CJ, Chedin F. 2019. Interplay between DNA sequence and negative superhelicity drives R-loop structures. Proc Natl Acad Sci U S A 116:6260–6269. doi: 10.1073/pnas.1819476116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tadokoro T, Kanaya S. 2009. Ribonuclease H: molecular diversities, substrate binding domains, and catalytic mechanism of the prokaryotic enzymes. FEBS J 276:1482–1493. doi: 10.1111/j.1742-4658.2009.06907.x [DOI] [PubMed] [Google Scholar]
- 64. Ohtani N, Haruki M, Morikawa M, Kanaya S. 1999. Molecular diversities of RNases H. J Biosci Bioeng 88:12–19. doi: 10.1016/s1389-1723(99)80168-6 [DOI] [PubMed] [Google Scholar]
- 65. Sutormin D, Galivondzhyan A, Musharova O, Travin D, Rusanova A, Obraztsova K, Borukhov S, Severinov K. 2022. Interaction between transcribing RNA polymerase and topoisomerase I prevents R-loop formation in E. coli. Nat Commun 13:4524. doi: 10.1038/s41467-022-32106-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang JC. 1996. DNA topoisomerases. Annu Rev Biochem 65:635–692. doi: 10.1146/annurev.bi.65.070196.003223 [DOI] [PubMed] [Google Scholar]
- 67. Cook DN, Ma D, Pon NG, Hearst JE. 1992. Dynamics of DNA supercoiling by transcription in Escherichia coli. Proc Natl Acad Sci U S A 89:10603–10607. doi: 10.1073/pnas.89.22.10603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ullsperger CJ, Vologodskii AV, Cozzarelli NR. 1995. Unlinking of DNA by topoisomerases during DNA replication, p 115–142. In Eckstein F, Lilley DMJ (ed), Nucleic acids and molecular biology. Springer Berlin Heidelberg, Berlin, Heidelberg. [Google Scholar]
- 69. Cebrián J, Castán A, Martínez V, Kadomatsu-Hermosa MJ, Parra C, Fernández-Nestosa MJ, Schaerer C, Hernández P, Krimer DB, Schvartzman JB. 2015. Direct evidence for the formation of precatenanes during DNA replication. J Biol Chem 290:13725–13735. doi: 10.1074/jbc.M115.642272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lucas I, Germe T, Chevrier-Miller M, Hyrien O. 2001. Topoisomerase II can unlink replicating DNA by precatenane removal. EMBO J 20:6509–6519. doi: 10.1093/emboj/20.22.6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Peter BJ, Ullsperger C, Hiasa H, Marians KJ, Cozzarelli NR. 1998. The structure of supercoiled intermediates in DNA replication. Cell 94:819–827. doi: 10.1016/s0092-8674(00)81740-7 [DOI] [PubMed] [Google Scholar]
- 72. Uemura T, Yanagida M. 1984. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J 3:1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Holm C, Goto T, Wang JC, Botstein D. 1985. DNA topoisomerase II is required at the time of mitosis in yeast. Cell 41:553–563. doi: 10.1016/s0092-8674(85)80028-3 [DOI] [PubMed] [Google Scholar]
- 74. Karp PD, Ong WK, Paley S, Billington R, Caspi R, Fulcher C, Kothari A, Krummenacker M, Latendresse M, Midford PE, Subhraveti P, Gama-Castro S, Muñiz-Rascado L, Bonavides-Martinez C, Santos-Zavaleta A, Mackie A, Collado-Vides J, Keseler IM, Paulsen I. 2018. The EcoCyc database. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0006-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aldred KJ, Kerns RJ, Osheroff N. 2014. Mechanism of quinolone action and resistance. Biochemistry 53:1565–1574. doi: 10.1021/bi5000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deweese JE, Osheroff MA, Osheroff N. 2008. DNA topology and topoisomerases: teaching a “knotty” subject. Biochem Mol Biol Educ 37:2–10. doi: 10.1002/bmb.20244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dasgupta T, Ferdous S, Tse-Dinh Y-C. 2020. Mechanism of type IA topoisomerases. Molecules 25:4769. doi: 10.3390/molecules25204769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. DiNardo S, Voelkel KA, Sternglanz R, Reynolds AE, Wright A. 1982. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell 31:43–51. doi: 10.1016/0092-8674(82)90403-2 [DOI] [PubMed] [Google Scholar]
- 79. Raji A, Zabel DJ, Laufer CS, Depew RE. 1985. Genetic analysis of mutations that compensate for loss of Escherichia coli DNA topoisomerase I. J Bacteriol 162:1173–1179. doi: 10.1128/jb.162.3.1173-1179.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ahmad M, Xue Y, Lee SK, Martindale JL, Shen W, Li W, Zou S, Ciaramella M, Debat H, Nadal M, Leng F, Zhang H, Wang Q, Siaw G-L, Niu H, Pommier Y, Gorospe M, Hsieh T-S, Tse-Dinh Y-C, Xu D, Wang W. 2016. RNA topoisomerase is prevalent in all domains of life and associates with polyribosomes in animals. Nucleic Acids Res 44:6335–6349. doi: 10.1093/nar/gkw508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ahmed W, Bhat AG, Leelaram MN, Menon S, Nagaraja V. 2013. Carboxyl terminal domain basic amino acids of mycobacterial topoisomerase I bind DNA to promote strand passage. Nucleic Acids Res 41:7462–7471. doi: 10.1093/nar/gkt506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lima CD, Wang JC, Mondragón A. 1994. Three-dimensional structure of the 67K N-terminal fragment of E. coli DNA topoisomerase I. Nature 367:138–146. doi: 10.1038/367138a0 [DOI] [PubMed] [Google Scholar]
- 83. Stupina VA, Wang JC. 2005. Viability of Escherichia coli topA mutants lacking DNA topoisomerase I. J Biol Chem 280:355–360. doi: 10.1074/jbc.M411924200 [DOI] [PubMed] [Google Scholar]
- 84. Pruss GJ, Manes SH, Drlica K. 1982. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell 31:35–42. doi: 10.1016/0092-8674(82)90402-0 [DOI] [PubMed] [Google Scholar]
- 85. Stockum A, Lloyd RG, Rudolph CJ. 2012. On the viability of Escherichia coli cells lacking DNA topoisomerase I. BMC Microbiol 12:26. doi: 10.1186/1471-2180-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tse-Dinh YC. 1985. Regulation of the Escherichia coli DNA topoisomerase I gene by DNA supercoiling. Nucleic Acids Res 13:4751–4763. doi: 10.1093/nar/13.13.4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhou Q, Zhou YN, Jin DJ, Tse-Dinh Y-C. 2017. Deacetylation of topoisomerase I is an important physiological function of E. coli CobB. Nucleic Acids Res 45:5349–5358. doi: 10.1093/nar/gkx250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brochu J, Vlachos-Breton É, Sutherland S, Martel M, Drolet M. 2018. Topoisomerases I and III inhibit R-loop formation to prevent unregulated replication in the chromosomal Ter region of Escherichia coli. PLoS Genet 14:e1007668. doi: 10.1371/journal.pgen.1007668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Leela JK, Raghunathan N, Gowrishankar J. 2021. Topoisomerase I essentiality, DnaA-independent chromosomal replication, and transcription-replication conflict in Escherichia coli. J Bacteriol 203:e0019521. doi: 10.1128/JB.00195-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Martel M, Balleydier A, Sauriol A, Drolet M. 2015. Constitutive stable DNA replication in Escherichia coli cells lacking type 1A topoisomerase activity. DNA Repair (Amst) 35:37–47. doi: 10.1016/j.dnarep.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 91. Dean F, Krasnow MA, Otter R, Matzuk MM, Spengler SJ, Cozzarelli NR. 1983. Escherichia coli type-1 topoisomerases: identification, mechanism, and role in recombination. Cold Spring Harb Symp Quant Biol 47:769–777. doi: 10.1101/SQB.1983.047.01.088 [DOI] [PubMed] [Google Scholar]
- 92. Wang H, Di Gate RJ, Seeman NC. 1996. An RNA topoisomerase. Proc Natl Acad Sci U S A 93:9477–9482. doi: 10.1073/pnas.93.18.9477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hiasa H, Marians KJ. 1994. Topoisomerase III, but not topoisomerase I, can support nascent chain elongation during theta-type DNA replication. J Biol Chem 269:32655–32659. doi: 10.1016/S0021-9258(18)31684-3 [DOI] [PubMed] [Google Scholar]
- 94. Harmon FG, DiGate RJ, Kowalczykowski SC. 1999. RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination. Mol Cell 3:611–620. doi: 10.1016/s1097-2765(00)80354-8 [DOI] [PubMed] [Google Scholar]
- 95. Perez-Cheeks BA, Lee C, Hayama R, Marians KJ. 2012. A role for topoisomerase III in Escherichia coli chromosome segregation. Mol Microbiol 86:1007–1022. doi: 10.1111/mmi.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhu Q, Pongpech P, DiGate RJ. 2001. Type I topoisomerase activity is required for proper chromosomal segregation in Escherichia coli. Proc Natl Acad Sci U S A 98:9766–9771. doi: 10.1073/pnas.171579898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lopez CR, Yang S, Deibler RW, Ray SA, Pennington JM, Digate RJ, Hastings PJ, Rosenberg SM, Zechiedrich EL. 2005. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Mol Microbiol 58:80–101. doi: 10.1111/j.1365-2958.2005.04812.x [DOI] [PubMed] [Google Scholar]
- 98. DiGate RJ, Marians KJ. 1989. Molecular cloning and DNA sequence analysis of Escherichia coli topB, the gene encoding topoisomerase III. J Biol Chem 264:17924–17930. doi: 10.1016/S0021-9258(19)84661-6 [DOI] [PubMed] [Google Scholar]
- 99. Stracy M, Wollman AJM, Kaja E, Gapinski J, Lee J-E, Leek VA, McKie SJ, Mitchenall LA, Maxwell A, Sherratt DJ, Leake MC, Zawadzki P. 2019. Single-molecule imaging of DNA gyrase activity in living Escherichia coli. Nucleic Acids Res 47:210–220. doi: 10.1093/nar/gky1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gellert M, Mizuuchi K, O’Dea MH, Nash HA. 1976. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A 73:3872–3876. doi: 10.1073/pnas.73.11.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Higgins NP, Peebles CL, Sugino A, Cozzarelli NR. 1978. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc Natl Acad Sci U S A 75:1773–1777. doi: 10.1073/pnas.75.4.1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vanden Broeck A, Lotz C, Ortiz J, Lamour V. 2019. Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex. Nat Commun 10:4935. doi: 10.1038/s41467-019-12914-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sutormin D, Rubanova N, Logacheva M, Ghilarov D, Severinov K. 2019. Single-nucleotide-resolution mapping of DNA gyrase cleavage sites across the Escherichia coli genome. Nucleic Acids Res 47:1373–1388. doi: 10.1093/nar/gky1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Basu A, Parente AC, Bryant Z. 2016. Structural dynamics and mechanochemical coupling in DNA gyrase. J Mol Biol 428:1833–1845. doi: 10.1016/j.jmb.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nöllmann M, Stone MD, Bryant Z, Gore J, Crisona NJ, Hong S-C, Mitelheiser S, Maxwell A, Bustamante C, Cozzarelli NR. 2007. Multiple modes of Escherichia coli DNA gyrase activity revealed by force and torque. Nat Struct Mol Biol 14:264–271. doi: 10.1038/nsmb1213 [DOI] [PubMed] [Google Scholar]
