Table of Content
Abbreviations 1613
Introduction 1614
NOAC eligibility and dosing 1615
Practical considerations for initiation and follow-up 1618
Pharmacokinetics and drug–drug interactions of NOACs 1621
NOACs in patients with chronic kidney disease or advanced liver disease 1633
NOAC plasma level measurements: Technical approach, indications, pitfalls 1636
Management of bleeding under NOAC therapy 1638
Patients requiring an urgent surgical intervention 1644
Patients undergoing a planned invasive procedure, surgery, or ablation 1644
Patients with atrial fibrillation and coronary artery disease 1647
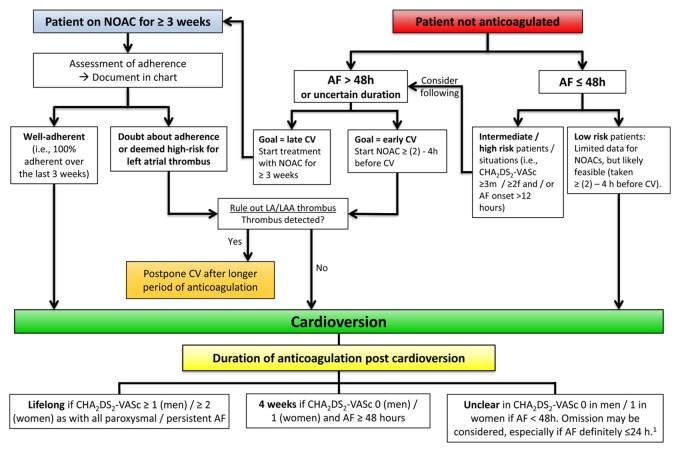
Cardioversion in a NOAC-treated patient 1651
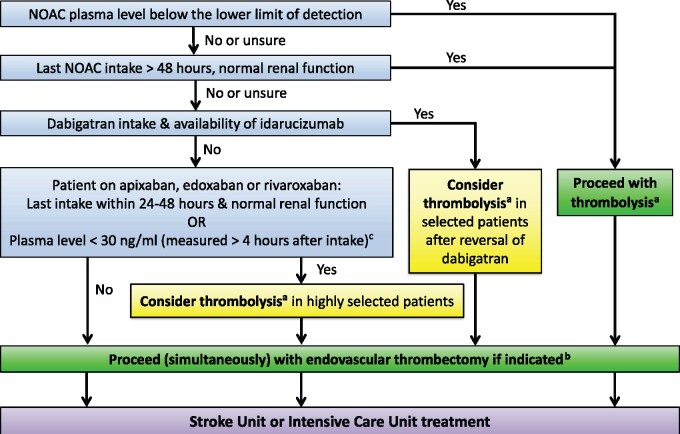
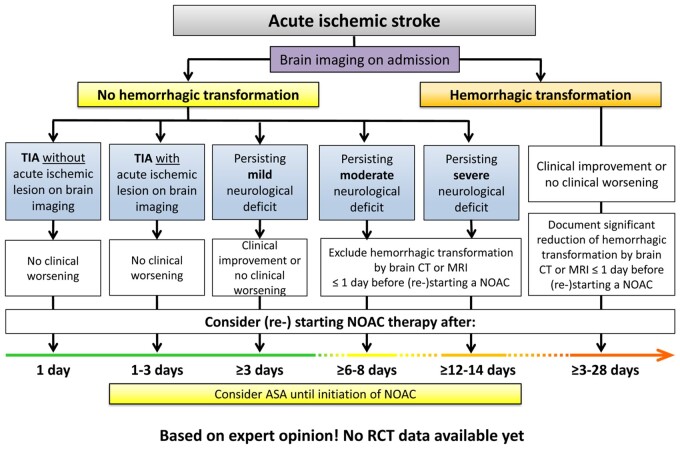
AF patients presenting with acute stroke while on NOACs 1652
NOACs in advanced age and frailty 1656
NOACs in high- and low body weights 1658
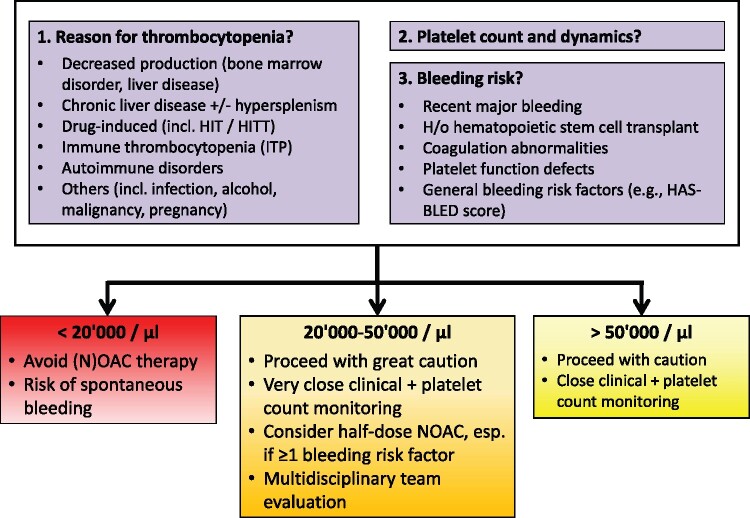
NOACs in other special populations 1660
NOACs in patients with atrial fibrillation and malignancy 1663
Optimizing dose adjustments of vitamin-K antagonists 1663
Abbreviations
- ACS
Acute coronary syndrome
- ACT
Activated clotting time
- AED
Antiepileptic drugs
- AF
Atrial fibrillation
- AFIRE
Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease
- AMPLIFY
Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy
- ANNEXA-4
Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors 4
- aPCC
Activated prothrombin complex concentrates
- aPTT
Activated prothrombin time
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- ATLANTIS
Anti-Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischemic and Hemorrhagic Events after Trans-Aortic Valve Implantation for Aortic Stenosis
- ATLAS ACS–TIMI
Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects with Acute Coronary Syndrome—Thrombolysis in Myocardial Infarction
- AUB
Abnormal uterine bleeding
- AUC
Area under the curve
- AUGUSTUS
Apixaban Versus Vitamin K Antagonist in Patients With Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention
- AXADIA
A Safety Study Assessing Oral Anticoagulation With Apixaban Versus Vitamin-K Antagonists in Patients With Atrial Fibrillation (AF) and End-Stage Kidney Disease (ESKD) on Chronic Hemodialysis Treatment
- AXAFA- AFNET
Anticoagulation using the direct factor Xa inhibitor apixaban during Atrial Fibrillation catheter Ablation: Comparison to vitamin K antagonist therapy—Atrial Fibrillation Network
- BCRP
Breast cancer resistance protein
- BID
twice daily
- BMI
Body mass index
- BMS
Bare metal stent
- BRIDGE
Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CCS
Chronic coronary syndrome
- CKD
Chronic kidney disease
- CKD-EPI
Chronic Kidney Disease—Epidemiology Collaboration
- CMB
Cerebral microbleeds
- COMPASS
Cardiovascular Outcomes for People Using Anticoagulation Strategies
- CORIDA
COncentration of RIvaroxaban, Dabigatran and Apixaban
- COVID-19
Coronavirus Disease of 2019
- CrCl
Creatinine clearance
- CRNM
Clinically relevant non-major bleeding
- CT
Computed tomography
- CV
Cardiovascular
- CYP
Cytochrome P (CYP)
- DAPT
Dual antiplatelet therapy
- DDI
Drug–drug interaction
- DES
Drug-eluting stent
- DOAC
Direct oral anticoagulant
- dTT
Diluted thrombin time
- EACTS
European Association for Cardio-Thoracic Surgery
- ECA
Ecarin chromogenic assay
- EHRA
European Heart Rhythm Association
- ELDERCARE- AF
Edoxaban low-dose for elder care AF patients
- ELIMINATE- AF
Evaluation of Edoxaban compared with VKA in subjects undergoing catheter ablation of non-valvular atrial fibrillation
- EMA
European Medicines Agency
- EMANATE
Eliquis evaluated in acute cardioversion compared to usual treatments for anticoagulation in subjects with NVAF
- ENAVLE
Efficacy and Safety of edoxabaN in Patients After Heart Valve Repair or Bioprosthetic vaLve Replacement
- ENGAGE AF-TIMI 48
Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation—Thrombolysis in Myocardial Infarction 48
- ENSURE-AF
Edoxaban versus warfarin in subjects undergoing cardioversion of Atrial Fibrillation
- ENTRUST AF-PCI
Evaluation of the Safety and Efficacy of an Edoxaban-Based Compared to a Vitamin K Antagonist-Based Antithrombotic Regimen in Subjects With Atrial Fibrillation Following Successful Percutaneous Coronary Intervention With Stent Placement
- ENVISAGE- TAVI
EdoxabaN Versus standard of care and theIr effectS on clinical outcomes in pAtients havinG undergone Transcatheter Aortic Valve Implantation–Atrial Fibrillation
- ESO
European Stroke Organization
- ESC
European Society of Cardiology
- FFP
Fresh frozen plasma
- GFR
Glomerular filtration rate
- GI
Gastrointestinal
- GP
General practitioner
- GRACE
Global Registry of Acute Coronary Events
- HCM
Hypertrophic cardiomyopathy
- HCP
Healthcare provider
- HIT/HITT
Heparin-induced thrombocytopenia ± thrombosis
- HMB
Heavy menstrual bleeding
- HPLC/MS
High performance liquid chromatography/mass spectrometry
- ICB
Intracerebral bleeding
- INR
International normalized ratio
- ISTH
International Society of Thrombosis and Hemostasis
- ITP
Immune thrombocytopenia
- J-ROCKET
Japanese ROCKET AF
- LAA
Left atrial appendage
- LMWH
Low molecular weight heparin
- MDRD
Modification of Diet in Renal Disease
- MI
Myocardial infarction
- MRI
Magnetic resonance imaging
- NOAC
Non-vitamin K antagonist oral anticoagulant
- NSAID
Non-steroidal anti-inflammatory drug
- NSTE-ACS
Non-ST-elevation acute coronary syndrome
- OAC
Oral anticoagulation
- PAUSE
Perioperative Anticoagulant Use for Surgery Evaluation
- PCC
Prothrombin complex concentrates
- PCI
Percutaneous coronary intervention
- PD
Pharmacodynamic
- PK
Pharmacokinetic
- P-gp
P-glycoprotein
- PIONEER AF-PCI
Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention
- POISE-2
Perioperative Ischemic Evaluation 2
- PPI
Proton pump inhibitor
- PT
Prothrombin time
- QD
Once daily
- RCT
Randomized clinical trial
- RE-CIRCUIT
Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy
- RE-DUAL PCI
Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention
- RE-LY
Randomized Evaluation of Long-Term Anticoagulation Therapy
- RENAL-AF
RENal Hemodialysis Patients ALlocated Apixaban Versus Warfarin in Atrial Fibrillation
- RE-VERSE AD
Reversal Effects of Idarucizumab in Patients on Active Dabigatran
- RIVER
Rivaroxaban for Valvular Heart Disease and Atrial Fibrillation
- ROCKET AF
Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation
- ROTEM
Rotational thromboelastometry
- rt-PA
Recombinant tissue-type plasminogen activator
- SAH
Subarachnoid haemorrhage
- SDH
Subdural haematoma
- SEE
Systemic embolic event
- SmPC
Summary of product characteristics
- STEMI
ST-elevation myocardial infarction
- TAVI
Transcatheter aortic valve implantation
- TOE
Transoesophageal echocardiogram
- TEG
Thromboelastography
- TIA
Transient ischaemic attack
- TSP
Transseptal puncture
- TT
Thrombin time
- TTR
Time in therapeutic range
- UFH
Unfractionated heparin
- ULN
Upper limit of normal
- VENTURE-AF
Active-controlled multi-center study with blind-adjudication designed to evaluate the safety of uninterrupted Rivaroxaban and uninterrupted vitamin K antagonists in subjects undergoing catheter ablation for non-valvular Atrial Fibrillation
- VHD
Valvular heart disease
- VKA
Vitamin K antagonist
- VTE
Venous thromboembolic event
- WOEST
What is the Optimal antiplatelet and anticoagulant therapy in patients with oral anticoagulation and coronary stenting
- X-VeRT
Explore the efficacy and safety of once daily oral rivaroxaban for the prevention of cardiovascular events in patients with non- valvular atrial fibrillation scheduled for cardioversion
Introduction
Non-vitamin K antagonist oral anticoagulants (NOACs) are considered by atrial fibrillation (AF) guidelines world-wide as the preferred choice of anticoagulants to prevent stroke in patients with AF.1–4 The term NOAC has been used for many years, is used by the current European Society of Cardiology (ESC) AF guidelines,1 and is widely recognized. Therefore, even though some authors refer to these drugs as ‘direct oral anticoagulants’ (DOACs),5 we prefer to continue to use the term NOAC. Ultimately, both terms are interchangeable when referring to the direct factor Xa inhibitors apixaban, edoxaban, and rivaroxaban as well as the direct thrombin inhibitor dabigatran.
NOACs have an improved efficacy/safety ratio and a predictable anticoagulant effect without the need for routine coagulation monitoring.6,7 However, the proper use of NOACs requires a carefully considered approach to many practical aspects. Each of the available NOACs is accompanied by the instructions for its proper use in many clinical situations [summary of product characteristics (SmPCs); patient cards; information leaflets for patients and physicians], but these are often slightly different (from drug to drug and from country to country), and physician education tools sometimes create confusion rather than clarity. Moreover, there are still several less well-researched aspects of NOAC use which are nonetheless relevant when these drugs are used by cardiologists, neurologists, geriatricians, general practitioners, and other healthcare providers (HCPs) in daily clinical practice. Based on these premises, the European Heart Rhythm Association (EHRA) set out to coordinate a unified way of informing physicians on the use of NOACs. The first edition of the ‘Practical Guide’ was published in 20138; a first update was published in 20159; and a fully revised new version in 2018.10,10a The EHRA Practical Guide’s purpose is to provide support for safe and effective use of NOACs in daily practice, thereby supplementing ESC and other international guidelines mainly focusing on the scientific evidence for treatment of patients with AF with anticoagulation in general and of NOACs in particular.1–4
A writing group formulated practical answers to 16 clinical scenarios, based on updated information. During the conception and writing of the 2021 Practical Guide, a public call was made to all EHRA members as well as to the Heads of the National Cardiac Societies to submit their suggestions additions, corrections, modifications, etc. to the 2018 version of the Guide, and these have been incorporated wherever possible and appropriate. We thank all participants for their input, which has further improved this Guide. As in the previous iterations, the writing group was assisted by medical experts from the manufacturers of the NOACs, who provided assurance that the latest information on the different NOACs was evaluated and provided feedback on the alignment of the text with the approved European SmPCs. However, the final responsibility of this document resided entirely with the EHRA writing group. In some instances, the authors opted to advise options that do not fully align with all SmPCs, with the goal of providing more uniform and simple practical advice (e.g. on the start of NOACs after cessation of vitamin K antagonist (VKA); on advice after a missed or forgotten dose; on perioperative management and others). Obviously, local regulations and HCPs’ freedoms for prescription may vary and final responsibility of use lies with the prescribing healthcare professional.
An EHRA website—www.NOACforAF.eu—accompanies the Practical Guide. The Practical Guide is summarized in a Key Message booklet which can be obtained through EHRA and ESC, and which is available in the ‘EHRA Key Messages’ app. The website also provides EHRA members with a downloadable slide kit on the Practical Guide.
We hope that the current edition further improves the practical tool that EHRA envisioned. The authors realize that there will always be grey areas, unaddressed questions, gaps in knowledge, and hence areas of uncertainty and debate. Therefore, readers can continue to address their suggestions for change or improvement to the website or via EHRANOACguide2021@escardio.org.
NOAC eligibility and dosing
NOAC eligibility
NOACs are approved for stroke prevention in ‘non-valvular’ AF. Most SmPCs base eligibility on the CHADS2 score as it was commonly used in the phase III randomized clinical trials (RCTs). Given the consistent efficacy and safety, the indication for NOAC therapy has subsequently been broadened to patients qualifying for anticoagulation according to the CHA2DS2-VASc score,1 with some regional differences (e.g. Canada, Japan).
In order to avoid confusion, the use of the term ‘non-valvular’ is strongly discouraged in the ESC guidelines on the management of patients with AF, and reference is made to the specific underlying valvular heart disease.1,11,12 However, the term is still found in the individual SmPCs of each of the NOACs due to the original wording used in the exclusion criteria of the RCTs on which their regulatory approval was based. When it is used, the term ‘non-valvular AF’ refers to AF in the absence of a mechanical prosthetic heart valve or moderate to severe mitral stenosis (usually of rheumatic origin) (Table 1),1,12,13 which were exclusion criteria for all phase III NOAC vs. warfarin trials in AF. However, there is no RCT indicating that NOACs are less efficacious in patients with rheumatic mitral stenosis, and no rational base on which to hypothesize a differential response to NOACs vs. VKA.14 Indeed, the lack of eligibility only stems from exclusion of these patients from the pivotal RCTs. The INVICTUS-program investigating the use of VKA, Rivaroxaban or Aspirin in patients with rheumatic heart disease is currently ongoing (NCT02832531). Until these and other trials are completed, such patients should be treated with VKA as a standard of care. However, if therapy with VKA is truly impossible (e.g. no means of monitoring, no stable international normalized ratio (INR) even when using self-monitoring and management etc.) use of a NOAC may be an option which physicians could carefully evaluate, also in view of the lack of other studied, safe and effective alternatives, after informed consent of the patient regarding the off-label use in this situation.
Table 1.
Selected indications and contraindications for NOAC therapy in AF patients
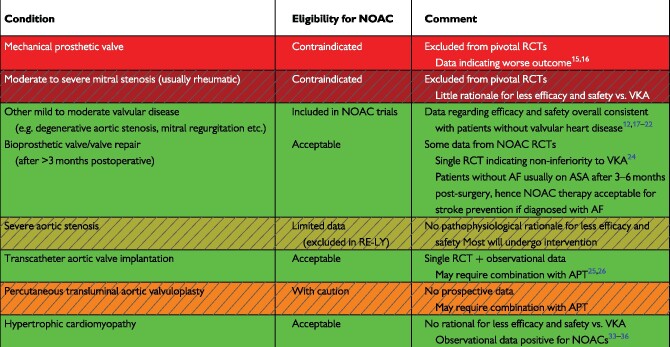
|
Hatched, limited data; See text for details.
AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; RCT, randomized clinical trial; VKA, vitamin K antagonist.