- 106. Champoux JJ. 2001. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70:369–413. doi: 10.1146/annurev.biochem.70.1.369 [DOI] [PubMed] [Google Scholar]
- 107. Zawadzki P, Stracy M, Ginda K, Zawadzka K, Lesterlin C, Kapanidis AN, Sherratt DJ. 2015. The localization and action of topoisomerase IV in Escherichia coli chromosome segregation is coordinated by the SMC complex, MukBEF. Cell Rep 13:2587–2596. doi: 10.1016/j.celrep.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zechiedrich EL, Cozzarelli NR. 1995. Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev 9:2859–2869. doi: 10.1101/gad.9.22.2859 [DOI] [PubMed] [Google Scholar]
- 109. Ullsperger C, Cozzarelli NR. 1996. Contrasting enzymatic activities of topoisomerase IV and DNA gyrase from Escherichia coli. J Biol Chem 271:31549–31555. doi: 10.1074/jbc.271.49.31549 [DOI] [PubMed] [Google Scholar]
- 110. Peng H, Marians KJ. 1995. The interaction of Escherichia coli topoisomerase IV with DNA. J Biol Chem 270:25286–25290. doi: 10.1074/jbc.270.42.25286 [DOI] [PubMed] [Google Scholar]
- 111. El Sayyed H, Le Chat L, Lebailly E, Vickridge E, Pages C, Cornet F, Cosentino Lagomarsino M, Espéli O. 2016. Mapping topoisomerase IV binding and activity sites on the E. coli genome. PLoS Genet 12:e1006025. doi: 10.1371/journal.pgen.1006025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ali Azam T, Iwata A, Nishimura A, Ueda S, Ishihama A. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J Bacteriol 181:6361–6370. doi: 10.1128/JB.181.20.6361-6370.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Azam TA, Ishihama A. 1999. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J Biol Chem 274:33105–33113. doi: 10.1074/jbc.274.46.33105 [DOI] [PubMed] [Google Scholar]
- 114. Rouvière-Yaniv J, Yaniv M, Germond JE. 1979. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell 17:265–274. doi: 10.1016/0092-8674(79)90152-1 [DOI] [PubMed] [Google Scholar]
- 115. Haykinson MJ, Johnson RC. 1993. DNA looping and the helical repeat in vitro and in vivo: effect of HU protein and enhancer location on Hin invertasome assembly. EMBO J 12:2503–2512. doi: 10.1002/j.1460-2075.1993.tb05905.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lavoie BD, Chaconas G. 1993. Site-specific HU binding in the Mu transpososome: conversion of a sequence-independent DNA-binding protein into a chemical nuclease. Genes Dev 7:2510–2519. doi: 10.1101/gad.7.12b.2510 [DOI] [PubMed] [Google Scholar]
- 117. Hammel M, Amlanjyoti D, Reyes FE, Chen J-H, Parpana R, Tang HYH, Larabell CA, Tainer JA, Adhya S. 2016. HU multimerization shift controls nucleoid compaction. Sci Adv 2:e1600650. doi: 10.1126/sciadv.1600650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li S, Waters R. 1998. Escherichia coli strains lacking protein HU are UV sensitive due to a role for HU in homologous recombination. J Bacteriol 180:3750–3756. doi: 10.1128/JB.180.15.3750-3756.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Huisman O, Faelen M, Girard D, Jaffé A, Toussaint A, Rouvière-Yaniv J. 1989. Multiple defects in Escherichia coli mutants lacking HU protein. J Bacteriol 171:3704–3712. doi: 10.1128/jb.171.7.3704-3712.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wada M, Kano Y, Ogawa T, Okazaki T, Imamoto F. 1988. Construction and characterization of the deletion mutant of hupA and hupB genes in Escherichia coli. J Mol Biol 204:581–591. doi: 10.1016/0022-2836(88)90357-9 [DOI] [PubMed] [Google Scholar]
- 121. Pinson V, Takahashi M, Rouviere-Yaniv J. 1999. Differential binding of the Escherichia coli HU, homodimeric forms and heterodimeric form to linear, gapped and cruciform DNA. J Mol Biol 287:485–497. doi: 10.1006/jmbi.1999.2631 [DOI] [PubMed] [Google Scholar]
- 122. Pontiggia A, Negri A, Beltrame M, Bianchi ME. 1993. Protein HU binds specifically to kinked DNA. Mol Microbiol 7:343–350. doi: 10.1111/j.1365-2958.1993.tb01126.x [DOI] [PubMed] [Google Scholar]
- 123. Shindo H, Furubayashi A, Shimizu M, Miyake M, Imamoto F. 1992. Preferential binding of E.coli histone-like protein HUα to negatively supercoiled DNA. Nucleic Acids Res 20:1553–1558. doi: 10.1093/nar/20.7.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bonnefoy E, Takahashi M, Yaniv JR. 1994. DNA-binding parameters of the HU protein of Escherichia coli to cruciform DNA. J Mol Biol 242:116–129. doi: 10.1006/jmbi.1994.1563 [DOI] [PubMed] [Google Scholar]
- 125. Castaing B, Zelwer C, Laval J, Boiteux S. 1995. HU protein of Escherichia coli binds specifically to DNA that contains single-strand breaks or gaps. J Biol Chem 270:10291–10296. doi: 10.1074/jbc.270.17.10291 [DOI] [PubMed] [Google Scholar]
- 126. Kar S, Edgar R, Adhya S. 2005. Nucleoid remodeling by an altered HU protein: reorganization of the transcription program. Proc Natl Acad Sci U S A 102:16397–16402. doi: 10.1073/pnas.0508032102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Berger M, Farcas A, Geertz M, Zhelyazkova P, Brix K, Travers A, Muskhelishvili G. 2010. Coordination of genomic structure and transcription by the main bacterial nucleoid-associated protein HU. EMBO Rep 11:59–64. doi: 10.1038/embor.2009.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bermúdez I, García-Martínez J, Pérez-Ortín JE, Roca J. 2010. A method for genome-wide analysis of DNA helical tension by means of psoralen-DNA photobinding. Nucleic Acids Res 38:e182. doi: 10.1093/nar/gkq687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lioy VS, Cournac A, Marbouty M, Duigou S, Mozziconacci J, Espéli O, Boccard F, Koszul R. 2018. Multiscale structuring of the E. coli chromosome by nucleoid-associated and condensin proteins. Cell 172:771–783. doi: 10.1016/j.cell.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 130. Walker DM, Freddolino PL, Harshey RM. 2020. A well-mixed E. coli genome: widespread contacts revealed by tracking Mu transposition. Cell 180:703–716. doi: 10.1016/j.cell.2020.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kamashev D, Rouviere-Yaniv J. 2000. The histone-like protein HU binds specifically to DNA recombination and repair intermediates. EMBO J 19:6527–6535. doi: 10.1093/emboj/19.23.6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Murtin C, Engelhorn M, Geiselmann J, Boccard F. 1998. A quantitative UV laser footprinting analysis of the interaction of IHF with specific binding sites: re-evaluation of the effective concentration of IHF in the cell. J Mol Biol 284:949–961. doi: 10.1006/jmbi.1998.2256 [DOI] [PubMed] [Google Scholar]
- 133. Grainger DC, Hurd D, Goldberg MD, Busby SJW. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res 34:4642–4652. doi: 10.1093/nar/gkl542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kasho K, Oshima T, Chumsakul O, Nakamura K, Fukamachi K, Katayama T. 2021. Whole-genome analysis reveals that the nucleoid protein IHF predominantly binds to the replication origin oriC specifically at the time of initiation. Front Microbiol 12:697712. doi: 10.3389/fmicb.2021.697712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rice PA, Yang S, Mizuuchi K, Nash HA. 1996. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell 87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3 [DOI] [PubMed] [Google Scholar]
- 136. Huo Y-X, Zhang Y-T, Xiao Y, Zhang X, Buck M, Kolb A, Wang Y-P. 2009. IHF-binding sites inhibit DNA loop formation and transcription initiation. Nucleic Acids Res 37:3878–3886. doi: 10.1093/nar/gkp258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yoshua SB, Watson GD, Howard JAL, Velasco-Berrelleza V, Leake MC, Noy A. 2021. Integration host factor bends and bridges DNA in a multiplicity of binding modes with varying specificity. Nucleic Acids Res 49:8684–8698. doi: 10.1093/nar/gkab641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lin J, Chen H, Dröge P, Yan J. 2012. Physical organization of DNA by multiple non-specific DNA-binding modes of integration host factor (IHF). PLoS ONE 7:e49885. doi: 10.1371/journal.pone.0049885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lee SY, Lim CJ, Dröge P, Yan J. 2015. Regulation of bacterial DNA packaging in early stationary phase by competitive DNA binding of Dps and IHF. Sci Rep 5:18146. doi: 10.1038/srep18146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Johnson RC, Bruist MF, Simon MI. 1986. Host protein requirements for in vitro site-specific DNA inversion. Cell 46:531–539. doi: 10.1016/0092-8674(86)90878-0 [DOI] [PubMed] [Google Scholar]
- 141. Ross W, Thompson JF, Newlands JT, Gourse RL. 1990. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J 9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kelly A, Goldberg MD, Carroll RK, Danino V, Hinton JCD, Dorman CJ. 2004. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology (Reading) 150:2037–2053. doi: 10.1099/mic.0.27209-0 [DOI] [PubMed] [Google Scholar]