In contrast, for AF in the context of mechanical heart valves, particularly in the setting of mechanical mitral valve replacement, NOAC therapy should be discouraged unless new evidence reverses existing data that NOACs may be inferior to VKA for stroke prevention.15,16 Conversely, patients with degenerative valvular heart disease were variously included in the phase III trials, and NOACs demonstrated comparable relative efficacy and safety vs. warfarin in patients with vs. without valvular disease [except for a higher risk of bleeding with rivaroxaban vs. warfarin in patients with valvular heart disease in a post hoc analysis of the ‘Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation’ (ROCKET-AF) trial].12,17–23 NOACs may therefore be used in patients with AF and most forms of valvular heart disease (Table 1).1,12
Until recently, oral anticoagulation (OAC) in patients with AF and biological valves or after valve repair constituted a grey area, even though these patients were included in some of the landmark NOAC trials.12,17,19,20 In the ‘Rivaroxaban for Valvular Heart Disease and Atrial Fibrillation’ (RIVER) trial rivaroxaban was non-inferior to warfarin regarding the mean time until the combined endpoint of death, major cardiovascular events, or major bleeding at 12 months in 1005 patients with AF or flutter and a bioprosthetic mitral valve.24 Similarly, edoxaban was non-inferior in 220 patients included in the ‘Efficacy and Safety of edoxabaN in Patients After Heart Valve Repair or Bioprosthetic vaLve Replacement’ (ENAVLE) trial (presented at ACC 2020). Today, NOACs hence appear as a valid option for the management of concomitant AF especially after the immediate 8–12 weeks after surgery.
For patients after transcatheter aortic valve implantation (TAVI), who have an indication for anticoagulation (e.g. AF), a small RCT of 157 patients comparing OAC alone with a combination of OAC plus clopidogrel, indicated a benefit from OAC alone in terms of reduced bleeding without compromising ischaemic events.25 A possibly even greater advantage was seen with the use of NOACs in this study (vs. VKA), but the study was underpowered to address this question. Observational data similarly found a lower rate of early thromboembolic- and bleeding events (as well as all-cause death in a more recent analysis) with NOACs vs. VKA after TAVI but residual confounding is likely.26,27 Dedicated trials are ongoing looking at the specific efficacy and safety of NOACs in this setting [e.g. ‘Anti-Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischaemic and Hemorrhagic Events after Trans-Aortic Valve Implantation for Aortic Stenosis’ (ATLANTIS),28 ‘EdoxabaN vs. standard of care and theIr effectS on clinical outcomes in pAtients havinG undergonE Transcatheter Aortic Valve Implantation–Atrial Fibrillation’ (ENVISAGE-TAVI)].29 It is important to remember that while OAC (including NOAC) monotherapy may be considered after TAVI in patients with AF, OAC is currently not indicated in patients without an established indication for OAC in such patients.30
In both obstructive and non-obstructive hypertrophic cardiomyopathy (HCM), AF is associated with a high rate of thromboembolism.31,32 Despite the absence of dedicated RCTs, increasing evidence from observational studies indicates that NOACs may be safe and effective in this condition.33–36 Indeed, there does not seem to be a mechanistic rationale why NOACs should be inferior to warfarin in HCM. On the contrary, AF in HCM shares many similarities of HFpEF related AF, for which NOACs are non-inferior to VKA.37–39 Moreover, NOACs demonstrate a sustained efficacy over VKA also in other high-risk subgroups (e.g. patients with a high CHA2DS2-VASc score). As such, patients with HCM may be eligible for NOAC therapy.
NOACs are contraindicated in pregnancy, and reliable contraceptive measures need to be in place in women of child-bearing age before starting NOAC therapy (see Supplementary material online). Paediatric patients have been excluded from the pivotal stroke prevention RCTs and AF with need for OAC is rare in this population. NOAC therapy should be discouraged in children but can be considered in fully grown adolescents with body weight > 50 kg. Of note, body weight adjusted treatment with rivaroxaban has proven safe and effective for children with acute venous thromboembolism compared to standard anticoagulants over 3 months40; also dose-adjusted treatment with Dabigatran revealed a favourable safety profile for secondary prevention of venous thromboembolism in children 3 months to 18 years.41
Patients with ‘non-valvular’ AF and antiphospholipid syndrome should be treated with VKA rather than NOACs, as a higher rate of thromboembolic events and major bleeding was observed with rivaroxaban vs. warfarin in these patients.42
Dosing
With four NOACs available in different dosages for different indications and with different dose reduction criteria, identification of the correct dose has become more complicated. Table 2 gives an overview of currently available NOACs and their doses in the different indications, including the relevant dose-reduction criteria.
Table 2.
OACs and approved/studied doses across indications
| Stroke prevention in atrial fibrillation (SPAF) | ||
|---|---|---|
| Standard dose | Comments/dose reduction | |
| Apixaban47 | 5 mg BID | 2.5 mg BID if two out of three fulfilled: weight ≤60 kg, age ≥80 years, serum
creatinine ≥133 µmol/L (1.5 mg/dL) (or single criterion: if CrCl 15–29 mL/min) |
| Dabigatran48 | 150 mg BID/110 mg BID | No pre-specified dose-reduction criteria in phase III triala |
| Edoxaban49 | 60 mg QD | 30 mg QD if: weight ≤60 kg or CrCl 15–49 mL/min or concomitant therapy with strong P-Gp inhibitor (see ‘Pharmacokinetics and drug-drug interactions of NOACs' section) |
| Rivaroxaban46 | 20 mg QD | 15 mg QD if CrCl ≤15–49 mL/min |
‘SmPc’ refers to European SmPc.
BID, twice daily; CrCl, creatinine clearance; GI, gastrointestinal; NOAC, non-vitamin K antagonist oral anticoagulant; QD, once daily.
SmPC: 110 mg BID if age ≥80 years, concomitant verapamil, increased risk of GI bleeding.
NOAC dosing in AF patients post-ACS/PCI (see ‘Patients with atrial fibrillation and coronary artery disease' section)
| Standard dose | Comments/dose reduction | |
|---|---|---|
| Apixaban244 | 5 mg BID | Dose reduction as for SPAF |
| Dabigatran247 | 150 mg BID or 110 mg BID | 110mg as for SPAF403 |
| Edoxaban245 | 60 mg QD | Dose reduction as for SPAF |
| Rivaroxaban246 | 15 mg QD | Dose reduction to 10 mg QD if CrCl 30–49 mL/min |
In addition to single/dual antiplatelet therapy, where applicable. See ‘Patients with atrial fibrillation and coronary artery disease' section for details.
BID, twice daily; CrCl, creatinine clearance; QD, once daily; SPAF, stroke prevention in atrial fibrillation.
Even in settings with optimal patient education (see ‘Practical considerations for initiation and follow-up' section) dosing errors are common in daily practice, and patients need to be informed on what to do in such cases. In order to provide a more uniform and simple practical advice, the writing group acknowledges that some of the below advice does not fully align with all European SmPCs.
Missed dose
A forgotten dose may be taken until half of the dosing interval has passed. Hence, for NOACs with a twice daily (BID) dosing regimen (i.e., intake every 12 h), a forgotten full dose can be taken up until 6 h after the scheduled intake. For NOACs with a once daily (QD) dosing regimen, a forgotten dose can be taken up until 12 h after the scheduled intake. After these time points, the dose should be skipped, and the next scheduled dose should be taken.
Double dose
For NOACs with a BID dosing regimen, the next planned dose (i.e. after 12 h) may be skipped, with the regular BID dosing regimen restarted 24 h after the double dose intake.
For NOACs with a QD dosing regimen, the patient should continue the normal dosing regimen, i.e. without skipping the next daily dose.
Uncertainty about dose intake
For NOACs with a BID dosing regimen, it is generally advisable to not take another tablet/capsule, but to continue with the regular dose regimen, i.e. starting with the next dose at the 12 h interval.
For NOACs with a QD dosing regimen, when thromboembolic risk is high (CHA2DS2-VASc ≥3), it may generally be advisable to take another tablet 6–8 h after the original (uncertain) intake and then continue the planned dose regimen. In case the thromboembolic risk is low (CHA2DS2-VASc ≤2) we advise to wait until the next scheduled dose.
Practical considerations for initiation and follow-up
Choice of anticoagulant therapy and initiation
Indication for anticoagulation and choice between VKA and NOAC
After the indication for OAC is established, NOACs are preferred over VKAs in all NOAC-eligible AF patients (see ‘NOAC eligibility and dosing' section).1,2
When starting a NOAC, knowledge of current kidney and liver function is required as all NOACs are eliminated to some extent via the kidneys, and renal function affects NOAC dosing. Importantly, kidney function should be assessed using the Cockcroft–Gault formula as it was used in the four pivotal phase III trial (see ‘NOACs in patients with chronic kidney disease or advanced liver disease' section for details). Indeed, use of other formulas including ‘Modification of Diet in Renal Disease’ (MDRD) and ‘Chronic Kidney Disease—Epidemiology Collaboration’ (CKD-EPI) may overestimate kidney function particularly in older patients and in those with low body weights.43
A baseline haematological profile should be obtained for reference during future follow-up.
Bleeding risk, as estimated using the HAS-BLED score, is not in itself a reason to deny OAC to AF patients at risk of stroke or reduce the dose of the NOAC. Instead, particularly patients at high bleeding risk (e.g. HAS-BLED ≥3) should have their modifiable bleeding risk factors identified and addressed,1,44 and should be scheduled for an earlier and more frequent clinical follow-up.45
Similarly, frailty, cognitive decline and risk of falling should not generally be a reason not to anticoagulate patients. Care needs to be taken to minimize the risk of falling and to ensure optimal compliance and adherence. This topic is dealt with in detail in the ‘NOACs in advanced age and frailty' section.
Choosing the type and dose of NOACs
With four NOACs available in different dosages for different indications and with different dose reduction criteria, identification of the correct dose has become more complicated and is one of the key challenges in the daily use and individualization of treatment (see ‘NOAC eligibility and dosing' section). Local factors, such as regulatory approval, formulary restrictions, and the cost of therapy, may influence NOAC availability in specific healthcare settings.
All NOACs have been tested in large randomized prospective trials and have shown efficacy and safety of the respective agents. Testing of different doses, however, was carried out differently. In the ‘Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation’ (ARISTOTLE) trial (using apixaban) and ROCKET-AF (using rivaroxaban) trials, patients received a standard dose which was reduced in the presence of predefined patient characteristics.46,47 In contrast, in the ‘Randomized Evaluation of Long-Term Anticoagulation Therapy’ (RE-LY) trial (with dabigatran) and ‘Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation—Thrombolysis in Myocardial Infarction 48’ (ENGAGE AF-TIMI 48) trial (with edoxaban) both a lower and a higher dose were tested in fully powered patient cohorts (without further dose reduction for dabigatran, and with further dose reduction for edoxaban in certain patients).48,49 Dose reduction of NOACs is primarily recommended according to the published and approved dose reduction criteria (see ‘NOAC eligibility and dosing' section).1 Whenever possible, the tested and approved dose of NOACs should be used to provide optimal benefit for the patient.
There is a wealth of published data to confirm that in daily clinical practice—i.e. outside the controlled clinical trial setting—NOACs are at least as safe and efficacious as warfarin.50–55 However, some patterns have emerged from large observational studies indicating a higher than anticipated off-label dosing of NOACs.51,56–68 This is related to the fact that HCPs mostly worry about the risk of bleeding (as an iatrogenic event), whereas the risk of a stroke is often viewed as a possible ‘natural course of the disease’. However, various large trials and observational series indicate that high-risk patients derive a particularly pronounced benefit from anticoagulation.47,49,53,69–71 Involving the patient into the decision process and discussing together the options of anticoagulation (‘shared decision making’) is key in order to adequately assess patients’ needs, as for patients—in contrast to physicians—the risk of stroke usually outweighs the risk of a bleed.72–74
In addition, it is important to consider co-medications, some of which may be contraindicated or result in unfavourable drug–drug interactions (see ‘Pharmacokinetics and drug–drug interactions of NOACs' section). Also, patient age and frailty (see ‘NOACs in advanced age and frailty' section), weight (see ‘NOACs in high- and low body weights' section), renal function (see ‘NOACs in patients with chronic kidney disease or advanced liver disease' section), and other comorbidities influence the choice. Proton pump inhibitors (PPIs) may be considered to reduce the risk for gastrointestinal (GI) bleeding and accompanying hospitalizations, especially in those with a history of GI bleeding or ulcer and patients requiring concomitant use of (dual) antiplatelet therapy.75–80 This gastroprotective effect was especially demonstrated in patients receiving antiplatelet or VKA therapy,81–83 while data on the preventive effects in NOAC treated patients are limited.79 Decision aids are available to guide clinicians about which NOAC may be best suited for a specific target group.84–87
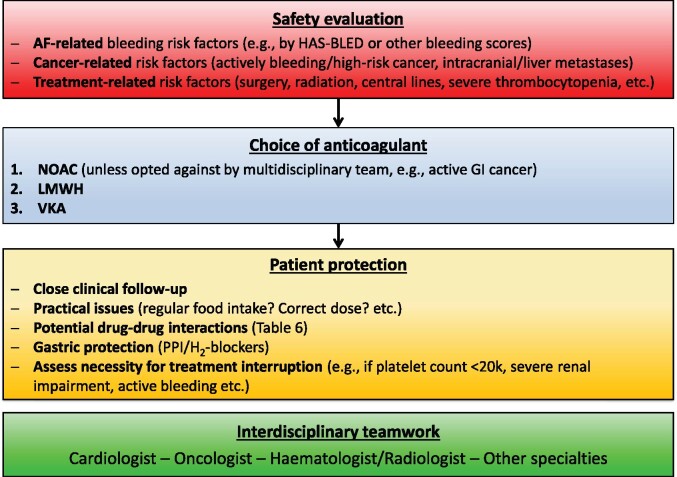
Practical considerations regarding adherence and persistence
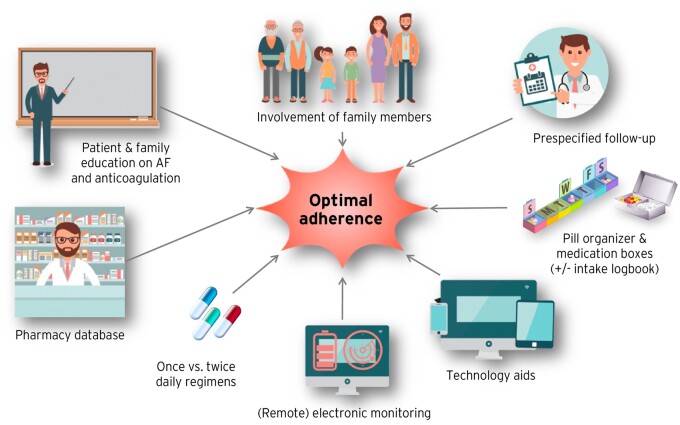
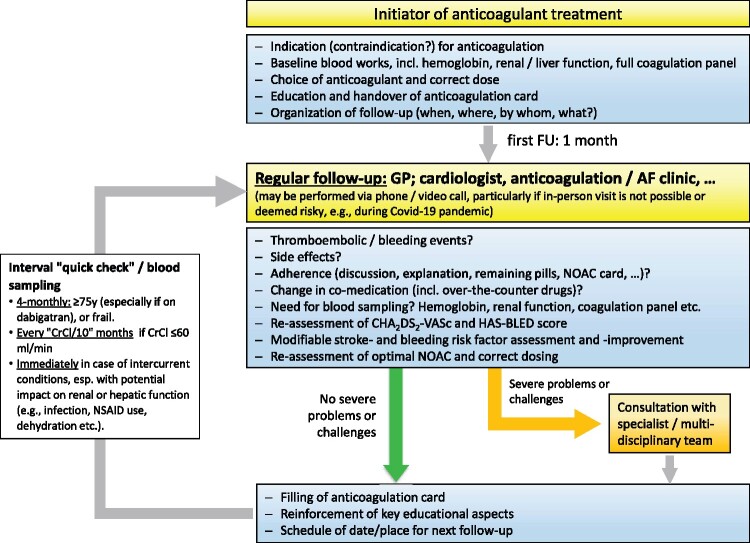
Practical considerations to assure adherence and persistence with NOAC therapy are summarized in Figure 1 and discussed in the Supplementary material online. Figure 2 shows the EHRA NOAC card (details see Supplementary material online), Figure 3 shows the structured follow-up scheme of NOAC treated patients.
Figure 1.
Selection of possibilities to increase adherence to NOACs. AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant.
Figure 2.
The EHRA NOAC card. A patient information card is crucial, both for the patient (instructions on correct intake; contact information in case of questions) as for healthcare providers. This generic and universal card should document each visit, each relevant observation or examination, and any medication change. EHRA, European Heart Rhythm Association; NOAC, non-vitamin K antagonist oral anticoagulant.
Figure 3.
Initiation and structured follow-up of patients on NOACs. It is crucial to ensure a structured follow-up of patients on NOACs. The anticoagulation card, as proposed in Figure 2, is intended to document each visit so that every person following up on the patient is well-informed. Moreover, written communication between different healthcare providers is required to inform them about the follow-up plan and execution. AF, atrial fibrillation; CrCl, creatinine clearance; GP, General Practitioner; NOAC, non-vitamin K antagonist oral anticoagulant.
Organization of follow-up and continued care
The organization of follow-up and continued care is summarized in Figure 3 and Table 3, and is discussed in detail in the Supplementary material online.
Table 3.
Checklist during follow-up contacts of AF patients on anticoagulation
| Interval | Comments | |
|---|---|---|
| 1. Adherence | Each visit |
|
| 2. Thromboembolism | Each visit |
|
| 3. Bleeding | Each visit |
|
| 4. Other side effects | Each visit |
|
| 5. Co-medications | Each visit |
|
| 6. Blood sampling (including haemoglobin, renal, and liver function) | Yearly |
|
| 4-monthly |
|
|
| Variable |
|
|
| If needed |
|
|
| 7. Re-assess stroke risk | Each visit |
|
| 8. Assessing and minimizing modifiable risk factors for bleeding | Each visit |
|
| ||
| 9. Assessing for optimal NOAC and correct dosing1 | Each visit |
|
AF, atrial fibrillation; CrCl, creatinine clearance; INR, international normalized ratio; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
For frequency of visits: see Figure 3.
Treatment of DVT/PE
| Initial therapy | Remainder of treatment phase | |
|---|---|---|
| Apixaban498 | 10 mg BID, 7 days | 5 mg BID, no dose reduction |
| Dabigatran499 | Heparin/LMWH | 150 mg BID, no dose reductiona |
| Edoxaban500 | Heparin/LMWH | 60 mg QD, same dose reduction as for SPAF (see above) |
| Rivaroxaban501,502 | 15 mg BID, 21 days | 20 mg QD, no dose reductionb |
BID, twice daily; GI, gastrointestinal; LMWH, low molecular weight heparin; QD, once daily; SPAF, stroke prevention in atrial fibrillation.
Per SmPC: 110 mg BID if age ≥80 years, concomitant verapamil, increased risk of GI bleeding [based on pharmacokinetic/pharmacodynamic (PK/PD) analyses; not studied in this setting].