- 143. Filutowicz M, Ross W, Wild J, Gourse RL. 1992. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol 174:398–407. doi: 10.1128/jb.174.2.398-407.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hengen PN, Bartram SL, Stewart LE, Schneider TD. 1997. Information analysis of Fis binding sites. Nucleic Acids Res 25:4994–5002. doi: 10.1093/nar/25.24.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yuan HS, Finkel SE, Feng JA, Kaczor-Grzeskowiak M, Johnson RC, Dickerson RE. 1991. The molecular structure of wild-type and a mutant Fis protein: relationship between mutational changes and recombinational enhancer function or DNA binding. Proc Natl Acad Sci U S A 88:9558–9562. doi: 10.1073/pnas.88.21.9558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bailly C, Waring MJ, Travers AA. 1995. Effects of base substitutions on the binding of a DNA-bending protein. J Mol Biol 253:1–7. doi: 10.1006/jmbi.1995.0530 [DOI] [PubMed] [Google Scholar]
- 147. Pan CQ, Finkel SE, Cramton SE, Feng JA, Sigman DS, Johnson RC. 1996. Variable structures of Fis-DNA complexes determined by flanking DNA-protein contacts. J Mol Biol 264:675–695. doi: 10.1006/jmbi.1996.0669 [DOI] [PubMed] [Google Scholar]
- 148. Zhang J, Zeuner Y, Kleefeld A, Unden G, Janshoff A. 2004. Multiple site-specific binding of Fis protein to Escherichia coli nuoA-N promoter DNA and its impact on DNA topology visualised by means of scanning force microscopy. Chembiochem 5:1286–1289. doi: 10.1002/cbic.200400022 [DOI] [PubMed] [Google Scholar]
- 149. Schneider R, Lurz R, Lüder G, Tolksdorf C, Travers A, Muskhelishvili G. 2001. An architectural role of the Escherichia coli chromatin protein FIS in organising DNA. Nucleic Acids Res 29:5107–5114. doi: 10.1093/nar/29.24.5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gourse RL, de Boer HA, Nomura M. 1986. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell 44:197–205. doi: 10.1016/0092-8674(86)90498-8 [DOI] [PubMed] [Google Scholar]
- 151. Finkel SE, Johnson RC. 1992. The Fis protein: it’s not just for DNA inversion anymore. Mol Microbiol 6:3257–3265. doi: 10.1111/j.1365-2958.1992.tb02193.x [DOI] [PubMed] [Google Scholar]
- 152. Bradley MD, Beach MB, de Koning APJ, Pratt TS, Osuna R. 2007. Effects of Fis on Escherichia coli gene expression during different growth stages. Microbiology (Reading) 153:2922–2940. doi: 10.1099/mic.0.2007/008565-0 [DOI] [PubMed] [Google Scholar]
- 153. Schneider R, Travers A, Muskhelishvili G. 1997. FIS modulates growth phase-dependent topological transitions of DNA in Escherichia coli. Mol Microbiol 26:519–530. doi: 10.1046/j.1365-2958.1997.5951971.x [DOI] [PubMed] [Google Scholar]
- 154. Schneider R, Travers A, Kutateladze T, Muskhelishvili G. 1999. A DNA architectural protein couples cellular physiology and DNA topology in Escherichia coli. Mol Microbiol 34:953–964. doi: 10.1046/j.1365-2958.1999.01656.x [DOI] [PubMed] [Google Scholar]
- 155. Schneider R, Travers A, Muskhelishvili G. 2000. The expression of the Escherichia coli fis gene is strongly dependent on the superhelical density of DNA. Mol Microbiol 38:167–175. doi: 10.1046/j.1365-2958.2000.02129.x [DOI] [PubMed] [Google Scholar]
- 156. Navarre WW, McClelland M, Libby SJ, Fang FC. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev 21:1456–1471. doi: 10.1101/gad.1543107 [DOI] [PubMed] [Google Scholar]
- 157. Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236–238. doi: 10.1126/science.1128794 [DOI] [PubMed] [Google Scholar]
- 158. Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, Hinton JCD. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog 2:e81. doi: 10.1371/journal.ppat.0020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rimsky S, Zuber F, Buckle M, Buc H. 2001. A molecular mechanism for the repression of transcription by the H-NS protein. Mol Microbiol 42:1311–1323. doi: 10.1046/j.1365-2958.2001.02706.x [DOI] [PubMed] [Google Scholar]
- 160. Falconi M, Gualtieri MT, La Teana A, Losso MA, Pon CL. 1988. Proteins from the prokaryotic nucleoid: primary and quaternary structure of the 15-kD Escherichia coli DNA binding protein H-NS. Mol Microbiol 2:323–329. doi: 10.1111/j.1365-2958.1988.tb00035.x [DOI] [PubMed] [Google Scholar]
- 161. Kahramanoglou C, Seshasayee ASN, Prieto AI, Ibberson D, Schmidt S, Zimmermann J, Benes V, Fraser GM, Luscombe NM. 2011. Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res. 39:2073–2091. doi: 10.1093/nar/gkq934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bouffartigues E, Buckle M, Badaut C, Travers A, Rimsky S. 2007. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat Struct Mol Biol 14:441–448. doi: 10.1038/nsmb1233 [DOI] [PubMed] [Google Scholar]
- 163. Ueguchi C, Mizuno T. 1993. The Escherichia coli nucleoid protein H-NS functions directly as a transcriptional repressor. EMBO J 12:1039–1046. doi: 10.1002/j.1460-2075.1993.tb05745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Schröder O, Wagner R. 2000. The bacterial DNA-binding protein H-NS represses ribosomal RNA transcription by trapping RNA polymerase in the initiation complex. J Mol Biol 298:737–748. doi: 10.1006/jmbi.2000.3708 [DOI] [PubMed] [Google Scholar]
- 165. Kotlajich MV, Hron DR, Boudreau BA, Sun Z, Lyubchenko YL, Landick R. 2015. Bridged filaments of histone-like nucleoid structuring protein pause RNA polymerase and aid termination in bacteria. Elife 4:e04970. doi: 10.7554/eLife.04970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dame RT, Wyman C, Wurm R, Wagner R, Goosen N. 2002. Structural basis for H-NS-mediated trapping of RNA polymerase in the open initiation complex at the rrnB P1. J Biol Chem 277:2146–2150. doi: 10.1074/jbc.C100603200 [DOI] [PubMed] [Google Scholar]
- 167. Forrest D, Warman EA, Erkelens AM, Dame RT, Grainger DC. 2022. Xenogeneic silencing strategies in bacteria are dictated by RNA polymerase promiscuity. Nat Commun 13:1149. doi: 10.1038/s41467-022-28747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Figueroa-Bossi N, Sánchez-Romero MA, Kerboriou P, Naquin D, Mendes C, Bouloc P, Casadesús J, Bossi L. 2022. Pervasive transcription enhances the accessibility of H-NS-silenced promoters and generates bistability in Salmonella virulence gene expression. Proc Natl Acad Sci U S A 119:e2203011119. doi: 10.1073/pnas.2203011119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Rangarajan AA, Schnetz K. 2018. Interference of transcription across H-NS binding sites and repression by H-NS. Mol Microbiol 108:226–239. doi: 10.1111/mmi.13926 [DOI] [PubMed] [Google Scholar]
- 170. Bloch V, Yang Y, Margeat E, Chavanieu A, Augé MT, Robert B, Arold S, Rimsky S, Kochoyan M. 2003. The H-NS dimerization domain defines a new fold contributing to DNA recognition. Nat Struct Biol 10:212–218. doi: 10.1038/nsb904 [DOI] [PubMed] [Google Scholar]
- 171. Dame RT, Noom MC, Wuite GJL. 2006. Bacterial chromatin organization by H-NS protein unravelled using dual DNA manipulation. Nature 444:387–390. doi: 10.1038/nature05283 [DOI] [PubMed] [Google Scholar]
- 172. Esposito D, Petrovic A, Harris R, Ono S, Eccleston JF, Mbabaali A, Haq I, Higgins CF, Hinton JCD, Driscoll PC, Ladbury JE. 2002. H-NS oligomerization domain structure reveals the mechanism for high order self-association of the intact protein. J Mol Biol 324:841–850. doi: 10.1016/s0022-2836(02)01141-5 [DOI] [PubMed] [Google Scholar]
- 173. Liu Y, Chen H, Kenney LJ, Yan J. 2010. A divalent switch drives H-NS/DNA-binding conformations between stiffening and bridging modes. Genes Dev 24:339–344. doi: 10.1101/gad.1883510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. van der Valk RA, Vreede J, Qin L, Moolenaar GF, Hofmann A, Goosen N, Dame RT. 2017. Mechanism of environmentally driven conformational changes that modulate H-NS DNA-bridging activity. Elife 6:e27369. doi: 10.7554/eLife.27369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Arold ST, Leonard PG, Parkinson GN, Ladbury JE. 2010. H-NS forms a superhelical protein scaffold for DNA condensation. Proc Natl Acad Sci U S A 107:15728–15732. doi: 10.1073/pnas.1006966107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Amit R, Oppenheim AB, Stavans J. 2003. Increased bending rigidity of single DNA molecules by H-NS, a temperature and osmolarity sensor. Biophys J 84:2467–2473. doi: 10.1016/S0006-3495(03)75051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lim CJ, Kenney LJ, Yan J. 2014. Single-molecule studies on the mechanical interplay between DNA supercoiling and H-NS DNA architectural properties. Nucleic Acids Res 42:8369–8378. doi: 10.1093/nar/gku566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Shen BA, Hustmyer CM, Roston D, Wolfe MB, Landick R. 2022. Bacterial H-NS contacts DNA at the same irregularly spaced sites in both bridged and hemi-sequestered linear filaments. iScience 25:104429. doi: 10.1016/j.isci.2022.104429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Boudreau BA, Hron DR, Qin L, van der Valk RA, Kotlajich MV, Dame RT, Landick R. 2018. StpA and Hha stimulate pausing by RNA polymerase by promoting DNA-DNA bridging of H-NS filaments. Nucleic Acids Res 46:5525–5546. doi: 10.1093/nar/gky265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rashid F-Z, Dame RT. 2023. Three-dimensional chromosome re-modelling: the integral mechanism of transcription regulation in bacteria. Mol Microbiol 120:60–70. doi: 10.1111/mmi.15062 [DOI] [PubMed] [Google Scholar]
- 181. Duan B, Ding P, Navarre WW, Liu J, Xia B. 2021. Xenogeneic silencing and bacterial genome evolution: mechanisms for DNA recognition imply multifaceted roles of xenogeneic silencers. Mol Biol Evol 38:4135–4148. doi: 10.1093/molbev/msab136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Dorman CJ. 2007. H-NS, the genome sentinel. Nat Rev Microbiol 5:157–161. doi: 10.1038/nrmicro1598 [DOI] [PubMed] [Google Scholar]
- 183. Dorman CJ. 2014. H-NS-like nucleoid-associated proteins, mobile genetic elements and horizontal gene transfer in bacteria. Plasmid 75:1–11. doi: 10.1016/j.plasmid.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 184. Singh K, Milstein JN, Navarre WW. 2016. Xenogeneic silencing and its impact on bacterial genomes. Annu Rev Microbiol 70:199–213. doi: 10.1146/annurev-micro-102215-095301 [DOI] [PubMed] [Google Scholar]
- 185. Soucy SM, Huang J, Gogarten JP. 2015. Horizontal gene transfer: building the web of life. Nat Rev Genet 16:472–482. doi: 10.1038/nrg3962 [DOI] [PubMed] [Google Scholar]
- 186. Porwollik S, McClelland M. 2003. Lateral gene transfer in Salmonella. Microbes Infect 5:977–989. doi: 10.1016/s1286-4579(03)00186-2 [DOI] [PubMed] [Google Scholar]
- 187. Higashi K, Tobe T, Kanai A, Uyar E, Ishikawa S, Suzuki Y, Ogasawara N, Kurokawa K, Oshima T. 2016. H-NS facilitates sequence diversification of horizontally transferred DNAs during their integration in host chromosomes. PLoS Genet 12:e1005796. doi: 10.1371/journal.pgen.1005796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Rovinskiy NS, Agbleke AA, Chesnokova ON, Higgins NP. 2019. Supercoil levels in E. coli and Salmonella chromosomes are regulated by the C-terminal 35−38 amino acids of GyrA. Microorganisms 7:81. doi: 10.3390/microorganisms7030081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Cameron ADS, Stoebel DM, Dorman CJ. 2011. DNA supercoiling is differentially regulated by environmental factors and FIS in Escherichia coli and Salmonella enterica. Mol Microbiol 80:85–101. doi: 10.1111/j.1365-2958.2011.07560.x [DOI] [PubMed] [Google Scholar]
- 190. Rovinskiy N, Agbleke AA, Chesnokova O, Pang Z, Higgins NP. 2012. Rates of gyrase supercoiling and transcription elongation control supercoil density in a bacterial chromosome. PLoS Genet 8:e1002845. doi: 10.1371/journal.pgen.1002845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kavita K, de Mets F, Gottesman S. 2018. New aspects of RNA-based regulation by Hfq and its partner sRNAs. Curr Opin Microbiol 42:53–61. doi: 10.1016/j.mib.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Nogueira T, Springer M. 2000. Post-transcriptional control by global regulators of gene expression in bacteria. Curr Opin Microbiol 3:154–158. doi: 10.1016/s1369-5274(00)00068-0 [DOI] [PubMed] [Google Scholar]
- 193. Gottesman S, Storz G. 2015. RNA reflections: converging on Hfq. RNA 21:511–512. doi: 10.1261/rna.050047.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Andrade JM, Dos Santos RF, Chelysheva I, Ignatova Z, Arraiano CM. 2018. The RNA‐binding protein Hfq is important for ribosome biogenesis and affects translation fidelity. EMBO J 37:e97631. doi: 10.15252/embj.201797631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Sharma IM, Korman A, Woodson SA. 2018. The Hfq chaperone helps the ribosome mature. EMBO J 37:e99616. doi: 10.15252/embj.201899616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. McQuail J, Switzer A, Burchell L, Wigneshweraraj S. 2020. The RNA-binding protein Hfq assembles into foci-like structures in nitrogen starved Escherichia coli J Biol Chem 295:12355–12367. doi: 10.1074/jbc.RA120.014107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. McQuail J, Carpousis AJ, Wigneshweraraj S. 2022. The association between Hfq and RNase E in long-term nitrogen-starved Escherichia coli. Mol Microbiol 117:54–66. doi: 10.1111/mmi.14782 [DOI] [PubMed] [Google Scholar]
- 198. Goldberger O, Szoke T, Nussbaum-Shochat A, Amster-Choder O. 2022. Heterotypic phase separation of Hfq is linked to its roles as an RNA chaperone. Cell Rep 41:111881. doi: 10.1016/j.celrep.2022.111881 [DOI] [PubMed] [Google Scholar]
- 199. Kajitani M, Kato A, Wada A, Inokuchi Y, Ishihama A. 1994. Regulation of the Escherichia coli hfq gene encoding the host factor for phage Qβ. J Bacteriol 176:531–534. doi: 10.1128/jb.176.2.531-534.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Amemiya HM, Goss TJ, Nye TM, Hurto RL, Simmons LA, Freddolino PL. 2022. Distinct heterochromatin‐like domains promote transcriptional memory and silence parasitic genetic elements in bacteria. EMBO J. 41:e108708. doi: 10.15252/embj.2021108708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Beaufay F, Amemiya HM, Guan J, Basalla J, Meinen BA, Chen Z, Mitra R, Bardwell JCA, Biteen JS, Vecchiarelli AG, Freddolino PL, Jakob U. 2021. Polyphosphate drives bacterial heterochromatin formation. Sci Adv 7:eabk0233. doi: 10.1126/sciadv.abk0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Strunnikov AV, Hogan E, Koshland D. 1995. SMC2, a Saccharomyces cerevisiae gene essential for chromosome segregation and condensation, defines a subgroup within the SMC family. Genes Dev 9:587–599. doi: 10.1101/gad.9.5.587 [DOI] [PubMed] [Google Scholar]
- 203. Hirano T. 2005. Condensins: organizing and segregating the genome. Curr Biol 15:R265–75. doi: 10.1016/j.cub.2005.03.037 [DOI] [PubMed] [Google Scholar]
- 204. Cobbe N, Heck MMS. 2004. The evolution of SMC proteins: phylogenetic analysis and structural implications. Mol Biol Evol 21:332–347. doi: 10.1093/molbev/msh023 [DOI] [PubMed] [Google Scholar]
- 205. Soppa J. 2001. Prokaryotic structural maintenance of chromosomes (SMC) proteins: distribution, phylogeny, and comparison with MukBs and additional prokaryotic and eukaryotic coiled-coil proteins. Gene 278:253–264. doi: 10.1016/s0378-1119(01)00733-8 [DOI] [PubMed] [Google Scholar]
- 206. Bürmann F, Lee B-G, Than T, Sinn L, O’Reilly FJ, Yatskevich S, Rappsilber J, Hu B, Nasmyth K, Löwe J. 2019. A folded conformation of MukBEF and cohesin. Nat Struct Mol Biol 26:227–236. doi: 10.1038/s41594-019-0196-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Melby TE, Ciampaglio CN, Briscoe G, Erickson HP. 1998. The symmetrical structure of structural maintenance of chromosomes (SMC) and MukB proteins: long, antiparallel coiled coils, folded at a flexible hinge. J Cell Biol 142:1595–1604. doi: 10.1083/jcb.142.6.1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffé A. 1989. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol 171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Yamanaka K, Ogura T, Niki H, Hiraga S. 1996. Identification of two new genes, mukE and mukF, involved in chromosome partitioning in Escherichia coli. Mol Gen Genet 250:241–251. doi: 10.1007/BF02174381 [DOI] [PubMed] [Google Scholar]
- 210. Niki H, Jaffé A, Imamura R, Ogura T, Hiraga S. 1991. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J 10:183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Sawitzke JA, Austin S. 2000. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc Natl Acad Sci U S A 97:1671–1676. doi: 10.1073/pnas.030528397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Niki H, Imamura R, Kitaoka M, Yamanaka K, Ogura T, Hiraga S. 1992. E.coli MukB protein involved in chromosome partition forms a homodimer with a rod-and-hinge structure having DNA binding and ATP/GTP binding activities. EMBO J 11:5101–5109. doi: 10.1002/j.1460-2075.1992.tb05617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Fennell-Fezzie R, Gradia SD, Akey D, Berger JM. 2005. The MukF subunit of Escherichia coli condensin: architecture and functional relationship to kleisins. EMBO J 24:1921–1930. doi: 10.1038/sj.emboj.7600680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Badrinarayanan A, Lesterlin C, Reyes-Lamothe R, Sherratt D. 2012. The Escherichia coli SMC complex, MukBEF, shapes nucleoid organization independently of DNA replication. J Bacteriol 194:4669–4676. doi: 10.1128/JB.00957-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Woo J-S, Lim J-H, Shin H-C, Suh M-K, Ku B, Lee K-H, Joo K, Robinson H, Lee J, Park S-Y, Ha N-C, Oh B-H. 2009. Structural studies of a bacterial condensin complex reveal ATP-dependent disruption of intersubunit interactions. Cell 136:85–96. doi: 10.1016/j.cell.2008.10.050 [DOI] [PubMed] [Google Scholar]
- 216. Mäkelä J, Sherratt DJ. 2020. Organization of the Escherichia coli chromosome by a MukBEF axial core. Mol Cell 78:250–260. doi: 10.1016/j.molcel.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. She W, Wang Q, Mordukhova EA, Rybenkov VV. 2007. MukEF is required for stable association of MukB with the chromosome. J Bacteriol 189:7062–7068. doi: 10.1128/JB.00770-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zawadzka K, Zawadzki P, Baker R, Rajasekar KV, Wagner F, Sherratt DJ, Arciszewska LK. 2018. MukB ATPases are regulated independently by the N- and C-terminal domains of MukF kleisin. Elife 7:e31522. doi: 10.7554/eLife.31522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Badrinarayanan A, Reyes-Lamothe R, Uphoff S, Leake MC, Sherratt DJ. 2012. In vivo architecture and action of bacterial structural maintenance of chromosome proteins. Science 338:528–531. doi: 10.1126/science.1227126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Nolivos S, Sherratt D. 2014. The bacterial chromosome: architecture and action of bacterial SMC and SMC-like complexes. FEMS Microbiol Rev 38:380–392. doi: 10.1111/1574-6976.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kumar R, Grosbart M, Nurse P, Bahng S, Wyman CL, Marians KJ. 2017. The bacterial condensin MukB compacts DNA by sequestering supercoils and stabilizing topologically isolated loops. J Biol Chem 292:16904–16920. doi: 10.1074/jbc.M117.803312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kumar R, Nurse P, Bahng S, Lee CM, Marians KJ. 2017. The MukB-topoisomerase IV interaction is required for proper chromosome compaction. J Biol Chem 292:16921–16932. doi: 10.1074/jbc.M117.803346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kumar R, Bahng S, Marians KJ. 2022. The MukB-topoisomerase IV interaction mutually suppresses their catalytic activities. Nucleic Acids Res 50:2621–2634. doi: 10.1093/nar/gkab1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Murayama Y, Uhlmann F. 2015. DNA entry into and exit out of the cohesin ring by an interlocking gate mechanism. Cell 163:1628–1640. doi: 10.1016/j.cell.2015.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bürmann F, Funke LFH, Chin JW, Löwe J. 2021. Cryo-EM structure of MukBEF reveals DNA loop entrapment at chromosomal unloading sites. Mol Cell 81:4891–4906. doi: 10.1016/j.molcel.2021.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Kim E, Gonzalez AM, Pradhan B, van der Torre J, Dekker C. 2022. Condensin-driven loop extrusion on supercoiled DNA. Nat Struct Mol Biol 29:719–727. doi: 10.1038/s41594-022-00802-x [DOI] [PubMed] [Google Scholar]