Per SmPc: 15 mg if risk of bleeding outweighs risk for recurrent DVT and PE (based on PK/PD analyses; not studied in this setting).
Long-term prevention of recurrent DVT/PE
| Standard dose | Comments/dose adjustment | |
|---|---|---|
| Apixaban503 | 2.5 mg BID | |
| Dabigatran504 | 150 mg BID | No pre-specified dose-reduction criteria in clinical triala |
| Edoxaban473,500,505 | 60 mg QDb | |
| Rivaroxaban506 | 10 mg QD | c |
BID, twice daily; QD, once daily.
SmPC: 110 mg BID if age ≥80 years, concomitant verapamil (both based on pharmacokinetics/pharmacodynamics analyses; not studied in this setting).
Not specifically studied, follow-up data available up to 12 months in phase III trial.
SmPc: 20 mg QD in patients at high risk of recurrence.
VTE prevention post-major orthopaedic surgery
| Standard dose | Comments/dose reduction | |
|---|---|---|
| Apixaban507 | 2.5 mg BID | |
| Dabigatran508,509 | 220 mg QD/150 mg QD | a |
| Edoxaban510,511 | 30 mg QD | Not approved in Europe (only studied in Asia) |
| Rivaroxaban512–515 | 10 mg QD |
BID, twice daily; QD, once daily.
SmPc: 1× 150 mg if CrCl 30–50 mL/min; concomitant verapamil, amiodarone, quinidine; age >75 years.
Secondary prevention of atherothrombotic events post-ACS in patients without AF (i.e. no OAC indication)
| Standard dose | Comments/dose reduction | |
|---|---|---|
| Rivaroxaban115 | 2.5 mg BID | In addition to aspirin ± P2Y12 inhibitor |
BID, twice daily.
Secondary prevention of atherothrombotic events in patients with chronic coronary syndrome and/or symptomatic peripheral artery disease patients without AF (i.e. no OAC indication)
| Standard dose | Comments/dose reduction | |
|---|---|---|
| Rivaroxaban516 | 2.5 mg BID | In addition to aspirin |
AF, atrial fibrillation; BID, twice daily; OAC, oral anticoagulation.
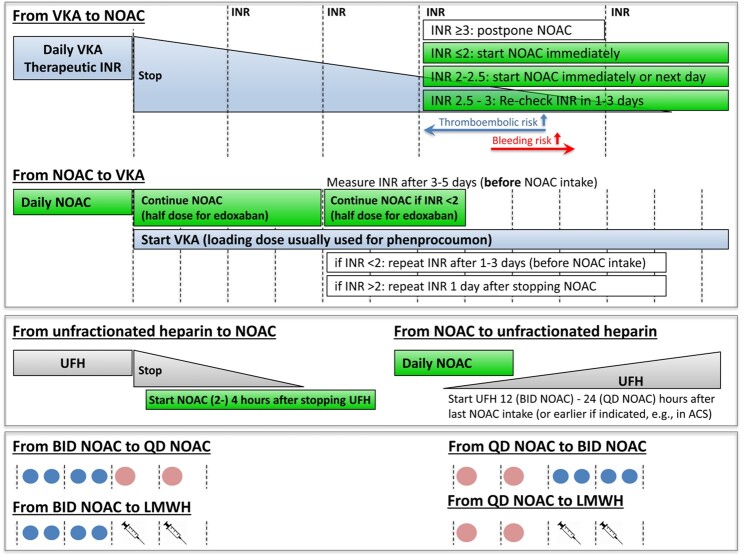
Switching between anticoagulant regimens
Practical advice on how to switch between anticoagulant regimens is summarized in Figure 4 and discussed in detail in the Supplementary material online.
Figure 4.
Switching between NOACs and other anticoagulants. ACS, acute coronary syndrome; BID, twice daily; INR, international normalized ratio; LMWH, low molecular weight heparin; NOAC, non-vitamin K antagonist oral anticoagulant; QD, once daily; UFH, unfractionated heparin; VKA, vitamin K antagonist.
Special considerations for NOAC use during the ‘coronavirus disease of 2019’ (COVID-19) pandemic
In addition to the general preference of NOACs over VKA for stroke prevention in AF due to efficacy and safety,1,6 NOAC therapy comes with some potentially important practical advantages over VKA-based anticoagulation during the coronavirus disease of 2019 (COVID-19) pandemic, including the lack of necessity for frequent clinic/office visits for INR monitoring. Community teams for at home INR controls may equally be limited during these periods. As a result, both the individual’s risk for contracting the virus as well as the workload on the healthcare system would be reduced.
Nevertheless, NOAC therapy also comes with its inherent challenges necessitating a well-planned and executed follow-up scheme (Figure 3) to optimize efficacy and safety of the drugs (see above). Conversely, any ‘file and forget’ NOAC use needs to be avoided also during a high-tide pandemic situation. Unfortunately, this is particularly true for high-risk AF patients—who almost inevitably would also potentially be high-risk COVID-19 patients in case of exposure and infection, likely primarily due to concomitant risk factors and comorbidities.88–90 Careful and wise decision-making regarding the type of NOAC, dose and follow-up scheme is essential. Importantly, since plasma level assessment of NOACs or coagulation tests are not needed, large parts of the regular follow-up routine may be performed via telemonitoring, including assessment of any thromboembolic or bleeding events, side effects, adherence, clinical factors precipitating a relevant decline in renal function [e.g. dehydration, intercurrent illnesses, non-steroidal anti-inflammatory drug (NSAID) use, …] etc. By doing so, in-person consultation may be reduced to a minimum and only be scheduled if physical examination and/or blood sampling (renal function, haemoglobin etc.) is required. Nevertheless, clear communication, ideally in writing (e.g. with Email follow-up) is key in order to avoid misunderstandings in these frequently older patients not accustomed to this way of consultation.
If patients on NOACs are infected with COVID-19 and particularly in case of severe infection requiring hospitalization, increasing evidence indicates a benefit for continuing anticoagulation to stave off COVID-19 complications.91 However, clinical deterioration (particularly of renal function) as well as administration of concomitant medication (see ‘Pharmacokinetics and drug–drug interactions of NOACs' section) needs to be carefully observed and therapy adjusted accordingly. Assessment via a multidisciplinary expert team including cardiologist, intensive care specialists, haematologists, neurologist etc. and, if in doubt, conversion to low-molecular or unfractionated heparin (UFH) is advisable. Further specific guidance can be found in the ‘ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic’.92
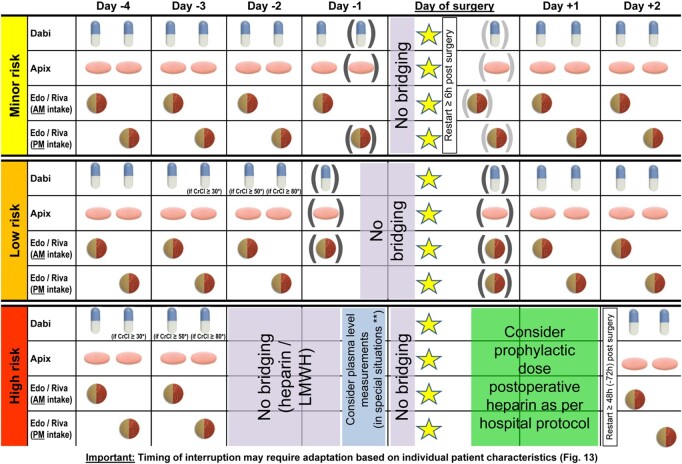
Covid-19 vaccines are usually administered by intramuscular (i.m.) injection. In patients on NOACs it is advisable to follow the scheme for ‘minor risk’ interventions as outlined in the ‘Patients undergoing a planned invasive procedure, surgery, or ablation' section (as well as in the Supplementary material online):
Leave out the morning dose of the NOAC prior to i.m. injection;
Use a fine-gauge needle for injection;
Apply firm pressure for 2–5 min after the injection;
In QD NOACs: take the left-out morning dose 3 h after the injection (esp. in case of high stroke risk and QD NOAC); and
In BID NOACs: re-start NOAC with the next scheduled dose.
Pharmacokinetics and drug–drug interactions of NOACs
Treatment with VKAs requires careful consideration of multiple food- and drug–drug interactions (DDIs). Despite fewer interactions with NOACs, physicians need to consider the pharmacokinetic interactions of accompanying drugs and comorbidities when prescribing NOACs. This section aims to provide a simple, non-exhaustive guide to deal with such situations. However, every patient may require more specific consideration, especially when a combination of interfering factors is present. The considerations on DDIs given in this chapter are based on extensive research using Stockleys Drug Interactions (https://about.medicinescomplete.com/publication/stockleys-drug-interactions/), UpToDate (https://www.uptodate.com/home/drugs-drug-interaction), the Phil database (https://phil.apb.be/nl-BE/product/2756153), as well as numerous published studies, reviews, and case reports. Knowledge regarding interactions (with effect on plasma levels and/or on clinical effects of NOAC drugs) is expanding, so that new information is likely going to modify existing advice.
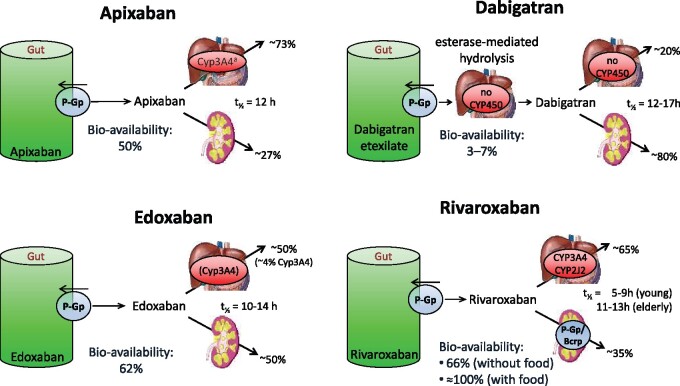
The absorption, distribution, metabolism, and excretion of the different NOACs are summarized in Table 4 and Figure 5.9 An important interaction mechanism for most NOACs consists of significant GI re-secretion over a P-glycoprotein (P-gp) transporter after absorption in the gut. P-gp is also involved in active renal secretion of NOACs.93 Competitive inhibition of the P-gp pathway will result in increased plasma levels, which needs to be considered since many drugs used in AF patients are P-gp inhibitors (e.g. verapamil, dronedarone, amiodarone, ranolazine, and quinidine). CYP3A4-type cytochrome P450-dependent elimination is relevantly involved in the hepatic clearance of rivaroxaban and apixaban.94 Strong cytochrome P (CYP) 3A4 inhibition or induction may affect plasma concentrations, and should be evaluated in context (see Tables 5–9 and colour coding, discussed below). Non-metabolic clearance of apixaban is diverse (including excretion of the unchanged compound by >50%).95 In general, NOAC use is not advisable in combination with drugs that are strong inhibitors of both P-gp and/or CYP3A4. Conversely, strong inducers of P-gp and/or CYP3A4 (such as rifampicin, carbamazepine, etc.) will markedly reduce NOAC plasma levels; concomitant use with NOACs should be avoided or used with great caution and surveillance.
Table 4.
Absorption and metabolism of the different NOACs
| Dabigatran106,376 | Apixaban517 | Edoxaban518 | Rivaroxaban519,520 | |
|---|---|---|---|---|
| Bioavailability | 3–7% | 50% | 62% | 15 mg/20 mg: 66% without food, 100% with food |
| Prodrug | Yes | No | No | No |
| Clearance non-renal/renal of absorbed dose | 20%/80% | 73%/27% | 50%/50% | 65%/35% |
| Plasma protein binding | 35% | 87% | 55% | 95% |
| Dialysability | 50–60% | 14% | NA | NA |
| (In part dialysable) | (Not dialysable) | (Not dialysable) | (Not dialysable) | |
| Metabolism | Glucoronic acid conjugation | CYP3A4 (25%), CYP1A2, CYP2J2, CYP2C8, CYP2C9 CYP2C19 | CYP3A4 (<4% of elimination) | CYP2A4 (18%)519, CYP2J2 |
| Absorption with food | No effect | No effect | 6–22% more; minimal effect on exposure | +39% more (see above) |
| Absorption with H2B/PPI | −12% to 30% (not clinically relevant) | No effect | No effect | No effect |
| Time to peak levels (h) | 3 | 3 | 2–4 | 2–4 |
| Elimination half-life (h) | 12–17 | 12 | 10–14 | 5–9 (young) 11–13 h (elderly) |
NOAC, non-vitamin K antagonist oral anticoagulant.
Figure 5.
Absorption and metabolism of the different NOACs. There are interaction possibilities at the level of absorption or first transformation, and at the level of metabolization and excretion. aAlso via CYP1A2, CYP2J2, CYP2C8, CYP2C9, and CYP2C19. NOAC, non-vitamin K antagonist oral anticoagulant.
Table 5.
Effect of drug-drug interactions and clinical factors on NOAC plasma levels and anticoagulant effects
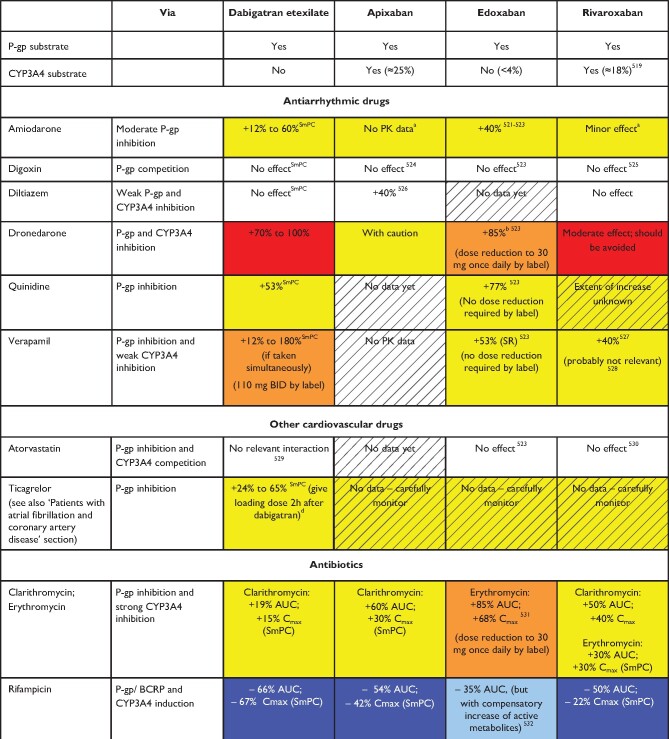
|
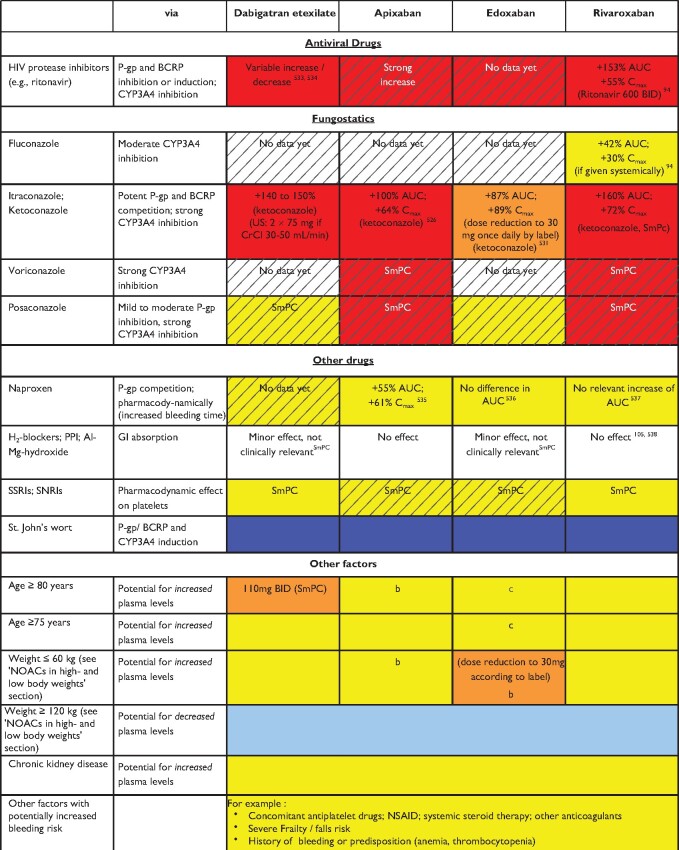
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion. The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
White: No relevant drug–drug interaction anticipated.
Yellow: Caution required, especially in case of polypharmacy or in the presence of ≥2 yellow/bleeding risk factors (see Figure 6).
Orange: Lower dose (dabigatran) or dose reduction (edoxaban) recommended according to label.
Red: Contraindicated/not advisable due to increased plasma levels.
Blue (dark): Contraindicated due to reduced NOAC plasma levels.
Blue (light): Caution required, especially in case of polypharmacy or in the presence of ≥2 light blue interactions due to reduced NOAC plasma levels.
AUC, area under the curve; BCRP, breast cancer resistance protein; BID, twice daily; CrCl, creatinine clearance; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; PK, pharmacokinetic; PPI, proton pump inhibitor.
Based on in vitro investigations, comparing the IC50 for P-gp inhibition to maximal plasma levels at therapeutic dose, and/or on interaction analysis of efficacy and safety endpoints in the Phase-3 clinical trials.46,47 No direct PK interaction data available.
Dose reduction based on published criteria (see Table 2).
Age had no significant effect after adjusting for weight and renal function.
Data from Phase I study. Interpret in the light of data from Re-DUAL PCI (see ‘Patients with atrial fibrillation and coronary artery disease' section for details).247
Specific dosing algorithms for the different NOACs have been evaluated in large phase III clinical RCTs and resulted in documented efficacy and safety of the respective agents. Of note, only one phase III study prospectively used concomitant therapy with certain drugs as a dose reduction criterion (dose reduction of edoxaban in ENGAGE-AF in patients treated with potent P-gp inhibitors verapamil, quinidine, or dronedarone). Dose reduction of all NOACs is primarily recommended along the published dose reduction criteria (see ‘NOAC eligibility and dosing' section, Table 2). Whenever possible, the tested and approved dosing regimen of NOACs should be used.1
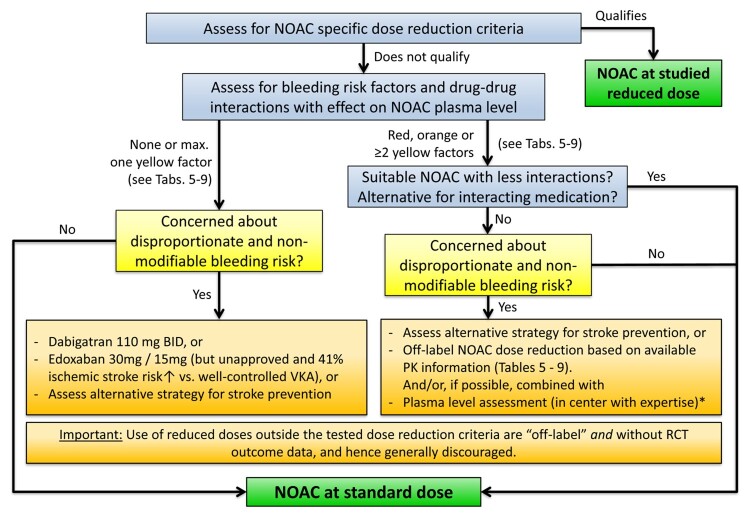
However, there may be a clinical rationale for using a lower dose of a NOAC in patients with a particularly high bleeding risk and/or when a higher plasma level of the drug can be anticipated based on a combination of factors even if the label-recommended criteria for dose reduction are not fulfilled.1,96–99 Prospective clinical trial data only exist for ‘lower doses’ of dabigatran (110 mg BID) and edoxaban (lower dose edoxaban regimen: 30/15 mg QD; but not approved for stroke prevention). For edoxaban 30/15 mg QD a 41% higher ischaemic stroke risk compared to a well-controlled warfarin arm [median time in therapeutic range (TTR) > 68%] was observed leading to non-approval of this dosing regimen. At the same time, a reduction in haemorrhagic stroke, major bleeding, cardiovascular-, and all-cause mortality was observed compared with warfarin.49,98 This was confirmed in a recent direct comparison of the lower-dose edoxaban regimen (30 mg/15 mg) and higher-dose edoxaban regimen (60 mg/30 mg).100 For dabigatran 110 mg BID, a similar stroke risk and significantly reduced major bleeding vs. warfarin was observed.48 These data represent the only available RCT-based evidence of a ‘lower dose’ of a NOAC for stroke prevention in AF on hard clinical endpoints.48,49 In contrast, no ‘lower dose’ arm was included (only ‘dose reduction’) in ROCKET-AF (for rivaroxaban) or ARISTOTLE (for apixaban) and as such, no clinical outcome data are available for the use of these reduced doses outside the tested dose reduction algorithms. The ‘Japanese ROCKET’ (J-ROCKET) study demonstrated a safety profile of 15 mg QD rivaroxaban as standard dose for stroke prevention in AF in Japanese patients as compared to VKA but was not powered for efficacy outcomes.101 In the ELDERCARE-AF trial, Japanese patients ≥80 years of age deemed unsuitable for anticoagulation receiving a very low and unapproved dose of 15 mg QD edoxaban showed a 4.4%/year absolute risk reduction in stroke/systemic embolism as compared to placebo, at the cost of a non-significant 1.5%/year absolute increase in the risk of major bleeding.102 Whether these findings translate to non-Japanese populations remains to be determined.
The use of plasma level measurements for NOAC dose-adjustment or in the setting of ‘off label’ lower dose prescription (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section) is discouraged for the vast majority of patients due to the lack of outcome data to support such an approach. Indeed, an increased risk of bleeding frequently goes along with an increased risk of stroke due to the overlapping risk factors (including advanced age, frailty etc.), and inappropriate use of a reduced dose may result in sub-optimal stroke prevention.103 However, in rare cases of potentially substantial DDIs or special situations in which a certain NOAC is preferred for certain reasons (e.g. patients after transplantation, patients on HIV medication etc.) this may be considered (Figure 6).104 Importantly, this approach should be limited to centres with extensive experience in the performance and interpretation of such assays as well as in the care of NOAC-treated patients (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section).
Figure 6.
NOAC selection based on drug–drug interactions and/or risk of bleeding. Dose reduction of all NOACs is primarily recommended along the published dose reduction criteria (see ‘NOAC eligibility and dosing' section, Table 2). Whenever possible, the tested and approved dosing regimen of NOACs should be used. See text for details. *Use of plasma level measurements to guide dosing is generally discouraged and should only be used in rare cases of potentially substantial interactions or special situations, and only in centers with great experience in the performance and interpretation of such assays as well as the care of NOAC-treated patients (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section). BID, twice daily; NOAC, non-vitamin K antagonist oral anticoagulant; PK, pharmacokinetic; RCT, randomized clinical trial; VKA, vitamin K antagonist.
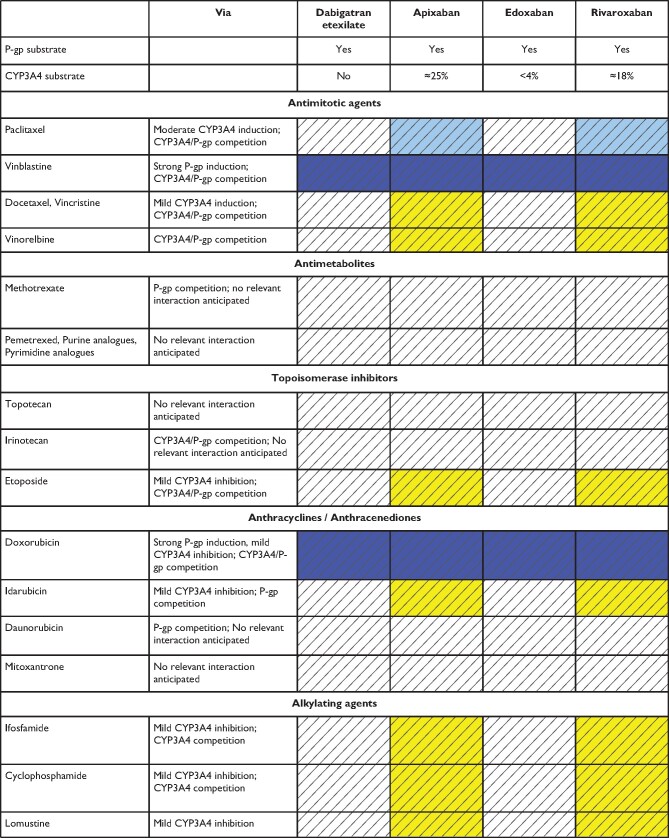
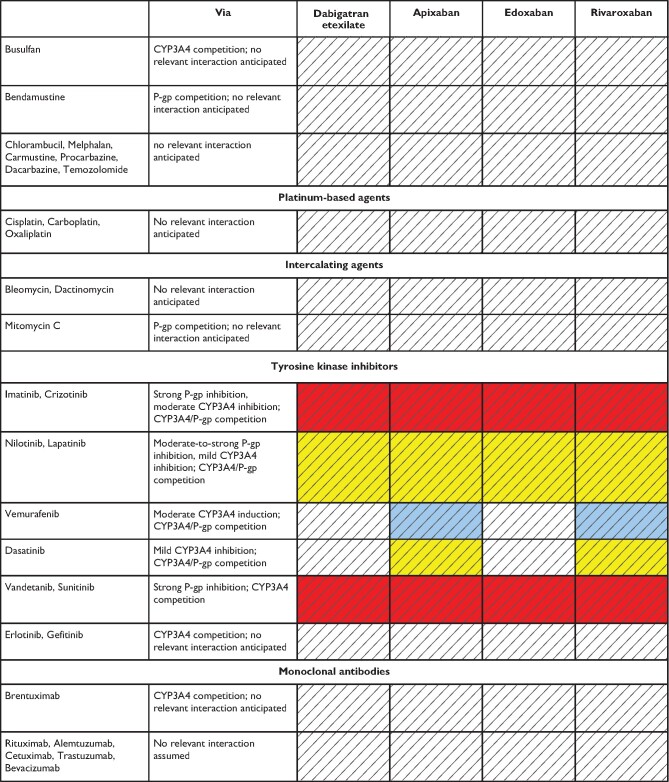
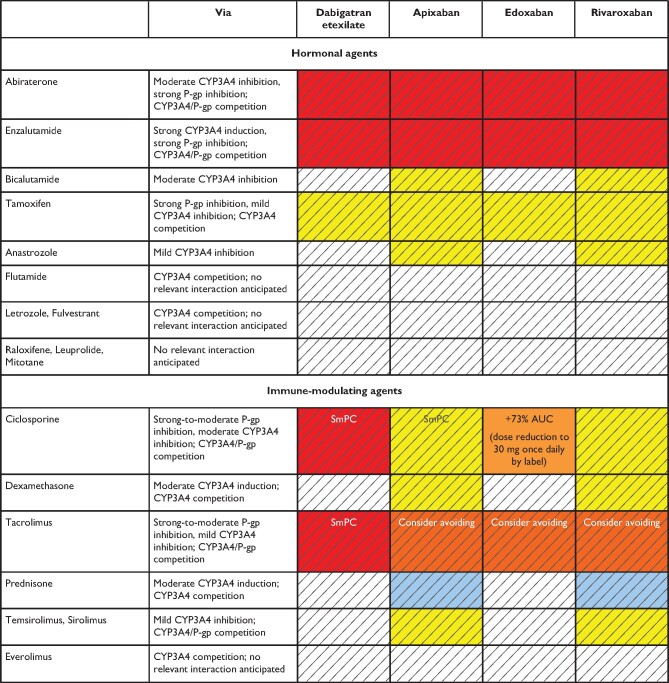
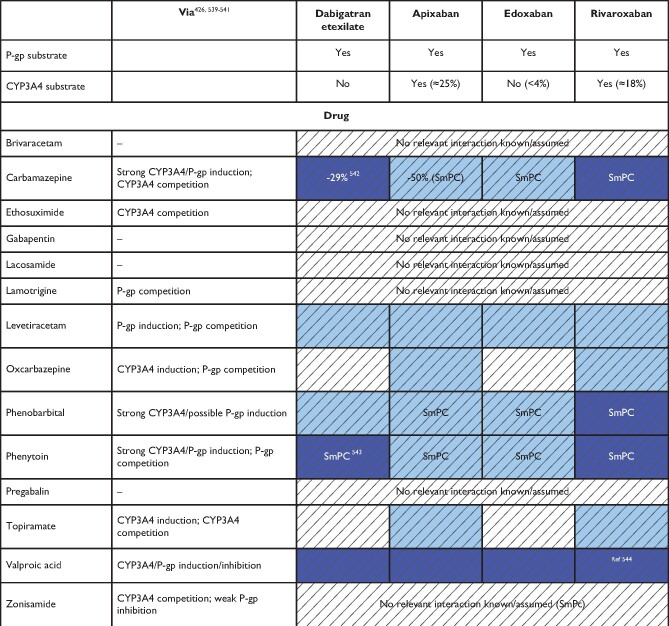
In summary, possible DDIs, especially when combined with other clinical risk factors affecting NOAC plasma levels are important aspects for choosing a specific NOAC for a specific patient. Table 5 gives an overview of the effect of various frequently used agents on NOAC plasma levels; Table 6 focuses on common cancer drugs (see also ‘NOACs in patients with atrial fibrillation and malignancy' section), Table 7 on antiepileptic drugs (AEDs) (see also ‘NOACs in other special populations' section) and Table 8 on common herbal products. There are several major limitations particularly regarding the assessment of NOACs—herbal drug interactions including the possibility of several hypothetical pharmacokinetic and pharmacodynamic pathways, unknown mechanisms of interaction, and the inherent variation in composition. As such, firm advice regarding the safety of use is difficult to give. Particularly in patients with additional risk factors, plasma level measurements may be considered (including its inherent limitations, as discussed above).
Table 6.
Anticipated effects of common anti-cancer drugs on non-vitamin K antagonist oral anticoagulants plasma levels
|
|
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion. The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
White: No relevant drug–drug interaction anticipated.
Yellow: Caution required, especially in case of polypharmacy or in the presence of ≥2 yellow/bleeding risk factors (see Figure 6).
Orange: Consider avoiding concomitant use, careful monitoring required if combined. See Figure 6.
Red: Contraindicated/not advisable due to increased plasma levels.
Orange: Dose reduction (edoxaban) recommended according to label.
Blue (dark): Contraindicated/not advisable due to reduced NOAC plasma levels.
Blue (light): Caution required, especially in case of polypharmacy or in the presence of ≥2 light blue interactions due to reduced NOAC plasma levels.
Where no data or SmPC instructions were available, expert opinion was generally based on the following principles:
• Strong CYP3A4 and/or P-gp inducer—should not be used (dark blue).
• Moderate CYP3A4 or P-gp inducer—use with caution or avoid (light blue).
• Strong CYP3A4 and/or inhibitor—should not be used (red).
• Moderate CYP3A4 and/or P-gp inhibitor—use with caution or avoid (orange).
• Mild CYP3A4 and/or P-gp inducers or inhibitors—caution required especially with polypharmacy or in the presence of ≥2 bleeding risk factors (yellow).
Purine analogues: Mercaptopurine, Thioguanine, Pentostatin, Cladribine, Clofarabine, Fludarabine.
Pyrimidine analogues: Fluorouracil, Capecitabine, Cytarabine, Gemcitabine, Azacitadine, Decitabine.
Table 7.
Anticipated effects of common antiepileptic drugs on non-vitamin K antagonist oral anticoagulants plasma levels
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion.426 The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
White: No relevant drug–drug interaction anticipated.
Blue (dark): Contraindicated due to reduced NOAC plasma levels.
Blue (light): Caution required, especially in case of polypharmacy or in the presence of ≥2 light blue interactions due to reduced NOAC plasma levels.
Table 8.
Anticipated effects of common herbal medicines on non-vitamin K antagonist oral anticoagulants plasma levels
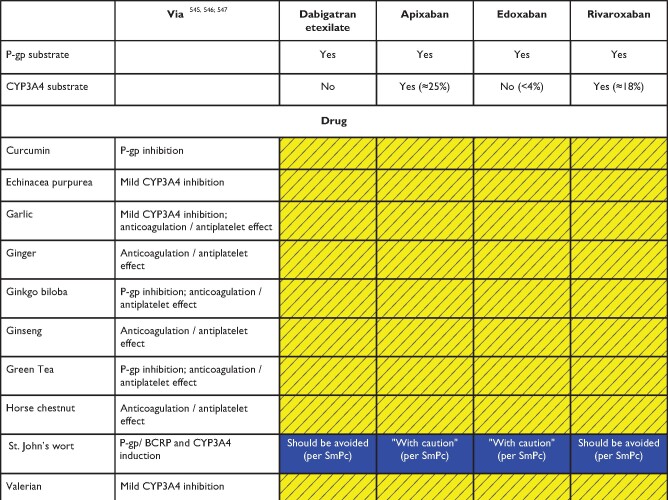
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion. The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
Major limitations regarding the assessment of NOACs—herbal drug interactions include the possibility of several hypothetical pharmacokinetic and pharmacodynamic pathways, unknown mechanisms of interaction, and the inherent variation in composition.
White: No relevant drug–drug interaction anticipated.
Yellow: Caution required, especially in case of polypharmacy or in the presence of ≥2 yellow/bleeding risk factors (see Figure 6).
Blue (dark): Contraindicated/not advisable due to reduced NOAC plasma levels.
Where no data or SmPC instructions were available, expert opinion was generally based on the following principles:
• Strong CYP3A4 and/or P-gp inducer—should not be used (dark blue).
• Mild CYP3A4 and/or P-gp inducers or inhibitors or pharmacodynamic interaction—caution is needed especially with polypharmacy or in the presence of ≥2 bleeding risk factors (yellow).
Taking into consideration these factors as well as the setup and results from the large randomized NOAC outcome trials the algorithm shown in Figure 6 may assist in a rational selection of a specific NOAC and/or a ‘reduced dose’ based on DDIs and other clinical risk factors. Unfortunately, for many potential interactions with drugs that are often used in AF patients no detailed information is available yet (hatched in Tables 5–9).
Food intake, antacids, and nasogastric tube administration
Rivaroxaban for stroke prevention in AF (20 mg/15 mg QD) needs to be taken with food since the area under the curve (AUC) of the plasma concentration increases by 39% to a very high bioavailability of almost 100%.105 There is no relevant food interaction with the other NOACs. The concomitant use of PPIs and H2-blockers leads to a reduction in the bioavailability of dabigatran, but without effect on clinical efficacy.106,107 There is also no relevant antacid interaction for the other NOACs.105,108,109 There are no pharmacokinetic data on fish oil supplements for any of the NOACs, but interaction is unlikely.
Data have shown that administration in crushed form, e.g. via a nasogastric tube, does not alter the bioavailability for apixaban, rivaroxaban, and edoxaban.110–113 In contrast, dabigatran capsules must not be opened as this results in a substantial increase in drug bioavailability (+75% per SmPC).
Interactions of specific drug classes and considerations for polypharmacy are discussed in the Supplementary material online.
Pharmacodynamic interactions
Apart from the pharmacokinetic interactions, co-administration of NOACs with other anticoagulants, platelet inhibitors (e.g. aspirin, clopidogrel, ticlopidine, prasugrel, ticagrelor; see also ‘Patients with atrial fibrillation and coronary artery disease' section), and NSAIDs increases the risk of bleeding.114–116 Therefore, such combinations should be carefully balanced against the potential benefit in each clinical situation. Co-administration of NOACs with dual antiplatelet drugs requires active measures to prevent bleeding (see ‘Patients with atrial fibrillation and coronary artery disease' section).
NOACs in patients with chronic kidney disease or advanced liver disease
Atrial fibrillation and chronic kidney disease
AF and chronic kidney disease (CKD) are not only frequent comorbidities but also strongly interacting diseases: AF facilitates the development and progression of CKD, and, vice versa, the prevalence and incidence of AF increase with decreasing renal function.117–120 Patients with AF and CKD have a markedly increased morbidity and mortality especially due to their excessive risk for both thromboembolic and severe bleeding events, making risk stratification and treatment challenging.121,122 This is of particular relevance since all four available NOACs are in part eliminated by the kidneys: dabigatran has the greatest extent of renal elimination (80%), while 50%, 35%, and 27% of edoxaban, rivaroxaban, and apixaban, respectively, are cleared via the kidneys.
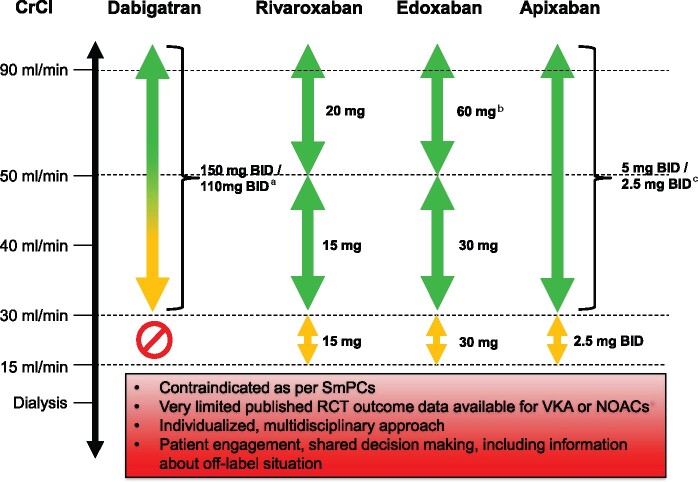
Further details regarding the available data on NOACs in patients with CKD are discussed in detail in the Supplementary material online. Basic information on the diagnosis/staging of CKD and assessment of renal function is provided in Table 10. Practical considerations for the use of NOACs based on renal function are summarized in Figure 7.