- 227. Petrushenko ZM, Lai C-H, Rybenkov VV. 2006. Antagonistic interactions of kleisins and DNA with bacterial condensin MukB. J Biol Chem 281:34208–34217. doi: 10.1074/jbc.M606723200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kawalek A, Wawrzyniak P, Bartosik AA, Jagura-Burdzy G. 2020. Rules and exceptions: the role of chromosomal ParB in DNA segregation and other cellular processes. Microorganisms 8:0. doi: 10.3390/microorganisms8010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Badrinarayanan A, Le TBK, Laub MT. 2015. Bacterial chromosome organization and segregation. Annu Rev Cell Dev Biol 31:171–199. doi: 10.1146/annurev-cellbio-100814-125211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Livny J, Yamaichi Y, Waldor MK. 2007. Distribution of centromere-like parS sites in bacteria: insights from comparative genomics. J Bacteriol 189:8693–8703. doi: 10.1128/JB.01239-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Niki H, Yano K. 2016. In vitro topological loading of bacterial condensin MukB on DNA, preferentially single-stranded DNA rather than double-stranded DNA. Sci Rep 6:29469. doi: 10.1038/srep29469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ludtke DN, Eichorn BG, Austin SJ. 1989. Plasmid-partition functions of the P7 prophage. J Mol Biol 209:393–406. doi: 10.1016/0022-2836(89)90005-3 [DOI] [PubMed] [Google Scholar]
- 233. Funnell BE. 1988. Mini-P1 plasmid partitioning: excess ParB protein destabilizes plasmids containing the centromere parS. J Bacteriol 170:954–960. doi: 10.1128/jb.170.2.954-960.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Funnell BE, Gagnier L. 1995. Partition of P1 plasmids in Escherichia coli mukB chromosomal partition mutants. J Bacteriol 177:2381–2386. doi: 10.1128/jb.177.9.2381-2386.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Mercier R, Petit M-A, Schbath S, Robin S, El Karoui M, Boccard F, Espéli O. 2008. The MatP/matS site-specific system organizes the terminus region of the E. coli chromosome into a macrodomain. Cell 135:475–485. doi: 10.1016/j.cell.2008.08.031 [DOI] [PubMed] [Google Scholar]
- 236. Nolivos S, Upton AL, Badrinarayanan A, Müller J, Zawadzka K, Wiktor J, Gill A, Arciszewska L, Nicolas E, Sherratt D. 2016. MatP regulates the coordinated action of topoisomerase IV and MukBEF in chromosome segregation. Nat Commun 7:10466. doi: 10.1038/ncomms10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hofmann A, Mäkelä J, Sherratt DJ, Heermann D, Murray SM. 2019. Self-organised segregation of bacterial chromosomal origins. Elife 8:e46564. doi: 10.7554/eLife.46564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Mäkelä J, Uphoff S, Sherratt DJ. 2021. Nonrandom segregation of sister chromosomes by Escherichia coli MukBEF. Proc Natl Acad Sci U S A 118:e2022078118. doi: 10.1073/pnas.2022078118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Fisher GL, Bolla JR, Rajasekar KV, Mäkelä J, Baker R, Zhou M, Prince JP, Stracy M, Robinson CV, Arciszewska LK, Sherratt DJ. 2021. Competitive binding of MatP and topoisomerase IV to the MukB hinge domain. Elife 10:e70444. doi: 10.7554/eLife.70444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Danilova O, Reyes-Lamothe R, Pinskaya M, Sherratt D, Possoz C. 2007. MukB colocalizes with the oriC region and is required for organization of the two Escherichia coli chromosome arms into separate cell halves. Mol Microbiol 65:1485–1492. doi: 10.1111/j.1365-2958.2007.05881.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Espéli O, Borne R, Dupaigne P, Thiel A, Gigant E, Mercier R, Boccard F. 2012. A MatP-divisome interaction coordinates chromosome segregation with cell division in E. coli. EMBO J 31:3198–3211. doi: 10.1038/emboj.2012.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Vora T, Hottes AK, Tavazoie S. 2009. Protein occupancy landscape of a bacterial genome. Mol Cell 35:247–253. doi: 10.1016/j.molcel.2009.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Freddolino PL, Amemiya HM, Goss TJ, Tavazoie S. 2021. Dynamic landscape of protein occupancy across the Escherichia coli chromosome. PLoS Biol 19:e3001306. doi: 10.1371/journal.pbio.3001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Scholz SA, Diao R, Wolfe MB, Fivenson EM, Lin XN, Freddolino PL. 2019. High-resolution mapping of the Escherichia coli chromosome reveals positions of high and low transcription. Cell Syst 8:212–225. doi: 10.1016/j.cels.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Schilling B, Basisty N, Christensen DG, Sorensen D, Orr JS, Wolfe AJ, Rao CV. 2019. Global lysine acetylation in Escherichia coli results from growth conditions that favor acetate fermentation. J Bacteriol 201:e00768-18. doi: 10.1128/JB.00768-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Sultan A, Jers C, Ganief TA, Shi L, Senissar M, Køhler JB, Macek B, Mijakovic I. 2021. Phosphoproteome study of Escherichia coli devoid of Ser/Thr kinase YeaG during the metabolic shift from glucose to malate. Front Microbiol 12:657562. doi: 10.3389/fmicb.2021.657562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Hansen A-M, Chaerkady R, Sharma J, Díaz-Mejía JJ, Tyagi N, Renuse S, Jacob HKC, Pinto SM, Sahasrabuddhe NA, Kim M-S, Delanghe B, Srinivasan N, Emili A, Kaper JB, Pandey A. 2013. The Escherichia coli phosphotyrosine proteome relates to core pathways and virulence. PLoS Pathog 9:e1003403. doi: 10.1371/journal.ppat.1003403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Bignaud A, Cockram C, Borde C, Groseille J, Allemand E, Thierry A, Marbouty M, Mozziconacci J, Espéli O, Koszul R. 2024. Transcription-induced domains form the elementary constraining building blocks of bacterial chromosomes. Nat Struct Mol Biol 31:489–497. doi: 10.1038/s41594-023-01178-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Le TB, Laub MT. 2016. Transcription rate and transcript length drive formation of chromosomal interaction domain boundaries. EMBO J 35:1582–1595. doi: 10.15252/embj.201593561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Booker BM, Deng S, Higgins NP. 2010. DNA topology of highly transcribed operons in Salmonella enterica serovar Typhimurium . Mol Microbiol 78:1348–1364. doi: 10.1111/j.1365-2958.2010.07394.x [DOI] [PubMed] [Google Scholar]
- 251. Sarnataro S, Chiariello AM, Esposito A, Prisco A, Nicodemi M. 2017. Structure of the human chromosome interaction network. PLoS One 12:e0188201. doi: 10.1371/journal.pone.0188201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Le TBK, Imakaev MV, Mirny LA, Laub MT. 2013. High-resolution mapping of the spatial organization of a bacterial chromosome. Science 342:731–734. doi: 10.1126/science.1242059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Niki H, Yamaichi Y, Hiraga S. 2000. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev 14:212–223. doi: 10.1101/gad.14.2.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Wang X, Liu X, Possoz C, Sherratt DJ. 2006. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev 20:1727–1731. doi: 10.1101/gad.388406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Valens M, Penaud S, Rossignol M, Cornet F, Boccard F. 2004. Macrodomain organization of the Escherichia coli chromosome. EMBO J 23:4330–4341. doi: 10.1038/sj.emboj.7600434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Cagliero C, Grand RS, Jones MB, Jin DJ, O’Sullivan JM. 2013. Genome conformation capture reveals that the Escherichia coli chromosome is organized by replication and transcription. Nucleic Acids Res 41:6058–6071. doi: 10.1093/nar/gkt325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Gaal T, Bratton BP, Sanchez-Vazquez P, Sliwicki A, Sliwicki K, Vegel A, Pannu R, Gourse RL. 2016. Colocalization of distant chromosomal loci in space in E. coli: a bacterial nucleolus. Genes Dev 30:2272–2285. doi: 10.1101/gad.290312.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Crisona NJ, Weinberg RL, Peter BJ, Sumners DW, Cozzarelli NR. 1999. The topological mechanism of phage λ integrase. J Mol Biol 289:747–775. doi: 10.1006/jmbi.1999.2771 [DOI] [PubMed] [Google Scholar]
- 259. Espeli O, Mercier R, Boccard F. 2008. DNA dynamics vary according to macrodomain topography in the E. coli chromosome. Mol Microbiol 68:1418–1427. doi: 10.1111/j.1365-2958.2008.06239.x [DOI] [PubMed] [Google Scholar]