Table 10.
Criteria for diagnosing CKD; estimation of renal function and categories of renal dysfunction
| Decreased GFRa | GFR <60 mL/min/1.73 m2 | ||
| Markers of kidney damage (≥1) | Excessive albuminuria (AER ≥30 mg/24 h; ACR ≥30 mg/g or ≥3 mg/mmol) | ||
| Urine sediment abnormalities | |||
| Electrolyte or other abnormality caused by tubular disorders | |||
| Abnormal histology | |||
| Structural abnormalities detected by kidney imaging | |||
| History of kidney transplantation | |||
| GFR category | CKD stage | GFRa | Description |
| G1 | 1 | ≥90 | Normal or high |
| G2 | 2 | 60–89 | Mildly decreased |
| G3a | 3 | 45–59 | Mildly to moderately decreased |
| G3b | 30–44 | Moderately to severely decreased | |
| G4 | 4 | 15–29 | Severely decreased |
| G5 | 5 | <15 | Kidney failure (requires renal replacement therapy, dialysis or kidney transplantation) |
Online calculators available at (e.g.): www.kidney.org/professionals/kdoqi/gfr_calculator, www.nephron.com/cgi-bin/CGSI.cgi, www.mdcalc.com/creatinine-clearance-cockcroft-gault-equation, https://reference.medscape.com/calculator/creatinine-clearance-cockcroft-gault.
Popular Apps are NephroCalc, MedMath, MedCalc, Calculate by QxMD, and Archimedes.
(mL/min/1.73 m2).
Figure 7.
Use of NOACs according to renal function. a110 mg BID in patients at high risk of bleeding (per SmPc). bOther dose reduction criteria may apply (weight ≤ 60 kg, concomitant potent P-Gp inhibitor therapy). According to EMA, SmPc edoxaban should be used in ‘high CrCl only after a careful evaluation of the individual thromboembolic and bleeding risk’.473 See text for details. c2 × 2.5 mg only if at least two out of three fulfilled: age ≥80 years, body weight ≤60 kg, creatinine ≥1.5 mg/dL (133 µmol/L). Orange arrows indicate cautionary use; see text for details. BID, twice daily; CrCl, creatinine clearance; EMA, European Medicines Agency; NOAC, non-vitamin K antagonist oral anticoagulant; RCT, randomized clinical trial; VKA, vitamin K antagonist.
Table 9.
Anticipated effects of Medications used in the treatment of Covid-19 on non-vitamin K antagonist oral anticoagulants plasma levels
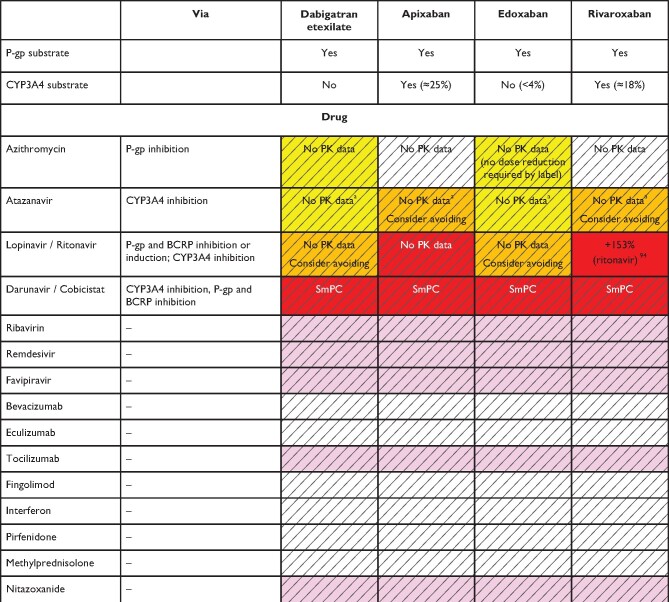
|
Colour coding is based on the respective NOAC SmPC, drug interaction databases, or expert opinion. The hatched colour coding indicates no clinical or PK data available. Some of the colour codes will likely require adaptation as more data become available over time.
White: No relevant drug–drug interaction anticipated.
Yellow: Caution required, especially in case of polypharmacy or in the presence of ≥2 yellow/bleeding risk factors (see Figure 6).
Orange: Consider avoiding concomitant use, careful monitoring required if combined. See Figure 6.
Red: Contraindicated/not advisable due to increased NOAC plasma levels.
Pink: No information retrievable.
Where no data or SmPC instructions were available, expert opinion was generally based on the following principles:
• Strong CYP3A4 and/or inhibitor—should not be used (red).
• Moderate CYP3A4 and/or P-gp inhibitor—use with caution or avoid (orange)
• Mild CYP3A4 and/or P-gp inducers or inhibitors—caution is needed especially with polypharmacy or in the presence of ≥2 bleeding risk factors (yellow).
The use of NOACs is not advisable when atazanavir is given in combination with its enhancers ritonavir or cobicistat.
Oral anticoagulant therapy in patients with severe CKD (CrCl of 15–29 mL/min)
There are no RCT data on the use of warfarin for thromboprophylaxis in AF patients with severe CKD or on dialysis, and all landmark trials with NOACs essentially excluded patients with a creatinine clearance (CrCl) of <30 mL/min (apart from few patients on apixaban with CrCl 25–30 mL/min).123 In the US (but not in Europe), a low dose dabigatran 75 mg BID has been approved for patients with severe CKD (a CrCl of 15–29 mL/min), based on pharmacokinetic simulations. Rivaroxaban, apixaban, and edoxaban (but not dabigatran) are approved in Europe for the use in patients with severe CKD (stage 4, i.e. a CrCl of 15–29 mL/min), with a reduced dose regimen (Figure 7). Observational data indicate a favourable efficacy and safety profile of all three FXa inhibitors compared to VKA in patients with severe renal dysfunction but these data need to be interpreted with caution based on the inherent high likelihood of substantial residual confounding.124–126 The 2020 ESC guidelines recommend the use of factor Xa inhibitors ‘with caution’ and at reduced doses for patients with CrCl 15–29 mL/min.1
Apixaban is least renally cleared (27%) and its dose is reduced by 50% under rather stringent conditions; furthermore, the rate of major bleeding with apixaban is reduced more (vs. warfarin) in patients with impaired renal function.123,127 Edoxaban is more renally cleared, but its dose reduction to 50% is applied more rapidly and was tested in a large subgroup. Rivaroxaban has an intermediate renal clearance (35%) and is reduced less (by 25%) under similar conditions as edoxaban. In view of the individual NOAC pharmacokinetics (27% renal clearance for apixaban), dose-reduction criteria (50% reduction for apixaban and edoxaban), and available evidence from RCTs, the use of either apixaban or edoxaban may be preferable in these patients, but direct head-to-head comparisons are missing. Given the important limitation of observational studies128 further randomized RCT-based data are urgently required for these difficult to treat patients.
Oral anticoagulant therapy in patients with end-stage CKD (CrCl of <15 mL/min and/or dialysis)
Numerous observational studies have reported conflicting results for the use of both VKA and NOACs in patients with end-stage renal disease regarding effectiveness and bleeding without a clear signal for a benefit of OAC.129–132 A propensity score matched analysis of 4,537 Medicare patients as well as a meta-analysis of 16 studies with 71 877 dialysis-dependent patients with AF (about 3000 with NOACs) did not demonstrate a benefit regarding the risk for stroke and thromboembolism but instead found a markedly increased incidence of bleeding complications in patients with OAC compared to those without.133,134
The use of VKA in end-stage CKD may in some cases result in calciphylaxis, a painful and often lethal condition caused by calcification and occlusion of cutaneous arteries and arterioles.135 Moreover, there is also an ongoing controversy about the clinical relevance of aggravated calcifications of the large vessels as well as those of the kidney itself under VKA.
The efficacy and safety of NOACs in patients with end-stage renal dysfunction and on dialysis is unclear and subject to ongoing studies. Plasma levels while on treatment with apixaban 2.5 mg BID136 (as well as with 5 mg, Pokorney et al., presented at ESC 2020), edoxaban 15 mg QD,137 and rivaroxaban 10 mg QD138 or 15 mg139 were found to be similar to patients with the full dose and normal renal function. Initial registry data had indicated a higher incidence of hospitalization or death from bleeding in dialysis-dependent patients with dabigatran or rivaroxaban as compared to VKA.140 More recent analyses indicated more similar thromboembolic- and bleeding rates with apixaban and rivaroxaban vs. VKA; however, residual confounding is likely to be substantial in these analyses precluding any definitive answer regarding efficacy and safety of NOACs in these patients.124,141–143 Furthermore, two randomized controlled trials have been initiated comparing apixaban vs. VKA [‘RENal Hemodialysis Patients ALlocated Apixaban vs. Warfarin in Atrial Fibrillation’ (RENAL-AF) in the US (NCT02942407), and ‘A Safety Study Assessing Oral Anticoagulation With Apixaban vs. Vitamin-K Antagonists in Patients With Atrial Fibrillation (AF) and End-Stage Kidney Disease (ESKD) on Chronic Hemodialysis Treatment’ (AXADIA) in Germany (NCT02933697)144]. Both studies lacked a third treatment arm without any OAC and both suffered from severe recruitment problems. RENAL-AF has been stopped prematurely after including 154 patients and reported similar rates of major and clinically relevant non-major bleeds as well as a (numerical) doubling of cardiovascular deaths with apixaban vs. warfarin (presented at AHA 2019). Of note, a large proportion of warfarin patients were outside the therapeutic range (TTR 44%) and about 50% of apixaban patients received 5 mg BID. A third, smaller trial (NCT03987711) comparing warfarin, apixaban, and no anticoagulation is currently ongoing. Despite the lack of data for NOACs (or OAC in general) in dialysis-dependent patients, their usage seems to be increasing.145
In summary, given the lack of strong evidence the decision to anticoagulate and (if so) whether to use a NOAC or VKA in patients with end-stage renal failure or on dialysis requires a high degree of individualization. Measurements of NOAC plasma levels (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section), although intuitively appealing for this situation, has equally never been prospectively investigated for hard clinical endpoints, and should hence be reserved to highly specialized centres. Patients need to be informed of the lack of data as well as the ‘off label’ character of whichever strategy or drug is chosen, including the uncertain benefit and the increased risk of complications. Ideally, such patients should be included in ongoing trials to improve the evidence base for this difficult to treat patient population.121,146 Of note, there are also no RCT data for the use of alternative stroke prevention strategies such as left atrial appendage (LAA) occluder implantation for these individuals.
There are no data on the use of NOACs in AF patients after kidney transplantation. If NOACs are used in such patients, the dosing regimen should be selected according to the estimated renal function, and caution is needed concerning possible DDIs between the NOAC and concomitant immunosuppressive therapies (see ‘Pharmacokinetics and drug-drug interactions of NOACs' section).
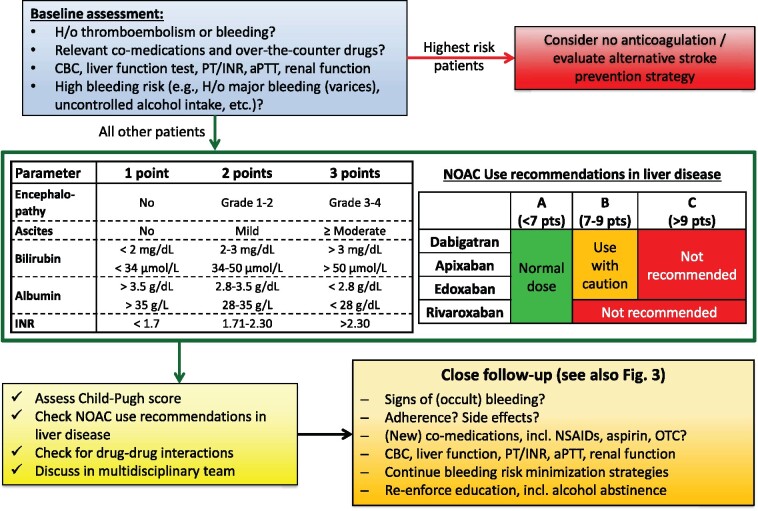
NOACs in liver disease
Practical considerations for the use of NOACs in liver disease are discussed in the Supplementary material online and are summarized in Figure 8.
Figure 8.
NOACs in patients with liver disease. APTT, activated prothrombin time; INR, international normalized ratio; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; OTC, over-the-counter; PT, prothrombin time.
NOAC plasma level measurements: technical approach, indications, pitfalls
Assessment of the anticoagulant effect of NOACs
The use of NOAC in daily clinical practice does not require monitoring of coagulation since all four phase III RCTs comparing NOACs to VKAs have been conducted without dose adjustments based on plasma level measurements.46–49 However, assessment of the anticoagulant effect of NOACs may be desirable in certain, rare situations (see below).
NOAC anticoagulant activity can be measured via specific coagulation assays developed for the quantification of NOAC plasma levels.147–149 Most routine coagulometers are capable of measuring NOAC plasma levels within ≤30 min. Institutions should strongly consider 24/7 availability of these tests for emergency situations. In contrast, point-of-care tests are being developed and are entering clinical practice, but are not yet widely available.150,151
Anti-FXa chromogenic assays are available to measure plasma concentrations of the FXa inhibitors using validated calibrators. Low and high plasma levels can be measured with acceptable inter-laboratory precision. The absence of anti-Xa activity with these assays excludes clinically relevant drug levels. Conversely, the diluted thrombin time (dTT) test as well as the ecarin chromogenic assay (ECA) display a direct linear relationship with dabigatran concentrations and are suitable for their quantitative assessment . Even though levels in clinical trials were measured using High Performance Liquid Chromatography/Mass Spectrometry (HPLC/MS), drug measurement and monitoring can be closely approximated using a calibrated dTT/ECA assay for dabigatran or chromogenic anti-FXa assay for FXa-inhibitors. These determinations have been demonstrated to be comparable to HPLC/MS.152–154 It is advisable to primarily use plasma concentrations rather than anti-FXa activity or dTT to gauge the level of anticoagulation in NOAC-treated patients to minimize inter- and intra-laboratory variability as well as other potential methodological limitations.155,156 An overview of the expected peak and trough levels in patients on NOACs can be found in Table 11. When interpreting a coagulation assay in a patient treated with a NOAC, it is important to know when the NOAC was administered relative to the time of blood sampling. The maximum effect of the NOAC on the clotting test will occur at its maximal plasma concentration, which is approximately 2–3 hours (±1 h) after intake for each of these drugs (Table 4).
Table 11.
Plasma levels and coagulation assays in patients treated with NOACs for stroke prevention in AF
| Dabigatran97,548,549 | Apixaban550 | Edoxaban98,100 | Rivaroxaban519,520,551 | |
|---|---|---|---|---|
| Expected plasma levels of NOACs in patients treated for AF* | ||||
| Peak levels | 52–383 | 69–321 | 101–288 | 178–343 |
| Trough levels | 28–215 | 34–230 | 12–43 | 12–137 |
| Expected impact of NOACs on routine coagulation tests148,150,158,549,552–554 | ||||
| PT | (↑) peak (↑) if supratherapeutic149 |
(↑) at peak |
↑ at therapeutic levels (if sensitive assay is used) Normal values do not exclude trough levels |
↑ at therapeutic levels (if sensitive assay is used) Normal values do not exclude trough levels |
| aPTT |
↑↑(↑) Normal values exclude supratherapeutic- but not therapeutic levels |
(↑) at peak | (↑) at peak | (↑) at peak |
| ACT |
↑(↑) Consistent with effect on aPTT |
(↑) | (↑) | (↑) |
| TT |
↑↑↑↑ Normal values exclude presence of Dabigatran |
– | – | – |
ACT, activated clotting time; AF, atrial fibrillation; aPTT, activated prothrombin time; NOAC, non-vitamin K antagonist oral anticoagulant; PT, prothrombin time.
[ng/ml] 5–95% percentiles for FXa inhibitors and 10–90% percentiles (ng/ml) for Dabigatran).
Impact of NOACs on other coagulation assays
Routine coagulation tests [prothrombin time (PT), activated prothrombin time (aPTT), activated clotting time (ACT)] generally do not provide an accurate assessment of NOAC anticoagulant effects and cannot be used to accurately to gauge anticoagulant activity (Table 11) or provide information on adherence to treatment. However, a normal aPTT excludes supratherapeutic levels in dabigatran-treated patients. The effect of apixaban, edoxaban, and rivaroxaban on the PT is highly dependent on the PT reagent used. Therefore, a normal PT does not necessarily exclude therapeutic levels of rivaroxaban, edoxaban and particularly apixaban.148,156,157 Point-of-care INR devices developed to monitor VKAs do not accurately reflect the anticoagulant status of NOAC treated patients.
There is not enough information to consider the use of thromboelastography or rotational thromboelastometry for adequately assessing NOAC activity, as they lose sensitivity at trough levels of the NOACs.156 Urine tests may be useful for detecting exposure to NOACs but levels do not correlate well with plasma concentrations.156,158
Impact of NOACs on thrombophilia testing
NOACs interfere with thrombophilia tests and the measurement of coagulation factors.159 Therefore, leaving a time window of at least 24 h is reasonable between the last intake of a NOAC and blood sampling to confidently assess coagulation parameters.147 This time window may need to be even longer for lupus anticoagulant measurements (≥48 h) or in the presence of factors potentially prolonging the anticoagulant effect such as CKD. In patients in whom interruption of anticoagulation is not feasible, ex vivo neutralization of the NOAC activity in plasma samples is possible in specialized haemostasis labs. This may allow for correct interpretation of thrombophilia tests, but requires good collaboration with the haemostasis lab and appropriate clinical information.160,161
Potential indications for NOAC plasma level measurements
No studies have investigated if measurement of drug levels and dose adjustment based on laboratory coagulation parameters, e.g. by dose reduction in case of higher than expected levels or by dose increase in case of lower than expected levels, improve the overall benefit of NOACs during long-term treatment. As such, routine monitoring of plasma levels and subsequent dose adaptation is generally discouraged.
However, laboratory assessment of drug exposure and anticoagulant effect may help clinicians in emergencies such as bleeding (see ‘Management of bleeding under NOAC therapy' section), urgent (see ‘Patients requiring an urgent surgical intervention' section), or certain elective procedures (see ‘Patients undergoing a planned invasive procedure, surgery, or ablation' section), suspected overdose, and acute stroke (see 'AF patients presenting with acute stroke while on NOACs' section). Also, in special situations during long-term care such as multiple possible DDIs (see ‘Pharmacokinetics and drug-drug interactions of NOAC' section), extremes of bodyweight (see ‘NOACs in high- and low body weights' section), or severely impaired renal function (see ‘NOACs in patients with chronic kidney disease or advanced liver disease section) plasma level measurements may aid in the clinical decision-making. This, however, should only be done under the guidance of a coagulation expert and in the knowledge that prospective randomized clinical outcome data still do not exist to support such a strategy (only observational data).104,162–164 Also patients need to be informed of and consent to this ‘off-label’ approach.