- 260. Thiel A, Valens M, Vallet-Gely I, Espéli O, Boccard F. 2012. Long-range chromosome organization in E. coli: a site-specific system isolates the Ter macrodomain. PLoS Genet 8:e1002672. doi: 10.1371/journal.pgen.1002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Schmitt AD, Hu M, Ren B. 2016. Genome-wide mapping and analysis of chromosome architecture. Nat Rev Mol Cell Biol 17:743–755. doi: 10.1038/nrm.2016.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Xie T, Fu L-Y, Yang Q-Y, Xiong H, Xu H, Ma B-G, Zhang H-Y. 2015. Spatial features for Escherichia coli genome organization. BMC Genomics 16:37. doi: 10.1186/s12864-015-1258-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. van Berkum NL, Lieberman-Aiden E, Williams L, Imakaev M, Gnirke A, Mirny LA, Dekker J, Lander ES. 2010. Hi-C: a method to study the three-dimensional architecture of genomes. J Vis Exp 39:e1869. doi: 10.3791/1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Wang X, Le TBK, Lajoie BR, Dekker J, Laub MT, Rudner DZ. 2015. Condensin promotes the juxtaposition of DNA flanking its loading site in Bacillus subtilis. Genes Dev 29:1661–1675. doi: 10.1101/gad.265876.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Marbouty M, Le Gall A, Cattoni DI, Cournac A, Koh A, Fiche J-B, Mozziconacci J, Murray H, Koszul R, Nollmann M. 2015. Condensin- and replication-mediated bacterial chromosome folding and origin condensation revealed by Hi-C and super-resolution imaging. Mol Cell 59:588–602. doi: 10.1016/j.molcel.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 266. Dupaigne P, Tonthat NK, Espéli O, Whitfill T, Boccard F, Schumacher MA. 2012. Molecular basis for a protein-mediated DNA-bridging mechanism that functions in condensation of the E. coli chromosome. Mol Cell 48:560–571. doi: 10.1016/j.molcel.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Conin B, Billault-Chaumartin I, El Sayyed H, Quenech’Du N, Cockram C, Koszul R, Espéli O. 2022. Extended sister-chromosome catenation leads to massive reorganization of the E. coli genome. Nucleic Acids Res 50:2635–2650. doi: 10.1093/nar/gkac105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Qian Z, Dimitriadis EK, Edgar R, Eswaramoorthy P, Adhya S. 2012. Galactose repressor mediated intersegmental chromosomal connections in Escherichia coli. Proc Natl Acad Sci U S A 109:11336–11341. doi: 10.1073/pnas.1208595109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Weickert MJ, Adhya S. 1993. The galactose regulon of Escherichia coli. Mol Microbiol 10:245–251. doi: 10.1111/j.1365-2958.1993.tb01950.x [DOI] [PubMed] [Google Scholar]
- 270. Agerschou ED, Christiansen G, Schafer NP, Madsen DJ, Brodersen DE, Semsey S, Otzen DE. 2016. The transcriptional regulator GalR self-assembles to form highly regular tubular structures. Sci Rep 6:27672. doi: 10.1038/srep27672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Nakai H, Doseeva V, Jones JM. 2001. Handoff from recombinase to replisome: insights from transposition. Proc Natl Acad Sci U S A 98:8247–8254. doi: 10.1073/pnas.111007898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Harshey RM. 2014. Transposable phage Mu. Microbiol Spectr 2. doi: 10.1128/microbiolspec.MDNA3-0007-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Crozat E, Tardin C, Salhi M, Rousseau P, Lablaine A, Bertoni T, Holcman D, Sclavi B, Cicuta P, Cornet F. 2020. Post-replicative pairing of sister ter regions in Escherichia coli involves multiple activities of MatP. Nat Commun 11:3796. doi: 10.1038/s41467-020-17606-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Wang X, Brandão HB, Le TBK, Laub MT, Rudner DZ. 2017. Bacillus subtilis SMC complexes juxtapose chromosome arms as they travel from origin to terminus. Science 355:524–527. doi: 10.1126/science.aai8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Helmstetter CE. 1968. DNA synthesis during the division cycle of rapidly growing Escherichia coli Br. J Mol Biol 31:507–518. doi: 10.1016/0022-2836(68)90424-5 [DOI] [PubMed] [Google Scholar]
- 276. Campos M, Surovtsev IV, Kato S, Paintdakhi A, Beltran B, Ebmeier SE, Jacobs-Wagner C. 2014. A constant size extension drives bacterial cell size homeostasis. Cell 159:1433–1446. doi: 10.1016/j.cell.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Tiruvadi-Krishnan S, Männik J, Kar P, Lin J, Amir A, Männik J. 2022. Coupling between DNA replication, segregation, and the onset of constriction in Escherichia coli. Cell Rep 38:110539. doi: 10.1016/j.celrep.2022.110539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Berger M, Wolde PRT. 2022. Robust replication initiation from coupled homeostatic mechanisms. Nat Commun 13:6556. doi: 10.1038/s41467-022-33886-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Wallden M, Fange D, Lundius EG, Baltekin Ö, Elf J. 2016. The synchronization of replication and division cycles in individual E. coli cells. Cell 166:729–739. doi: 10.1016/j.cell.2016.06.052 [DOI] [PubMed] [Google Scholar]
- 280. Ho P-Y, Amir A. 2015. Simultaneous regulation of cell size and chromosome replication in bacteria. Front Microbiol 6:662. doi: 10.3389/fmicb.2015.00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Jameson KH, Wilkinson AJ. 2017. Control of initiation of DNA replication in Bacillus subtilis and Escherichia coli. Genes (Basel) 8:22. doi: 10.3390/genes8010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kowalski D, Eddy MJ. 1989. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J 8:4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ryan VT, Grimwade JE, Nievera CJ, Leonard AC. 2002. IHF and HU stimulate assembly of pre-replication complexes at Escherichia coli oriC by two different mechanisms. Mol Microbiol 46:113–124. doi: 10.1046/j.1365-2958.2002.03129.x [DOI] [PubMed] [Google Scholar]
- 284. Grimwade JE, Ryan VT, Leonard AC. 2000. IHF redistributes bound initiator protein, DnaA, on supercoiled oriC of Escherichia coli. Mol Microbiol 35:835–844. doi: 10.1046/j.1365-2958.2000.01755.x [DOI] [PubMed] [Google Scholar]
- 285. Kasho K, Ozaki S, Katayama T. 2023. IHF and Fis as Escherichia coli cell cycle regulators: activation of the replication origin oriC and the regulatory cycle of the DnaA initiator. Int J Mol Sci 24:11572. doi: 10.3390/ijms241411572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Bramhill D, Kornberg A. 1988. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 52:743–755. doi: 10.1016/0092-8674(88)90412-6 [DOI] [PubMed] [Google Scholar]
- 287. Fang L, Davey MJ, O’Donnell M. 1999. Replisome assembly at oriC, the replication origin of E. coli, reveals an explanation for initiation sites outside an origin. Mol Cell 4:541–553. doi: 10.1016/s1097-2765(00)80205-1 [DOI] [PubMed] [Google Scholar]
- 288. Beattie TR, Reyes-Lamothe R. 2015. A replisome’s journey through the bacterial chromosome. Front Microbiol 6:562. doi: 10.3389/fmicb.2015.00562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Voet D, Voet JG. DNA replication, repair, and recombination. In Biochemistry [Google Scholar]
- 290. Lee CM, Wang G, Pertsinidis A, Marians KJ. 2019. Topoisomerase III acts at the replication fork to remove precatenanes. J Bacteriol 201:e00563-18. doi: 10.1128/JB.00563-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Lesterlin C, Gigant E, Boccard F, Espéli O. 2012. Sister chromatid interactions in bacteria revealed by a site-specific recombination assay. EMBO J 31:3468–3479. doi: 10.1038/emboj.2012.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Joshi MC, Bourniquel A, Fisher J, Ho BT, Magnan D, Kleckner N, Bates D. 2011. Escherichia coli sister chromosome separation includes an abrupt global transition with concomitant release of late-splitting intersister snaps. Proc Natl Acad Sci U S A 108:2765–2770. doi: 10.1073/pnas.1019593108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Wang X, Reyes-Lamothe R, Sherratt DJ. 2008. Modulation of Escherichia coli sister chromosome cohesion by topoisomerase IV. Genes Dev 22:2426–2433. doi: 10.1101/gad.487508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Joshi MC, Magnan D, Montminy TP, Lies M, Stepankiw N, Bates D. 2013. Regulation of sister chromosome cohesion by the replication fork tracking protein SeqA. PLoS Genet 9:e1003673. doi: 10.1371/journal.pgen.1003673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Steiner WW, Kuempel PL. 1998. Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J Bacteriol 180:6269–6275. doi: 10.1128/JB.180.23.6269-6275.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Shimokawa K, Ishihara K, Grainge I, Sherratt DJ, Vazquez M. 2013. FtsK-dependent XerCD-dif recombination unlinks replication catenanes in a stepwise manner. Proc Natl Acad Sci U S A 110:20906–20911. doi: 10.1073/pnas.1308450110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Kennedy SP, Chevalier F, Barre F-X. 2008. Delayed activation of Xer recombination at dif by FtsK during septum assembly in Escherichia coli. Mol Microbiol 68:1018–1028. doi: 10.1111/j.1365-2958.2008.06212.x [DOI] [PubMed] [Google Scholar]
- 298. Sutormin D, Galivondzhyan A, Gafurov A, Severinov K. 2023. Single-nucleotide resolution detection of Topo IV cleavage activity in the Escherichia coli genome with Topo-Seq. Front Microbiol 14:1160736. doi: 10.3389/fmicb.2023.1160736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Hojgaard A, Szerlong H, Tabor C, Kuempel P. 1999. Norfloxacin-induced DNA cleavage occurs at the dif resolvase locus in Escherichia coli and is the result of interaction with topoisomerase IV. Mol Microbiol 33:1027–1036. doi: 10.1046/j.1365-2958.1999.01545.x [DOI] [PubMed] [Google Scholar]
- 300. Li Y, Stewart NK, Berger AJ, Vos S, Schoeffler AJ, Berger JM, Chait BT, Oakley MG. 2010. Escherichia coli condensin MukB stimulates topoisomerase IV activity by a direct physical interaction . Proc Natl Acad Sci U S A 107:18832–18837. doi: 10.1073/pnas.1008678107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Ashley RE, Dittmore A, McPherson SA, Turnbough CL, Neuman KC, Osheroff N. 2017. Activities of gyrase and topoisomerase IV on positively supercoiled DNA. Nucleic Acids Res 45:9611–9624. doi: 10.1093/nar/gkx649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Aussel L, Barre FX, Aroyo M, Stasiak A, Stasiak AZ, Sherratt D. 2002. FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell 108:195–205. doi: 10.1016/s0092-8674(02)00624-4 [DOI] [PubMed] [Google Scholar]
- 303. Midonet C, Barre F-X. 2014. Xer site-specific recombination: promoting vertical and horizontal transmission of genetic information. Microbiol Spectr 2. doi: 10.1128/microbiolspec.MDNA3-0056-2014 [DOI] [PubMed] [Google Scholar]
- 304. Goodall DJ, Warecka D, Hawkins M, Rudolph CJ. 2023. Interplay between chromosomal architecture and termination of DNA replication in bacteria. Front Microbiol 14:1180848. doi: 10.3389/fmicb.2023.1180848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Chandler M, Bird RE, Caro L. 1975. The replication time of the Escherichia coli K12 chromosome as a function of cell doubling time. J Mol Biol 94:127–132. doi: 10.1016/0022-2836(75)90410-6 [DOI] [PubMed] [Google Scholar]
- 306. Baker TA, Bell SP. 1998. Polymerases and the replisome: machines within machines. Cell 92:295–305. doi: 10.1016/s0092-8674(00)80923-x [DOI] [PubMed] [Google Scholar]
- 307. Molina F, Skarstad K. 2004. Replication fork and SeqA focus distributions in Escherichia coli suggest a replication hyperstructure dependent on nucleotide metabolism. Mol Microbiol 52:1597–1612. doi: 10.1111/j.1365-2958.2004.04097.x [DOI] [PubMed] [Google Scholar]
- 308. Lemon KP, Grossman AD. 1998. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science 282:1516–1519. doi: 10.1126/science.282.5393.1516 [DOI] [PubMed] [Google Scholar]
- 309. Cass JA, Kuwada NJ, Traxler B, Wiggins PA. 2016. Escherichia coli chromosomal loci segregate from midcell with universal dynamics. Biophys J 110:2597–2609. doi: 10.1016/j.bpj.2016.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Lemon KP, Grossman AD. 2000. Movement of replicating DNA through a stationary replisome. Mol Cell 6:1321–1330. doi: 10.1016/s1097-2765(00)00130-1 [DOI] [PubMed] [Google Scholar]
- 311. Mangiameli SM, Veit BT, Merrikh H, Wiggins PA. 2017. The replisomes remain spatially proximal throughout the cell cycle in bacteria. PLoS Genet 13:e1006582. doi: 10.1371/journal.pgen.1006582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Reyes-Lamothe R, Possoz C, Danilova O, Sherratt DJ. 2008. Independent positioning and action of Escherichia coli replisomes in live cells. Cell 133:90–102. doi: 10.1016/j.cell.2008.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Bates D, Kleckner N. 2005. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell 121:899–911. doi: 10.1016/j.cell.2005.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Chen PJ, McMullin AB, Visser BJ, Mei Q, Rosenberg SM, Bates D. 2023. Interdependent progression of bidirectional sister replisomes in E. coli. Elife 12:e82241. doi: 10.7554/eLife.82241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Magnan D, Joshi MC, Barker AK, Visser BJ, Bates D. 2015. DNA replication initiation is blocked by a distant chromosome-membrane attachment. Curr Biol 25:2143–2149. doi: 10.1016/j.cub.2015.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Lies M, Visser BJ, Joshi MC, Magnan D, Bates D. 2015. MioC and GidA proteins promote cell division in E. coli. Front Microbiol 6:516. doi: 10.3389/fmicb.2015.00516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Gille H, Egan JB, Roth A, Messer W. 1991. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res 19:4167–4172. doi: 10.1093/nar/19.15.4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Ryan VT, Grimwade JE, Camara JE, Crooke E, Leonard AC. 2004. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol Microbiol 51:1347–1359. doi: 10.1046/j.1365-2958.2003.03906.x [DOI] [PubMed] [Google Scholar]
- 319. Flåtten I, Skarstad K. 2013. The Fis protein has a stimulating role in initiation of replication in Escherichia coli in vivo. PLoS One 8:e83562. doi: 10.1371/journal.pone.0083562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Kogoma T. 1997. Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol Mol Biol Rev 61:212–238. doi: 10.1128/mmbr.61.2.212-238.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Gowrishankar J. 2015. End of the beginning: elongation and termination features of alternative modes of chromosomal replication initiation in bacteria. PLoS Genet 11:e1004909. doi: 10.1371/journal.pgen.1004909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Dorman CJ, Dorman MJ. 2016. DNA supercoiling is a fundamental regulatory principle in the control of bacterial gene expression. Biophys Rev 8:89–100. doi: 10.1007/s12551-016-0238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Zechiedrich EL, Khodursky AB, Bachellier S, Schneider R, Chen D, Lilley DM, Cozzarelli NR. 2000. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J Biol Chem 275:8103–8113. doi: 10.1074/jbc.275.11.8103 [DOI] [PubMed] [Google Scholar]
- 324. Du S, Lutkenhaus J. 2017. Assembly and activation of the Escherichia coli divisome. Mol Microbiol 105:177–187. doi: 10.1111/mmi.13696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Haeusser DP, Margolin W. 2016. Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol 14:305–319. doi: 10.1038/nrmicro.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Männik J, Bailey MW. 2015. Spatial coordination between chromosomes and cell division proteins in Escherichia coli. Front Microbiol 6:306. doi: 10.3389/fmicb.2015.00306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Cornet F, Blanchais C, Dusfour-Castan R, Meunier A, Quebre V, Sekkouri Alaoui H, Boudsoq F, Campos M, Crozat E, Guynet C, Pasta F, Rousseau P, Ton Hoang B, Bouet J-Y. 2023. DNA segregation in enterobacteria. EcoSal Plus 11:eesp00382020. doi: 10.1128/ecosalplus.esp-0038-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Mulder E, Woldringh CL. 1989. Actively replicating nucleoids influence positioning of division sites in Escherichia coli filaments forming cells lacking DNA. J Bacteriol 171:4303–4314. doi: 10.1128/jb.171.8.4303-4314.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Woldringh CL, Mulder E, Huls PG, Vischer N. 1991. Toporegulation of bacterial division according to the nucleoid occlusion model. Res Microbiol 142:309–320. doi: 10.1016/0923-2508(91)90046-d [DOI] [PubMed] [Google Scholar]
- 330. Bernhardt TG, de Boer PAJ. 2005. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol Cell 18:555–564. doi: 10.1016/j.molcel.2005.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Cho H, McManus HR, Dove SL, Bernhardt TG. 2011. Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc Natl Acad Sci U S A 108:3773–3778. doi: 10.1073/pnas.1018674108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Tonthat NK, Arold ST, Pickering BF, Van Dyke MW, Liang S, Lu Y, Beuria TK, Margolin W, Schumacher MA. 2011. Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J 30:154–164. doi: 10.1038/emboj.2010.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Sun Q, Margolin W. 2004. Effects of perturbing nucleoid structure on nucleoid occlusion-mediated toporegulation of FtsZ ring assembly. J Bacteriol 186:3951–3959. doi: 10.1128/JB.186.12.3951-3959.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Dajkovic A, Lan G, Sun SX, Wirtz D, Lutkenhaus J. 2008. MinC spatially controls bacterial cytokinesis by antagonizing the scaffolding function of FtsZ. Curr Biol 18:235–244. doi: 10.1016/j.cub.2008.01.042 [DOI] [PubMed] [Google Scholar]
- 335. Shen B, Lutkenhaus J. 2010. Examination of the interaction between FtsZ and MinCN in E. coli suggests how MinC disrupts Z rings. Mol Microbiol 75:1285–1298. doi: 10.1111/j.1365-2958.2010.07055.x [DOI] [PubMed] [Google Scholar]
- 336. Rowlett VW, Margolin W. 2015. The Min system and other nucleoid-independent regulators of Z ring positioning. Front Microbiol 6:478. doi: 10.3389/fmicb.2015.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Lutkenhaus J. 2012. The ParA/MinD family puts things in their place. Trends Microbiol 20:411–418. doi: 10.1016/j.tim.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Lutkenhaus J. 2007. Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem 76:539–562. doi: 10.1146/annurev.biochem.75.103004.142652 [DOI] [PubMed] [Google Scholar]
- 339. Johnson JE, Lackner LL, de Boer PAJ. 2002. Targeting of DMinC/MinD and DMinC/DicB complexes to septal rings in Escherichia coli suggests a multistep mechanism for MinC-mediated destruction of nascent FtsZ rings. J Bacteriol 184:2951–2962. doi: 10.1128/JB.184.11.2951-2962.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Bailey MW, Bisicchia P, Warren BT, Sherratt DJ, Männik J. 2014. Evidence for divisome localization mechanisms independent of the Min system and SlmA in Escherichia coli. PLoS Genet 10:e1004504. doi: 10.1371/journal.pgen.1004504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Den Blaauwen T, Buddelmeijer N, Aarsman ME, Hameete CM, Nanninga N. 1999. Timing of FtsZ assembly in Escherichia coli. J Bacteriol 181:5167–5175. doi: 10.1128/JB.181.17.5167-5175.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Galli E, Gerdes K. 2010. Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol Microbiol 76:1514–1526. doi: 10.1111/j.1365-2958.2010.07183.x [DOI] [PubMed] [Google Scholar]
- 343. Sherratt DJ, Arciszewska LK, Crozat E, Graham JE, Grainge I. 2010. The Escherichia coli DNA translocase FtsK. Biochem Soc Trans 38:395–398. doi: 10.1042/BST0380395 [DOI] [PubMed] [Google Scholar]
- 344. Espeli O, Lee C, Marians KJ. 2003. A physical and functional interaction between Escherichia coli FtsK and topoisomerase IV. J Biol Chem 278:44639–44644. doi: 10.1074/jbc.M308926200 [DOI] [PubMed] [Google Scholar]