Management of bleeding under NOAC therapy
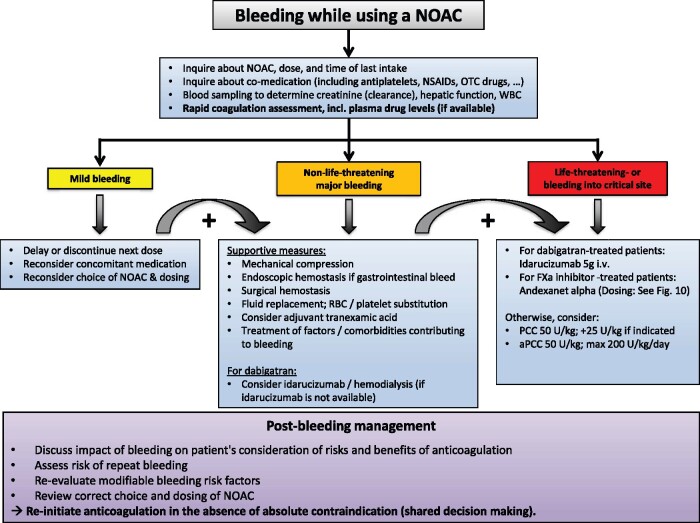
General aspects
The phase III trials have consistently shown that NOACs cause less intracranial and less life-threatening bleeds than warfarin, despite the absence of specific reversal agents in these trials. Not only was there a similar or even a reduced bleeding incidence, but patients experiencing a major (particularly extracranial) bleed under NOACs were also shown to have a more favourable outcome than for bleeding under VKA treatment.165–169 This is underlined by the reduction in all-cause mortality as well as life-threatening/fatal bleeds which was observed with NOACs vs. warfarin.6,46,49,165,170
Nevertheless, as more patients are being treated with NOACs, the absolute number of NOAC-related bleeding events increases. Importantly, any bleed is an opportunity to review the correct choice and dosing of the NOAC (see ‘NOAC eligibility and dosing' section) and to evaluate modifiable bleeding risk factors including sub-optimally treated hypertension, excessive alcohol intake and concomitant antiplatelet therapy, NSAIDs, glucocorticoids etc.1
To optimally manage NOAC-treated patients who present with a bleed we strongly suggest developing a hospital-wide policy in an interdisciplinary manner among cardiologists, haemostasis experts, emergency physicians/intensive care specialists, surgeons, and others. This protocol should describe the availability, timing, and indications of specific coagulation tests as well as the availability and use of specific and nonspecific reversal agents. Such a policy needs to be communicated well and be easily accessible (e.g. on an intranet site, in the emergency room, in pocket-sized leaflets etc.). In addition, a regular interdisciplinary review and discussion of patients experiencing severe bleeding complications (as well as strokes) is encouraged in order to share different subspecialty experiences as well as patient perception of such events and subsequent preferences.
Strategies to manage bleeding complications in patients treated with NOACs rely on a precise analysis of the clinical situation (Figure 9).
Figure 9.
Management of bleeding in patients taking NOACs. aPCC, activated prothrombin complex concentrates; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; OTC, over-the-counter; PCC, prothrombin complex concentrates; RBC, red blood cell; WBC, white blood cell.
-
The type of bleeding: nuisance/minor, major non-life threatening, or life-threatening.
-
The patient and his/her treatment, including:
– The exact time of last NOAC intake
– Prescribed dosing regimen
– Renal function
– Other factors influencing plasma concentrations (e.g. hepatic function, co-medications etc.)
– Other factors influencing haemostasis (e.g. concomitant use of antiplatelet drugs).
-
The patient’s thromboembolic risk
– Particularly when considering the use of prothrombotic agents, and regarding the necessity of (early) re-initiation of anticoagulant therapy
Both routine coagulation tests and assays that specifically measure NOAC plasma levels are important adjuncts in the assessment of NOAC related bleeds (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section).174 Normal results of dTT/ecarin clotting time (for dabigatran) or anti-Xa activity (for anti-FXa treated patients) exclude relevant levels of the respective anticoagulants. Importantly, conventional coagulation tests may be abnormal not only due to the effect of the NOAC itself, but for a variety of other reasons, particularly in the setting of severe bleeding and consumption coagulopathy. Conversely, it needs to be kept in mind that restoration of coagulation alone does not necessarily result in improved clinical outcome (e.g. in the context of intracranial haemorrhage).175,176
Practical advice for the management of nuisance/minor bleeding and non-life-threatening major bleeding is summarized in Figure 9 and discussed in the Supplementary material online.
Life-threatening bleeding or bleeding into a critical site
Patients with a life threatening bleed or bleeding into a critical site172,174,177,178 while treated with NOACs may benefit from its reversal in addition to the standard measures outlined above and in Figure 9. Although laboratory values (including a full coagulation panel) should be taken prior to any reversal measures in order to guide further treatment during the course, immediate actions are guided by clinical assessment without waiting for the results of laboratory measurements. Conversely and importantly, normalization of coagulation in itself is not necessarily sufficient to stop a bleed but may allow for more invasive interventions to control the bleeding source. Furthermore, even after direct reversal, significant NOAC concentrations may reappear in some patients and contribute to recurrent or continued bleeding (particularly after andexanet alpha due to its shorter half-life, less after idarucizumab administration),179,180 underlining the necessity for continued clinical and laboratory monitoring.
Idarucizumab
Idarucizumab is a humanized antibody fragment that specifically binds dabigatran. In the ‘Reversal Effects of Idarucizumab in Patients on Active Dabigatran’ (RE-VERSE-AD) study the drug was successfully used in patients on dabigatran presenting with major or life-threatening bleeding, or with the necessity of emergency surgery.181 This was confirmed in the observational RE-VECTO registry.182 Idarucizumab completely reversed the anticoagulant activity of dabigatran within minutes in almost all patients181 and is hence considered first-line therapy in such situations. A total of 5 g idarucizumab is administered intravenously in two ready-to-use doses of 2.5 g i.v., administered as two consecutive infusions over 5–10 min each or as a bolus injection.183 Continued clinical and laboratory monitoring is strongly advised, since a 5 g dose of idarucizumab may not completely neutralize an exceptionally high level of dabigatran (e.g. in case of overdose or CKD). Also, low levels of dabigatran may reappear after 12–24 h.
After 24 h, dabigatran can be re-started if clinically indicated and feasible, with normal kinetics. Other anticoagulants, including heparins, are not affected by idarucizumab.
If idarucizumab is not available, dialysis may be used to partially eliminate dabigatran from the circulation.184 However, starting and performing dialysis in a patient with a severe (potentially life-threatening) bleed may be challenging and may only be advisable if idarucizumab is not readily available.
Direct reversal of apixaban, edoxaban, or rivaroxaban (FXa-inhibitors)
Andexanet alfa is a recombinant, inactive human FXa analogue that non-specifically binds FXa inhibitors thereby preventing all FXa inhibitors (including low-molecular weight- and UFHs) from inhibiting FXa. In the ‘Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of FXA Inhibitors 4’ (ANNEXA-4) study, andexanet alpha was successfully used in major or life-threatening bleeding; in contrast to RE-VERSE-AD the trial did not include patients undergoing emergency surgery.185 The drug comes as a lyophilized powder which needs to be reconstituted before use. It is administered as a bolus over 15–30 min, followed by a 2-h infusion depending on the NOAC and on the timing since last intake (Figure 10). In the EU Andexanet alpha is only approved for the reversal of life-threatening or uncontrollable bleeding in patients taking apixaban or rivaroxaban. In view of the very similar mode of action and preliminary subanalyses from the ANNEXA-4 trial (Benz et al., presented at the International Stroke Conference meeting 2021) it can be assumed that it will have a similar effect in patients on edoxaban. Since anticoagulant activity may re-appear after cessation of the infusion it is currently less clear at what point in time and with which anticoagulant effect FXa inhibitors or heparin can be (re-)administered following andexanet alpha administration.
Figure 10.
Application and effect of idarucizumab and andexanet alpha. Per Andexanet Alpha SmPc.496 aOr unknown. Andexanet alpha is currently only approved for reversal of life-threatening uncontrollable bleeding in patients taking apixaban or rivaroxaban. In view of the very similar mode of action and preliminary subanalyses from the ANNEXA-4 trial (Benz et al., presented at at the International Stroke Conference meeting 2021), it can be assumed that it will have a similar effect in patients on edoxaban. The edoxaban dosing provided in this scheme is based on the (final) protocol of the ANNEXA-4 trial.185 dTT, diluted thrombin time.
Coagulation factors
Clinical trials and registry data with NOACs have shown that administration of coagulation factors is rarely needed.186,187 Indeed, any NOAC-antagonizing effect of a procoagulant has to be balanced carefully against the potential prothrombotic effect. Animal experiments as well as studies in healthy volunteers have indicated the potential usefulness of prothrombin complex concentrate (PCC) and activated PCC (aPCC) for the normalization of coagulation parameters under NOAC treatment as a surrogate for haemostatic support.188–194 As indicated above, data from the large phase III trials demonstrated that outcomes of bleeds under NOACs were similar (if not better) than in the VKA arm (with diverse bleeding treatments applied, including PCC/aPCC).165–167 The efficacy on clinical outcomes of PCCs or aPCCs in patients taking NOACs who are actively bleeding has not been firmly established in an RCT. However, several observational studies in patients with major bleedings have been published (with some inherent limitations including the retrospective, non-controlled setting as well as absence of a control group) indicating that (a)PCCs appeared to be efficacious in supporting haemostasis.195–199 Its usefulness in intracranial Haemorrhage, on the other hand, is uncertain (see ‘AF patients presenting with acute stroke while on NOACs' section).200 The administration of PCCs or aPCCs can hence be considered in a patient with a life-threatening bleed if immediate haemostatic support is required, especially in situations where a specific reversal agent is not available or too costly.201 The choice between PCC and aPCC may depend on their availability and the experience of the treatment centre. As indicated, aPCC induces a strong pro-coagulant effect and should only be used by physicians experienced in their use.
PCC and aPCC are preferred over recombinant activated factor VIIa (90 µg/kg) given the absence of any outcome data and the latter’s pronounced pro-coagulant effect.202,203 Fresh frozen plasma (FFP) is no longer considered a useful reversal strategy, primarily due to the plasma abundance of NOACs which will inhibit newly administered coagulation factors upon administration of FFP and the resulting large volume of FFP that would need to be administered to have any impact on coagulation.203 Vitamin K and protamine administration have no role in the management of a bleeding under NOACs; these may only be useful in the management of bleeding under NOACs when vitamin K deficiency is suspected or in case of concomitant treatment with heparins, respectively.
(Re-)initiating anticoagulation post-extracranial bleeding
In most cases of nuisance or minor bleeding anticoagulation can be re-started, sometimes simply by delaying or skipping a single dose. All other bleeds, particularly life-threatening bleeding episodes, require a careful re-assessment of the risks and benefits of re-initiating anticoagulation. In most cases of bleeds due to secondary (e.g. bleeding post-trauma) and/or reversible causes (e.g. genito-urinary bleeding due to cancer) anticoagulation can be resumed once the cause of the bleeding has been eliminated. As exemplified for gastro-intestinal bleeds many additional factors need to be taken into consideration (Figure 11). Conversely, for severe and life-threatening bleeds without a clear secondary or reversible/treatable cause, the risks of re-initiating anticoagulation may outweigh the benefits. In such cases, implantation of a LAA occluder or surgical LAA occlusion may be considered as a potential substitute for long-term anticoagulation,1 but RCT-based evidence for LAA occlusion after bleeding under OAC is currently also missing.
Figure 11.
(Re-) initiation of anticoagulation after GI bleeding. aWithout RCT evidence; ideally include patient in ongoing trial. GI, gastrointestinal; LAA, left atrial appendage; NOAC, non-vitamin K antagonist oral anticoagulant.
The approach after intracranial (intracerebral, subarachnoidal, subdural, or epidural) bleeding is outlined in the section on ‘AF patients presenting with acute stroke while on NOACs' .
Measures to consider in case of a (suspected) overdose without bleeding or a clotting test indicating a potential risk of bleeding
Excessive NOAC plasma concentrations potentially expose the patient to an increased risk of bleeding. This may occur when the patient has (intentionally) taken an overdose, but also intercurrent events such as an acute decline in renal function (especially with dabigatran) or administration of drugs with known DDIs (see ‘Pharmacokinetics and drug–drug interactions of NOACs' section) may increase NOAC plasma concentrations to supratherapeutic levels. In terms of management, it is important to distinguish between an overdose with resultant bleeding and without. In case of a suspected overdose, assessment of NOAC plasma levels can help determine its degree and possible bleeding risk (Table 11). Given the relatively short plasma half-life of NOACs, a ‘wait-and-see’ strategy can be used in most cases without active bleeding. The elimination half-life can be estimated taking into account age and renal function. As a result of limited absorption, a ceiling effect with little to no further increase in plasma exposure is seen at supra-therapeutic doses of ≥50 mg rivaroxaban.204 There are no data in this respect for the other FXa inhibitors or dabigatran.
In the case of recent acute ingestion of an overdose (especially when ≤2 h ago), the use of activated charcoal to reduce absorption may be considered for any NOAC (with a standard dosing scheme for adults of 30–50 g) although clinical data on its effectiveness are lacking.165,205,206
If a more aggressive normalization of plasma levels is deemed necessary, or rapid normalization is not expected (e.g. severely impaired renal function) the steps outlined in patients with an active bleed may need to be considered (Figure 9). Only in exceptional cases administration of coagulation factors (PCC, aPCC) awaiting clearance of the drugs should be considered; clearly in these situations balancing the benefit of normalizing coagulation in a non-bleeding patient needs to be carefully weighed against a possibly strong prothrombotic effect.
Patients requiring an urgent surgical intervention
If an emergency intervention is required, any NOAC should be discontinued immediately. Considerations for the specific management depends on the level or urgency (acute emergency, urgent or expedite)207 as summarized in Figure 12 and discussed in the Supplementary material online.
Figure 12.
NOAC management in the setting of unplanned surgery. aPTT, activated prothrombin time; dTT, diluted thrombin time; NOAC, non-vitamin K antagonist oral anticoagulant; PT, prothrombin time.
In all such situations, particularly prior to the application of any haemostatic agent, a full panel of coagulation assays (including PT, aPTT, anti-FXa, or dTT/ECA etc.) should be obtained to assess the patient’s coagulation status. Even if in an emergency situation the indication for application of reversal- and/or pro-haemostatic agents is governed by the patient's clinical presentation, results of these initial tests may have important implications for further treatment during the ensuing hours. Furthermore, assessment of NOAC plasma levels may be of great help in interpreting the patient’s anticoagulant status as well as the waning of any NOAC effect (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section).
Patients undergoing a planned invasive procedure, surgery, or ablation
General considerations
About one quarter of anticoagulated patients requires temporary cessation for a planned intervention within 2 years.187 Various societies have issued separate guidelines on the timing of NOAC interruption prior to surgery or interventions. It is impossible to summarize all recommendations, and HCPs are advised to check this guide’s schemes against the relevant recommendations of their country/healthcare setting and professional societies. Ever since its introduction, the EHRA practical guide intended to provide a unified approach which is as simplified as possible to allow for its broad implementation. Data from the PAUSE trial and drug-specific registries have meanwhile added to the evidence that such an approach may be safe and effective across many clinical scenarios, but also that additional individualization based on patient characteristics could further improve safety.208,209
While invasive surgical interventions require temporary discontinuation of NOACs, many less invasive procedures carry a relatively low bleeding risk and may be performed under minimally- or uninterrupted NOAC therapy (Table 12, Figures 13–15). However, patient characteristics (including age, stroke risk, history of bleeding complications, concomitant medication, kidney function etc.) as well as surgical factors need to be taken into account to determine when to discontinue and restart a NOAC (Figure 13). As such, the ‘default’ NOAC interruption periods provided in Figures 14 and 15 may require adaptation based on the individual benefit/risk ratio. It is strongly advisable to develop and implement institutional guidelines and hospital-wide policies concerning perioperative anticoagulation management in different surgical settings, which are widely communicated and readily available. All patients undergoing a planned intervention as well as caregivers (primary care physician etc.) should receive a written note indicating the anticipated date and time of the intervention as well as the date and time of last NOAC intake.
Table 12.
Classification of elective surgical interventions according to bleeding risk
| Minor risk interventions (i.e. infrequent bleeding and with low clinical impact) |
| Dental extractions (1–3 teeth), paradontal surgery, implant positioning, subgingival scalling/cleaning |
| Cataract or glaucoma intervention |
| Endoscopy without biopsy or resection |
| Superficial surgery (e.g. abscess incision; small dermatologic excisions, skin biopsy) |
| Pacemaker or ICD implantation (except complex procedures) |
| Electrophysiological study or catheter ablation (except complex procedures) |
| Routine elective coronary/peripheral artery intervention (except complex procedures) |
| Intramuscular injection (e.g. vaccination) |
| Low-risk interventions (i.e. infrequent bleeding or with non-severe clinical impact) |
| Complex dental procedures |
| Endoscopy with simple biopsy |
| Small orthopaedic surgery (foot, hand, arthroscopy, …) |
| High-risk interventions (i.e. frequent bleeding and/or with important clinical impact) |
| Cardiac surgery |
| Peripheral arterial revascularization surgery (e.g. aortic aneurysm repair, vascular bypass) |
| Complex invasive cardiological interventions, including lead extraction, (epicardial) VT ablation, chronic total occlusion PCI etc. |
| Neurosurgery |
| Spinal or epidural anaesthesia; lumbar diagnostic puncture |
| Complex endoscopy (e.g. multiple/large polypectomy, ERCP with sphincterotomy etc.) |
| Abdominal surgery (incl. liver biopsy) |
| Thoracic surgery |
| Major urologic surgery/biopsy (incl. kidney) |
| Extracorporeal shockwave lithotripsy |
| Major orthopaedic surgery |
For each patient, individual factors relating to bleeding and thromboembolic risk need to be taken into account and be discussed with the operating physician and the patient (see Figure 13).
Figure 13.
Perioperative NOAC management. NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug.
Figure 14.
Timing of last NOAC intake before an elective intervention. CrCl, creatinine clearance; LMWH, low molecular weight heparin; NOAC, non-vitamin K antagonist oral anticoagulant; UFH, unfractionated heparin.
Figure 15.