- 345. Stouf M, Meile J-C, Cornet F. 2013. FtsK actively segregates sister chromosomes in Escherichia coli. Proc Natl Acad Sci U S A 110:11157–11162. doi: 10.1073/pnas.1304080110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Japaridze A, van Wee R, Gogou C, Kerssemakers JWJ, van den Berg DF, Dekker C. 2023. MukBEF-dependent chromosomal organization in widened. Front Microbiol 14:1107093. doi: 10.3389/fmicb.2023.1107093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Bigot S, Saleh OA, Lesterlin C, Pages C, El Karoui M, Dennis C, Grigoriev M, Allemand J-F, Barre F-X, Cornet F. 2005. KOPS: DNA motifs that control E. coli chromosome segregation by orienting the FtsK translocase. EMBO J 24:3770–3780. doi: 10.1038/sj.emboj.7600835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Barre FX, Aroyo M, Colloms SD, Helfrich A, Cornet F, Sherratt DJ. 2000. FtsK functions in the processing of a Holliday junction intermediate during bacterial chromosome segregation. Genes Dev 14:2976–2988. doi: 10.1101/gad.188700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Nicolas E, Upton AL, Uphoff S, Henry O, Badrinarayanan A, Sherratt D. 2014. The SMC complex MukBEF recruits topoisomerase IV to the origin of replication region in live Escherichia coli. mBio 5:e01001-13. doi: 10.1128/mBio.01001-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Zhou M. 2022. DNA sliding and loop formation by E. coli SMC complex: MukBEF. Biochem Biophys Rep 31:101297. doi: 10.1016/j.bbrep.2022.101297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Holmes VF, Cozzarelli NR. 2000. Closing the ring: links between SMC proteins and chromosome partitioning, condensation, and supercoiling. Proc Natl Acad Sci U S A 97:1322–1324. doi: 10.1073/pnas.040576797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Fritsche M, Li S, Heermann DW, Wiggins PA. 2012. A model for Escherichia coli chromosome packaging supports transcription factor-induced DNA domain formation. Nucleic Acids Res 40:972–980. doi: 10.1093/nar/gkr779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Jun S, Mulder B. 2006. Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci U S A 103:12388–12393. doi: 10.1073/pnas.0605305103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Busiek KK, Margolin W. 2014. A role for FtsA in SPOR-independent localization of the essential Escherichia coli cell division protein FtsN. Mol Microbiol 92:1212–1226. doi: 10.1111/mmi.12623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Lutkenhaus J. 2009. FtsN--trigger for septation. J Bacteriol 191:7381–7382. doi: 10.1128/JB.01100-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Gerding MA, Liu B, Bendezú FO, Hale CA, Bernhardt TG, de Boer PAJ. 2009. Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J Bacteriol 191:7383–7401. doi: 10.1128/JB.00811-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Corbin BD, Geissler B, Sadasivam M, Margolin W. 2004. Z-ring-independent interaction between a subdomain of FtsA and late septation proteins as revealed by a polar recruitment assay. J Bacteriol 186:7736–7744. doi: 10.1128/JB.186.22.7736-7744.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Witz G, van Nimwegen E, Julou T. 2019. Initiation of chromosome replication controls both division and replication cycles in E. coli through a double-adder mechanism. Elife 8:e48063. doi: 10.7554/eLife.48063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Colin A, Micali G, Faure L, Cosentino Lagomarsino M, van Teeffelen S. 2021. Two different cell-cycle processes determine the timing of cell division in Escherichia coli. Elife 10:e67495. doi: 10.7554/eLife.67495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Dash S, Palma CSD, Baptista ISC, Bahrudeen MNM, Almeida BLB, Chauhan V, Jagadeesan R, Ribeiro AS. 2021. Positive supercoiling buildup is a trigger of E. coli ’s short-term response to cold shock . bioRxiv. doi: 10.1101/2021.12.22.473827 [DOI]
- 361. Rajkumari K, Kusano S, Ishihama A, Mizuno T, Gowrishankar J. 1996. Effects of H-NS and potassium glutamate on σS- and σ70-directed transcription in vitro from osmotically regulated P1 and P2 promoters of proU in Escherichia coli. J Bacteriol 178:4176–4181. doi: 10.1128/jb.178.14.4176-4181.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Falconi M, Colonna B, Prosseda G, Micheli G, Gualerzi CO. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J 17:7033–7043. doi: 10.1093/emboj/17.23.7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Dorman CJ. 2004. H-NS: a universal regulator for a dynamic genome. Nat Rev Microbiol 2:391–400. doi: 10.1038/nrmicro883 [DOI] [PubMed] [Google Scholar]
- 364. Ono S, Goldberg MD, Olsson T, Esposito D, Hinton JCD, Ladbury JE. 2005. H-NS is a part of a thermally controlled mechanism for bacterial gene regulation. Biochem J 391:203–213. doi: 10.1042/BJ20050453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. White-Ziegler CA, Davis TR. 2009. Genome-wide identification of H-NS-controlled, temperature-regulated genes in Escherichia coli K-12. J Bacteriol 191:1106–1110. doi: 10.1128/JB.00599-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Porter ME, Dorman CJ. 1994. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J Bacteriol 176:4187–4191. doi: 10.1128/jb.176.13.4187-4191.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Rafiei N, Cordova M, Navarre WW, Milstein JN. 2019. Growth phase-dependent chromosome condensation and heat-stable nucleoid-structuring protein redistribution in Escherichia coli under osmotic stress. J Bacteriol 201:e00469-19. doi: 10.1128/JB.00469-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Maslowska KH, Makiela-Dzbenska K, Fijalkowska IJ. 2019. The SOS system: a complex and tightly regulated response to DNA damage. Environ Mol Mutagen 60:368–384. doi: 10.1002/em.22267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Janion C. 2008. Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli. Int J Biol Sci 4:338–344. doi: 10.7150/ijbs.4.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Dillingham MS, Kowalczykowski SC. 2008. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol Mol Biol Rev 72:642–671, doi: 10.1128/MMBR.00020-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Ghodke H, Paudel BP, Lewis JS, Jergic S, Gopal K, Romero ZJ, Wood EA, Woodgate R, Cox MM, van Oijen AM. 2019. Spatial and temporal organization of RecA in the Escherichia coli DNA-damage response. Elife 8:e42761. doi: 10.7554/eLife.42761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Bell JC, Kowalczykowski SC. 2016. RecA: regulation and mechanism of a molecular search engine. Trends Biochem Sci 41:491–507. doi: 10.1016/j.tibs.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Lesterlin C, Ball G, Schermelleh L, Sherratt DJ. 2014. RecA bundles mediate homology pairing between distant sisters during DNA break repair. Nature 506:249–253. doi: 10.1038/nature12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Wiktor J, Gynnå AH, Leroy P, Larsson J, Coceano G, Testa I, Elf J. 2021. RecA finds homologous DNA by reduced dimensionality search. Nature 597:426–429. doi: 10.1038/s41586-021-03877-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Ambia-Garrido J, Vainrub A, Pettitt BM. 2010. A model for structure and thermodynamics of ssDNA and dsDNA near a surface: a coarse grained approach. Comput Phys Commun 181:2001–2007. doi: 10.1016/j.cpc.2010.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Kowalczykowski SC. 2015. An overview of the molecular mechanisms of recombinational DNA repair. Cold Spring Harb Perspect Biol 7:a016410. doi: 10.1101/cshperspect.a016410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Wolf SG, Frenkiel D, Arad T, Finkel SE, Kolter R, Minsky A. 1999. DNA protection by stress-induced biocrystallization. Nature 400:83–85. doi: 10.1038/21918 [DOI] [PubMed] [Google Scholar]
- 378. Frenkiel-Krispin D, Ben-Avraham I, Englander J, Shimoni E, Wolf SG, Minsky A. 2004. Nucleoid restructuring in stationary-state bacteria. Mol Microbiol 51:395–405. doi: 10.1046/j.1365-2958.2003.03855.x [DOI] [PubMed] [Google Scholar]
- 379. Karas VO, Westerlaken I, Meyer AS. 2015. The DNA-binding protein from starved cells (Dps) utilizes dual functions to defend cells against multiple stresses. J Bacteriol 197:3206–3215. doi: 10.1128/JB.00475-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Gupta A, Joshi A, Arora K, Mukhopadhyay S, Guptasarma P. 2023. The bacterial nucleoid-associated proteins, HU, and Dps, condense DNA into context-dependent biphasic or multiphasic complex coacervates. J Biol Chem 299:104637. doi: 10.1016/j.jbc.2023.104637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Janissen R, Arens MMA, Vtyurina NN, Rivai Z, Sunday ND, Eslami-Mossallam B, Gritsenko AA, Laan L, de Ridder D, Artsimovitch I, Dekker NH, Abbondanzieri EA, Meyer AS. 2018. Global DNA compaction in stationary-phase bacteria does not affect transcription. Cell 174:1188–1199. doi: 10.1016/j.cell.2018.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Kim J, Yoshimura SH, Hizume K, Ohniwa RL, Ishihama A, Takeyasu K. 2004. Fundamental structural units of the Escherichia coli nucleoid revealed by atomic force microscopy. Nucleic Acids Res 32:1982–1992. doi: 10.1093/nar/gkh512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Freddolino PL, Tavazoie S. 2012. Beyond homeostasis: a predictive-dynamic framework for understanding cellular behavior. Annu Rev Cell Dev Biol 28:363–384. doi: 10.1146/annurev-cellbio-092910-154129 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods for chromosome conformation calculation.