Stopping and re-initiation of NOAC therapy in elective surgery. Yellow star—Time point of the intervention/operation. Parentheses indicate optional pre-/postoperative intake, especially in patients not at high risk of drug accumulation/bleeding. Consider +24 h of interruption in situations likely resulting in increased plasma levels [e.g. body weight < 50 kg, significant interactions (see ‘Pharmacokinetics and drug-drug interactions of NOACs' section)]. *Intake of this dose of dabigatran if CrCl is in the indicated range; otherwise skip this dose. **Consider measurement of plasma levels in very special situations, e.g. highest risk neurosurgery/cardiac surgery, severely impaired renal function, combination of factors predisposing to higher NOAC levels (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section). Rivaroxaban needs to be taken with food for stroke prevention in AF, which needs to be considered (also) in the post-operative setting. AF, atrial fibrillation; CrCl, creatinine clearance; NOAC, non-vitamin K antagonist oral anticoagulant.
Laboratory testing before surgery or invasive procedures
Specific coagulation measurements (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section) prior to surgery or invasive procedures provide a direct assessment of the residual drug concentration210 and have been proposed in high-risk interventions or interventions in which even some bleeding may have severe consequences. Although theoretically reasonable, HCPs as well as patients need to be aware that adapting the duration of interruption based on residual NOAC levels is without prospectively validated evidence concerning its clinical impact, including the determination of ‘safe’ NOAC levels for different types of procedures. In the ‘Perioperative Anticoagulant Use for Surgery Evaluation’ (PAUSE) trial, patients undergoing low-risk procedures had a higher likelihood of mildly (≥30 ng/mL) or moderately (≥50 ng/mL) elevated NOAC levels due to shorter NOAC interruption times.211 For high-risk procedures, CrCl <50 mL/min, standard (vs. reduced) NOAC dose, body weight <70 kg and female sex were associated with elevated NOAC levels. In the prospective multicentre ‘COncentration of RIvaroxaban, Dabigatran and Apixaban’ (CORIDA) study, CrCl <50 mL/min and use of certain antiarrhythmic drugs (amiodarone, verapamil, diltiazem) were associated with elevated perioperative plasma levels.162 However, elevated NOAC levels were not independently predictive of an increased likelihood of bleeding in either PAUSE or CORIDA.162,211 Hence, although assessment of residual NOAC levels may be considered in certain selected patients, particularly before undergoing high-risk interventions, a ‘time-based’ interruption schedule as outlined above generally appears safe for the majority of patients and procedures.208,209 Of note, if NOACs are interrupted for >72 h the likelihood of any residual NOAC level appears very low162,211 usually precluding the necessity of NOAC level assessment outside scenarios with very high risk of drug accumulation (e.g. severely reduced renal function).
Interruption times based on bleeding risk classifications
Suggested interruptions times based on bleeding risk classifications (Table 12) are discussed in the Supplementary material online and are summarized in Figures 14 and 15.
Bridging
Pre-operative bridging with low-molecular weight heparin (LMWH) or UFH is not recommended in NOAC-treated patients since the predictable waning of the anticoagulation effect allows for properly timed short-term cessation of NOAC therapy before surgery. For patients on VKA, bridging with heparin/LMWH was associated with a significantly higher risk of major bleeding during cessation of OAC but did not reduce thromboembolic events.212 Similarly for NOACs, bridging is associated with an increased bleeding risk.187,213–215
Based on prior experience with VKA, the very few very high-risk situations in which bridging may be discussed include urgent surgery with a high bleeding risk in patients with a recent (≤3 months) thromboembolic event (including stroke, systemic embolism or venous thrombosis/pulmonary embolism) or who suffered an event during previous adequate interruption of NOAC therapy.216 In these instances, in addition to ‘timed’ NOAC interruption, switching to UFH or low-dose dabigatran—both with the possibility of rapid reversal—around the operation may be evaluated based on a multidisciplinary team decision. Further research on the optimal management in such high-risk patients is required as they were frequently excluded from or under-represented in the available trials addressing perioperative management of NOAC-treated patients.
In patients with chronic coronary artery disease (CAD) treatment with NOAC monotherapy is safe and effective and considered standard therapy for long-term management (see ‘Patients with atrial fibrillation and coronary artery disease' section).1 However, particularly patients with a high coronary risk may be at risk for peri-operative cardiovascular events during NOAC interruption due to the absence of any antithrombotic therapy.217,218 In the ‘Perioperative Ischaemic Evaluation 2’ (POISE-2) trial, peri-operative aspirin use did not reduce the risk of myocardial infarction (MI) or death but increased the risk of major bleeding in 10 010 patients at risk for vascular complications (one third with a history of vascular disease).219 However, whether these results translate to patients at very high risk of coronary events during perioperative interruption of NOAC therapy remains unclear. A strategy with initiation of aspirin therapy pre-operatively, performance of the operation under continued aspirin (with suspended NOAC), and re-initiation of NOAC therapy post-operatively (with discontinuation of aspirin therapy) may be evaluated and based on a multidisciplinary team decision. Again, further studies are required to help guide the perioperative management in these high-risk situations.
Restarting NOAC therapy after an invasive procedure
After a procedure with immediate and complete haemostasis, NOACs can generally be resumed 6–8 h after the end of the intervention. However, in some surgical interventions resuming full dose anticoagulation within the first 48–72 h after the procedure may carry a bleeding risk which outweighs the risk of AF-related embolism. In such cases, postoperative thromboprophylaxis using LMWH in prophylactic dose 6–8 h after surgery and delay of therapeutic anticoagulation by deferring restart of the NOAC ≥48–72 h can be considered. Similarly, in patients in whom oral drug intake is not possible (e.g. in the case of artificial ventilation, postoperative nausea and vomiting, ileus etc.) heparin administration should be considered. In contrast, there are no data on the safety and efficacy of the postoperative use of a reduced dose of NOACs (such as used for the prevention of venous thromboembolism after hip/knee replacement) in patients with AF undergoing a surgical procedure.
Special considerations for selected procedures
Special considerations for selected procedures are discussed in the Supplementary material online.
Special considerations for atrial fibrillation ablation procedures
Left atrial catheter ablation is an intervention with a risk of major groin bleedings as well as serious bleeding secondary to transseptal puncture (TSP) and manipulation/ablation in the left atrium (although the incidence of these complications has been decreasing, particularly in experienced centres).220 On the flipside, the intervention directly increases the risk of thromboembolic complications.220,221 Recent international consensus statements and guidelines recommend performing left atrial catheter ablation under uninterrupted anticoagulant treatment with VKA (target INR 2.0–2.5 if on VKAs),1,220 since such a strategy was associated with less thromboembolic events and less bleeding as compared to bridging with heparin.222 The efficacy and safety of uninterrupted NOAC vs. VKA therapy for AF ablation have been examined in dedicated RCTs for apixaban,223 dabigatran,224 edoxaban,225 and rivaroxaban.226 The last dose of once-daily based NOACs were recommended (rivaroxaban) or mandated (edoxaban) to be administered in the evening before the procedure, whereas twice-daily dosed NOACs (apixaban, dabigatran) were administered in the morning of the procedure.227 While substantial variations in the event rate in the VKA arm of these trials were observed, major bleedings were overall lower with NOACs without an increase in thromboembolic complications.225 A recent meta-analysis of 29 studies comprising over 12 000 patients confirmed a lower rate of bleeding events with NOACs vs. VKA at a similar (low) rate of thromboembolic complications.228 Taken together, uninterrupted NOAC therapy can be considered safe and effective in AF ablation and should likely be the preferred mode of anticoagulation for patients undergoing this procedure.
An institutional protocol for NOAC patients undergoing AF ablation should be developed to ensure a uniform approach. To mimic the trial situation as closely as possible, switching NOAC intake to the evening well in advance (e.g. 1 week) of the intervention may be reasonable for the once-daily based NOACs edoxaban and rivaroxaban.225,226 Whether opting to administer the last NOAC dose shortly before the procedure (i.e. ‘truly uninterrupted’) for BID dosed NOACs or to go for a short cessation period (last NOAC dose on the evening before the procedure), may depend on a number of factors including renal function, a routine practice of heparin administration prior to (first) TSP, and administration of protamine prior to sheath removal.9,220,229 Indeed, particular in the latter case, patients may be exposed to low anticoagulant levels following the procedure if the morning dose is withheld.227 RCT-based evidence comparing ‘truly’- and ‘minimally' interrupted NOAC strategies, however, is not available. In the RE-CIRCUIT trial, the five major bleeding events in the dabigatran arm all occurred in patients with ≤4 h (n = 2) or 4–8 h (n = 3) since last intake of dabigatran. Moreover, 19.6% of all patients on dabigatran had their last intake of the drug >8 h prior to the procedure resulting in a similar duration of interruption as in QD NOACs with last intake on the evening before the procedure. Skipping the morning dose on the day of the ablation may hence be a valid option in BID-dosed NOACs (Figure 16).
Figure 16.
NOAC management before and after AF ablation. ACT, activated clotting time; AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; TSP, transseptal puncture.
Routine exclusion of LA/LAA thrombus prior to AF ablation is recommended according to current expert consensus statements and guidelines also in NOAC treated patients, especially in patients presenting for the procedure without anticoagulation.1,230
During the ablation, intravenous heparin should be administered to achieve an ACT of 300–350 s.230 It has been noted that the total need for heparin and the time to target ACT was higher in some NOAC- (particularly FXa-inhibitor-) treated patients.226,232,233 Indeed, dabigatran readily prolongs ACT measurements whereas the effect of FXa inhibitors are variable depending on the assay used.234 The clinical implications of this, however, are currently unclear. It may hence be reasonable to use the same target ACT levels for heparin titration in NOAC-treated patients as in patients on (uninterrupted) VKA.
NOAC intake can be resumed 3–5 h after sheath removal if adequate haemostasis is established and pericardial effusion has been ruled out.229
Special considerations for cardiac surgery procedures
Cessation and re-initiation of NOACs around cardiac surgery
Elective cardiac surgery in patients on NOACs fall into the ‘red’ category of procedures with high risk (i.e. with a risk of frequent and/or high impact bleeding), as indicated in Table 12 and Figures 14 and 15. Hence, a standard interruption time of 48 hours applies, also according to the European Association for Cardio-Thoracic Surgery (EACTS) Guidelines,235 but longer interruption times of 72–96 h may be considered in patients at risk of NOAC accumulation (e.g. older patients, CKD etc.). Of note, if NOACs are interrupted for >72 h the likelihood of any residual NOAC level appears very low,162,211 usually precluding consideration of NOAC level assessment outside scenarios with very high risk of drug accumulation (e.g. severely reduced renal function). Importantly, and as for most other situations, pre-operative bridging with LMWH is not advised for elective patients on NOACs.
In patients on NOACs who need to urgently undergo cardiac surgery, i.e. without the possibility to interrupt treatment for the above-indicated intervals, assessment of NOAC plasma levels may be helpful for risk stratification (see Figure 12). EACTS guidelines suggest plasma levels <30 ng/mL as cut-off values below which operations may ‘safely’ be performed, but prospective outcome data are lacking.235 If higher values are measured and further waiting is impossible, reversal of dabigatran using idarucizumab may represent a valid treatment option.181 It is currently unclear if reversal of FXa inhibitors using andexanet alpha is similarly safe and effective in such situations, particularly given its potential pro-thrombogenic effect as well as its non-specific inhibitory effect on other FXa inhibitors including UFH (which may require the use of a direct thrombin inhibitor such as argatroban or bivalirudin during cardiopulmonary bypass).236 In view of these limitations, combined with the limited availability and high cost of andexanet alpha, FXa inhibitor ‘reversal’ using PCC or aPCC may be advisable, also carefully weighing its indication against its potential prothrombotic effect, until further data for andexanet alpha become available in the context of cardiac surgery procedures.235,237
Following cardiac surgery, the optimal time point for NOAC (re-)initiation depends on a number of factors, including adequate haemostasis as well as any additional interventions (planned and unplanned). Prophylactic UFH or LMWH is advisable in the initial postoperative period due to its rapid onset and offset as well as its reversibility, followed by therapeutic heparin 12–48 h postoperative, as discussed in the section on ‘Patients undergoing a planned invasive procedure, surgery, or ablation'.235 Once adequate haemostasis has been confirmed and no further interventions are planned, UFH or LMWH may be transitioned to a NOAC in eligible patients (Tables 1 and 4; excluding, importantly, patients after mechanical valve replacement as well as patients after bioprosthetic valve implantation or valve repair as discussed below).
NOAC management around interventions following cardiac surgery (including chest tube insertion, removal of temporary epicardial pacing wires)
There are no strong data to advise on how to best deal with interventions performed or planned to be performed shortly after cardiac surgery, including removal of temporary epicardial pacemaker wires. In most scenarios, a similar scheme as for ‘low bleeding risk’ interventions can be applied (Table 12, Figures 14 and 15), i.e. with a 24 h interruption of NOAC therapy. However, a host of other factors may influence the duration of NOAC interruption including thrombocytopenia, additional antiplatelet therapy, co-medications, deterioration of CKD etc. It may hence be advisable to not initiate NOAC therapy following cardiac surgery prior to temporary pacing wire removal or when any other intervention (drainage of pleural effusion etc.) is still anticipated.
NOAC use in post-operative AF
Post-operative AF is common following cardiac surgery, with incidences reported as high as 20–50%.1,238 The 2020 ESC AF guidelines (developed in collaboration with the EACTS) indicate that long-term OAC therapy may be considered in patients at risk for stroke with (newly developed) postoperative AF after cardiac surgery (Class IIb, level of evidence B), since both the short- and long-term risk of stroke may be substantially elevated in such patients.1,239 The timing of OAC/NOAC initiation follows the general principles after cardiac surgery as outlined above.
NOAC use in patients with AF after bioprosthetic valve implantation or valve repair
Traditionally, VKA have been the anticoagulants of choice during the first 1–3 months after bioprosthetic valve implantation or valve repair in patients with AF.235 As discussed in ‘NOAC eligibility and dosing' section, NOACs appear as a valid option after this period given data from the pivotal phase III studies as well as the dedicated RIVER trial.12,17,19,20,24 Results of the latter imply that patients may be treated with a NOAC even earlier after biological valve replacement, but the number of patients randomized <3 months post-operative was small (n = 95, on rivaroxaban). Further confirmatory data, also with other NOACs, are needed.
Practical aspects on the use of NOACs after TAVI implantation are covered in the ‘NOAC eligibility and dosing' section (see also Table 1).
NOACs after coronary artery bypass grafting
In patients without AF, dual antiplatelet therapy (DAPT) is frequently administered to patients following coronary artery bypass grafting (CABG), as it has been associated with improved vein graft patency and reduced mortality (although the level of evidence especially for the latter is weak).240–242 In patients with concomitant AF, the combination of a single antiplatelet agent (aspiring or clopidogrel) with a NOAC appears reasonable but—in contrast to patients after percutaneous coronary intervention (PCI)/acute coronary syndrome (ACS) (see ‘Patients with atrial fibrillation and coronary artery disease' section)—randomized trial evidence is not available. The combination of DAPT with a NOAC seems undesirable due to its inherent bleeding risk, but again, no prospective evidence is available. The timing of post-operative initiation of NOAC therapy follows the same principles as indicated above. One year post-CABG, NOACs may be continued as monotherapy, similar to other patients with chronic coronary syndrome (CCS).243
NOACs after surgical AF treatment ± LAA occlusion/exclusion
According to the 2020 ESC AF guidelines (developed in collaboration with EACTS), long-term OAC therapy is recommended in patients after AF surgery and appendage closure based on the patient’s thromboembolic risk as assessed by the CHA2DS2-VASc score and not on the ‘success’ of the procedure (no RCT data).1 Post-operative initiation of NOAC therapy follows the general principles after cardiac surgery as outlined above.
Patients with atrial fibrillation and coronary artery disease
The combination of AF and CAD is not only a common clinical scenario, it is also a complex setting to combine anticoagulation and antiplatelet therapy. According to the 2020 ESC guidelines AF patients with relevant CAD have at least a CHA2DS2-VASc score of 1 (and mostly higher due to the presence of other cardiovascular risk factors) and hence an indication for OAC. The convention is that a period of DAPT (i.e. aspirin and a P2Y12 inhibitor) is necessary to prevent stent thrombosis or recurrent events after an ACS and/or stenting for CAD—but that this is not sufficient for stroke prevention. Conversely, NOACs are essential for stroke prevention but on their own insufficient for preventing new coronary events in the immediate phase after ACS or stenting. The choice of antithrombotic drug combinations therefore represents a clinical conundrum: too little and risk a coronary event and/or stroke, too much and risk a bleeding event.
Triple vs. dual therapy
NOACs vs. VKA in dual vs. triple therapy
Four dedicated prospective RCTs have addressed the issue of using a NOAC or VKA in a variety of combinations with antiplatelet agents to reduce bleeding events after PCI and/or an ACS in patients with AF.244–247 In essence, these trials focused on bleeding as the primary endpoint, with coronary events and stroke as important secondary outcomes. On aggregate, these studies showed that dual therapy with a NOAC plus a P2Y12 inhibitor reduced the risk of bleeding compared to triple therapy with VKA, aspirin and a P2Y12 inhibitor (mostly clopidogrel). This bleeding risk reduction appeared to be driven by both receiving a NOAC instead of VKA as well as by omitting aspirin,244 and this benefit was also observed in medically managed ACS/PCI patients with AF.244,248
NOAC-based dual therapy also seems to be safe in terms of coronary ischaemic risk although the evidence is less strong as such events were relatively rare in all four studies which (as a result) were underpowered for thrombotic events analyses.244–247 While a recent network meta-analysis indicated that, on aggregate, a NOAC plus a P2Y12 inhibitor reduces bleeding risk without significantly increasing coronary thrombotic risk compared to any other regimen that includes DAPT,249 several other meta-analyses including the four NOAC RCTs indicate that there might be a small but statistically significant increase in the risk of coronary (but not stroke) events when omitting aspirin.250–253
Duration of triple therapy after ACS/PCI
According to the current 2020 ESC guidelines for AF as well as for non-ST-elevation acute coronary syndrome (NSTE-ACS), a short course of triple therapy is recommended for up to 1 week in all patients with AF undergoing PCI.1,254 In medically managed NST-ACS patients, combination of a NOAC with only a single antiplatelet agent (preferably clopidogrel) is recommended from the event onwards.254 However, the time frame of inclusion for the four aforementioned NOAC RCTs ranged from several hours after PCI up to >10 days. As such, a selection bias towards lower-risk patients cannot be excluded; furthermore, a variable course of triple therapy may have been given to a substantial number of patients subsequently randomized to NOAC-based dual therapy. Finally, although bleeding events were consistently reduced across the four NOAC trials by NOAC-based dual therapy this did not translate into a reduction in all-cause mortality (as compared to VKA-based triple therapy). Therefore, a low threshold for prolonging triple therapy with DAPT and a NOAC up to 30 days may be advisable in patients with a high atherothrombotic risk, including those after a complex PCI or with a history of stent thrombosis. In contrast, continuation of triple therapy beyond 30 days rarely seems warranted.255
The choice of anticoagulant as well as the duration of triple (and dual) therapy hence needs to be personalized based on atherothrombotic-, cardioembolic-, and bleeding risk.75 It is highly recommended to formally assess stroke and cardiac ischaemic event risk using validated tools such as the CHA2DS2-VASc and Global Registry of Acute Coronary Events (GRACE) scores.1,75 Estimating the bleeding risk should lead to efforts to correct or reduce reversible bleeding risk factors. Proton pump inhibitors should be encouraged in all patients with a combination of antiplatelets and anticoagulants.
NOAC dosing in the context of dual/triple therapy
It is unknown whether rivaroxaban 15 mg QD (dose reduced to 10 mg QD in patients with moderately reduced renal function) as used in the ‘Open-label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo Percutaneous Coronary Intervention’ (PIONEER) trial is sufficient for stroke prevention in patients with ACS and/or undergoing PCI as the trial (like the other three NOAC trials) was underpowered for individual efficacy outcomes.246 In contrast, approved stroke-preventive doses of NOACs were tested for apixaban (5 mg BID), dabigatran (110/150 mg BID), and edoxaban (60 mg QD) in the respective dual vs. triple therapy trials; in all three trials doses were reduced according to the respective standard criteria.244,245,247 NOAC dosing therefore should follow the general published and approved criteria with dose reduction be performed according to the individual NOAC’s dose reduction criteria.1
Adding a very low dose of rivaroxaban (2.5 mg BID) decreased ischaemic events including stent thrombosis as compared to DAPT alone in ACS patients without AF (albeit with an increase in bleeding).115 The same dose was used in the NOAC ‘triple’ therapy arm in the PIONEER study246; its protective effect against AF-related stroke, however, remains undetermined making this strategy unsuitable for AF patients after an ACS/PCI.
Choice of P2Y12 inhibitor
In the 2020 ESC AF guidelines, the use of ticagrelor or prasugrel as part of a triple therapy regimen is discouraged.1 Ticagrelor increases bleeding risk in patients on dual therapy when compared to clopidogrel.256 Although only few patients have been included with a P2Y12-inhibitor other than clopidogrel into the above-mentioned RCTs, the benefit in terms of reduced bleeding risk with NOAC-based dual therapy compared to VKA-based triple therapy appears to be maintained regardless of the type of P2Y12 inhibitor.256 In post-ACS patients at high coronary thrombotic risk and low bleeding risk in whom otherwise a VKA- or NOAC-based triple therapy would be warranted, dual therapy with a NOAC plus ticagrelor could be considered instead. Further data, including dedicated RCTs, are warranted in this area. Indeed, up to 40% of patients on clopidogrel may reach insufficient platelet inhibition.257 It is unknown whether measuring the antiplatelet response to clopidogrel when considering omitting aspirin, and adapting the strategy (e.g. switching to ticagrelor or re-introducing aspirin) will result in a net benefit in this setting.
Treatment of patients with chronic coronary syndrome
Until recently, there were only indirect data from the pivotal phase 3 NOAC trials as well as some observational data on whether it might be safe to transition to NOAC monotherapy in patients with CCS.258 The Japanese multi-centre, open-label 'Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease' (AFIRE) trial demonstrated that continuing rivaroxaban 15 mg QD monotherapy beyond 1 year after a revascularization procedure in AF patients not only decreased the risk of ISTH bleeding (primary safety outcome) but also demonstrated non-inferiority for the primary composite endpoint of cardiovascular events (stroke, systemic embolism, MI, unstable angina requiring revascularization) or death from any cause compared with the combination of rivaroxaban and antiplatelet therapy.259 Indeed, the trial was stopped prematurely due to an increased mortality in the combination therapy arm.259 Although it is formally unclear if these results translate to other NOACs, other doses, and other populations, these data suggest that most AF patients with chronic CAD should be transitioned to NOAC monotherapy without an antiplatelet agent as recommended in current ESC AF guidelines (Figure 17).1
Figure 17.
Anticoagulation therapy after elective PCI or ACS in patients with AF. ‘Shorten/de-intensify’: e.g. discontinuing Aspirin or P2Y12 inhibitor at an earlier stage. ‘Lengthen/intensify’: e.g. continuing triple combinations longer, or continuing P2Y12 inhibitor longer. A: aspirin 75–100 mg QD; C: clopidogrel 75 mg QD; Tica: Ticagrelor 90 mg BID. *If triple therapy needs to be continued after discharge clopidogrel is preferred over ticagrelor (due to lack of data). ACS, acute coronary syndrome; AF, atrial fibrillation; BID, twice daily; BMS, bare metal stent; DES, drug-eluting stent; LAD, left anterior descending artery; MI, myocardial infarction; NOAC, non-vitamin K antagonist oral anticoagulant; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; QD, once daily.
Creation of local standard operating procedures is strongly advised for the management of patients with AF and ACS or CCS, based on the available evidence and recent ESC AF- and Non-ST-Elevation Acute Coronary Syndrome (NSTE-ACS) Guidelines.1,254
Scenario 1: coronary interventions in atrial fibrillation patients on non-vitamin K antagonist oral anticoagulants
Performing a PCI (scheduled or not) under NOAC is different than under VKA for several reasons, and various aspects and uncertainties need to be taken into consideration, including:
timepoint of the last dose, adherence, and renal function;
variability in renal function in an acute setting;
singular factor II or Xa blockade vs. multifactor antagonism;
uncertainty about the extent of anticoagulation in the absence of established tests, and hence
uncertainty about stacking of additional periprocedural anticoagulants, etc.
Temporary discontinuation of the short-acting NOACs may allow for safe initiation of antiplatelet therapy and standard local anticoagulation practices peri-procedurally (Figure 18). In contrast, NOACs should be continued in non-invasively managed ACS patients.
Figure 18.
Acute management of elective PCI or ACS in AF patients treated with NOAC. ACS, acute coronary syndrome; ACT, activated clotting time; AF, atrial fibrillation; aPTT, activated prothrombin time; BMS, bare metal stent; CCS, chronic coronary syndrome; DES, drug-eluting stent; LMWH, low molecular weight heparin; NOAC, non-vitamin K antagonist oral anticoagulant; NSTE-ACS, non-ST-elevation acute coronary syndrome; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; UFH, unfractionated heparin.
New-generation drug-eluting stents are preferred to shorten exposure to dual or triple therapy after the procedure but also to avoid the need for repeat interventions. Sole balloon angioplasty or bypass surgery should always be considered as an alternative in patients in need for chronic anticoagulation since they can reduce the need for long-term dual or triple therapy. There is no longer a reason to opt for a bare metal stent as a strategy to reduce DAPT duration.260–262
The specific discussion of the possible scenarios (elective PCI, NSTE-ACS, ST-elevation myocardial infarction) is provided in the Supplementary material online and summarized in Figure 18.
Scenario 2: management of the patient with a recent acute coronary syndrome (<1 year) who develops new-onset atrial fibrillation
ACS guidelines recommended DAPT for up to 1 year after the acute event in patients without indication for OAC, and high-risk patients might require an even longer DAPT duration.263,264 In high bleeding-risk ACS patients, however, current ESC guidelines allow for shorter DAPT durations (3–6 months).75,76,265 If AF develops during the first year after an ACS and there is an indication for anticoagulation, a NOAC should be started and the need for continuing DAPT should be carefully weighed against the increased bleeding risk. Beyond 1 month after the event, aspirin can be stopped in the majority of such patients as discussed above.
Scenario 3: a chronic coronary syndrome patient (acute coronary syndrome ≥1 year ago) develops atrial fibrillation
Patients with a CCS developing AF should receive anticoagulation, depending on their CHA2DS2-VASc score (which per definition will be ≥1). A NOAC without any antiplatelet agent appears to be the preferred strategy for these patients as discussed above, based on the results of the four landmark NOAC trials (which included up to 15–20% of patients with a prior MI) and the ‘Atrial Fibrillation and Ischaemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease’ (AFIRE) trial.259 An additional antiplatelet agent should only be considered in individual patients with a very high ischaemic- and low bleeding risk.
Treatment of left ventricular thrombus after myocardial infarction in patients with atrial fibrillation
In the absence of randomized studies, it remains uncertain whether a NOAC is effective in the treatment of left ventricular thrombi complicating a large infarction. One observational study suggests that NOACs were associated with a higher incidence of thromboembolic events compared to VKA in (mostly non-AF) patients with a left ventricular thrombus, while others showed a similar rate of thrombus resolution.266–269 Although residual confounding can never be excluded in these settings, VKA should be viewed as standard of care for the treatment of patients with LV thrombus until more data are available. Only in very special situations (e.g. no VKA monitoring possible, no stable INR despite maximal efforts, etc.) NOACs may be evaluated after clear communication and consent from the patient about the lack of data and the off-label situation.
Cardioversion in a NOAC-treated patient
Based on current ESC guidelines,1 in patients with AF of >48 h (or unknown) duration undergoing electrical or pharmacological cardioversion, effective OAC needs to be established for at least 3 weeks prior to cardioversion or a pre-cardioversion transoesophageal echocardiography (TOE) needs to rule out left atrial thrombi, irrespective of CHA2DS2-VASc score.1,2,227 Different scenarios have to be distinguished: electrical cardioversion in a patient who is on chronic treatment with a NOAC and now requires cardioversion, and cardioversion in a patient not on anticoagulation (Figure 19).
Figure 19.
Cardioversion workflow in AF patients treated with NOAC, depending on the duration of the arrhythmia and prior anticoagulation. AF, atrial fibrillation; CV, cardiovascular; LA, left atrium; LAA, left atrial appendage; NOAC, non-vitamin K antagonist oral anticoagulant.
Considerations regarding the practical management of patients cardioverted after ≥3 weeks of NOAC treatment, as well as of patients with >48 h or ≤48 h AF without NOAC therapy are summarized in Figure 19 and in the Supplementary material online.
Duration of anticoagulation post-cardioversion
Oral anticoagulation post-cardioversion should be continued as per the recommendations provided in the ESC AF guidelines.1 The long-term management of patients post-cardioversion depends on the individual patient’s CHA2DS2-VASc score. Men and women with a CHA2DS2-VASc ≥2 and ≥3, respectively, have a Class I recommendation for long-term anticoagulation independent of the ‘success’ of cardioversion.1 This is also true for AF with a clear ‘trigger’ including pulmonary embolism, sepsis, or major surgery, since the trigger does not negate underlying structural or vascular factors associated with increased thromboembolic risk. For AF of ⪖48 h duration and a low CHA2DS2-VASc score (0 in men, 1 in women) anticoagulation needs to be continued for 4 weeks post-cardioversion.
In contrast, it is currently unknown how long (if at all) the latter patients should be anticoagulated if AF is of shorter duration (especially when <12-24 h). Indeed, these patients may in addition have shorter, self-limiting (i.e., ‘self-cardioverting’) episodes of AF for which the optimal anticoagulation strategy is currently unclear. Given the overall low risk of thromboembolism in these patients, longer and particularly life-long anticoagulation generally does not seem to be mandated.227 Current AF guidelines indicated the possibility to drop post-cardioversion anticoagulation in patients with a definite duration of AF ≤24 h and a very low stroke risk (CHA2DS2-VASc of 0 in men or 1 in women).1
Management of a patient with documented left atrial appendage thrombus
Patients in whom TOE identifies a left atrial thrombus should not undergo cardioversion. There are no (and likely never will be any) adequately powered prospective endpoint trials to investigate the best anticoagulation strategy (including NOAC vs. VKA) in this scenario. Previously, standard therapy consisted of VKA therapy (with heparin bridging if necessary) with rigorous follow-up and INR monitoring until resolution of the thrombus. One prospective study indicated a thrombus resolution rate of 41.5% (22 of 53 patients) with standard dose rivaroxaban (20 mg/d)270—comparable to a retrospective registry in which left atrial thrombus resolution was observed in 60 of 96 patients (62.5%) in heparin/warfarin treated patients.270 A small study also showed complete thrombus resolution with dabigatran 150 mg BID in 17 of 19 patients (89.5%) vs. 17 of 22 patients (77.3%) on warfarin.271 Another prospective study with dabigatran (NCT02256683) finished inclusion but study outcomes have not been reported yet. In the ‘Eliquis evaluated in acute cardioversion compared to usual treatments for anticoagulation in subjects with NVAF’ (EMANATE) trial, thrombus resolution rate was similar in patients treated with apixaban (52%, 12/23) as with LMWH/VKA (56%, 10/18).272 This is supported by observational evidence indicating a similar degree of thrombus resolution using a NOAC vs. a LMWH/VKA based regimen.227,273–275 Together, these data indicate that using NOACs for left atrial thrombus resolution may be an option (most data available for apixaban and rivaroxaban), particularly in patients where a VKA is not well tolerated or adequate INR control cannot be obtained.
If a thrombus persists during follow-up despite confirmed good adherence to the NOAC regimen an individualized management strategy is required. This may include switching to a different type of NOAC (direct thrombin inhibitor to FXa-inhibitor or vice versa) or INR-tailored VKA-therapy. Some centres have reported LAA closure in patients with a persistent thrombus.276 Finally, long-standing thrombi may become organized and fixed, allowing cardioversion if regaining sinus rhythm is considered to be of substantial benefit for the patient outweighing any residual thromboembolic risks. All of the aforementioned strategies are lacking strong evidence and further studies are clearly required in this field.
AF patients presenting with acute stroke while on NOACs
The incidence of ischaemic stroke is 1–2% per year in AF patients treated with a NOAC. Stroke may occur despite good adherence to drug treatment but NOAC plasma concentration may correlate both with stroke severity (as is the case with INR in patients on VKA) and large vessel occlusion.277 Case series and observational studies reveal an adequate NOAC dose at ischaemic stroke-onset is associated with milder severity and more favourable outcome compared to non-anticoagulated stroke patients with AF.278,279
Intracerebral bleeding (ICB) accounts for 8–15% of stroke in Europe and the USA. 15–25% of all ICBs are related to OAC.280,281 RCTs indicate an ICB incidence of 0.13–0.37% per year in AF patients on NOAC treatment, while the incidence of intracranial haemorrhage (ICH; also including subarachnoid, epidural and subdural haemorrhage) is 0.23–0.55% per year.47,170,282–284 A retrospective analysis of the USA ‘Get With the Guidelines-Stroke’ and a national Japanese database found a more favourable outcome with NOACs compared to VKA, contrasting previous studies reporting similar outcomes and a mortality rate of 25–40% after NOAC-related ICB.285,286 All stroke patients on NOAC treatment require immediate neurologist/stroke physician input to decide on the best therapeutic approach.
Management of NOAC treated AF patients in the acute phase of stroke
The management of AF patients on NOACs in the acute phase of ischaemic stroke is summarized in Figure 20 as well as in the Supplementary material online. The management of AF patients on NOACs in the acute phase of an intracranial bleeding is discussed in the Supplementary material online.
Figure 20.
Acute management of acute ischaemic stroke with relevant neurological deficit in a patient on NOAC. aSystemic thrombolysis only indicated if there are no (other) contra-indications for intravenous application of rt-PA according to its label. bEndovascular thrombectomy only indicated if there is a target vessel occlusion and procedure is indicated and feasible according to present evidence. cAccording to expert consensus.497 NOAC, non-vitamin K antagonist oral anticoagulant.
Management in the post-acute phase of stroke patients with AF
AF patients post-ischaemic stroke or transient ischaemic attack
Alternative (and treatable) causes of stroke have to be assessed in every AF patient.279,287 No RCT evidence exists favouring one NOAC over another or to switch one NOAC to another in patients with transient ischaemic attack (TIA) or ischaemic stroke on NOAC therapy. Treatment needs to be individualized with appropriate dosing and assessment of patient specific co-morbidities and co-medication (see ‘NOAC eligibility and dosing' section). Measurement of NOAC plasma levels at the time of hospital admission may help assess adherence at least at the time of stroke.
Since stroke-related disruption of the blood–brain barrier increases the risk of secondary haemorrhagic transformation, timing of (re-)starting OAC must balance the risk of recurrent ischaemic stroke vs. risk of parenchymal bleeding. Data from large RCTs are missing, as phase III trials of NOACs excluded patients within 7–30 days after stroke and within 3–6 months after severe stroke.280 As RCTs are ongoing, current recommendations are based on consensus opinion,11,288 observational studies,289–291 and an individual patient data analysis of prospective cohort studies.292 The 2020 ESC guidelines on the management of AF state that OAC ‘should be (re-)initiated as soon as considered possible from the neurological perspective (in most cases within the first 2 weeks)’.1 The 2019 AHA/ASA guidelines conclude that ‘for most patients with an [acute ischaemic stroke] in the setting of AF, it is reasonable to initiate OAC between 4 and 14 days after the onset of neurological symptoms’.288 A recent European Stroke Organisation (ESO) expert consensus concluded that ‘recommendations about the optimal time for initiating anticoagulation in patients with AIS’ could not be made.280
At present, several randomized trials [e.g. ELAN (NCT03148457), OPTIMAS (NCT03759938), TIMING (NCT02961348), START (NCT03021928), AREST (NCT02283294)] focusing on early vs. late (re-)starting of a NOAC after acute ischaemic stroke are underway with results expected in 2021/22.290 In the interim practical guidance is required for this common clinical dilemma. As first specified in the 2015 EHRA Practical Guide, OAC using a NOAC may be continued (according to prescription and label) or started the next day in TIA patients after exclusion of ICB/secondary haemorrhagic transformation by imaging, and considering the size of imaging-documented acute ischaemic brain lesion.9,11 If infarct size is not expected to substantially increase the risk of haemorrhagic transformation in patients with mild stroke, OAC may be initiated ≥3 days after AIS (Figure 21). In patients with moderate stroke, anticoagulation may be started ≥6–8 days and in patients with severe stroke at ≥12–14 days, after excluding secondary haemorrhagic transformation by repeating brain imaging [using computed tomography (CT) or magnetic resonance imaging (MRI)]. As indicated before, these time frames and actions represent expert opinion-driven practical advice until more evidence becomes available. A multidisciplinary team approach appears mandatory in these challenging situations.
Figure 21.
(Re-) initiation of anticoagulation after TIA/stroke. Without proven evidence/RCT data available, based on expert opinion. Consider inclusion of patient in an ongoing trial. (Re-)start only in the absence of contraindications and if stroke size is not expected to substantially increase the risk of secondary haemorrhagic transformation. Consider shorter delays to (re-)start a NOAC in case of a very high risk of stroke recurrence [e.g. LA(A) thrombus] and no haemorrhagic transformation on follow-up brain imaging (using CT or MRI). CT, computed tomography; LA, left atrium; LAA, left atrial appendage; MRI, magnetic resonance imaging; NOAC, non-vitamin K antagonist oral anticoagulant; RCT, randomized clinical trial; TIA, transient ischaemic attack.
A patient-centred decision to (re-)start OAC should also consider if left atrial (appendage) thrombus is present or if there is evidence of cerebral amyloid angiopathy. However, although MRI-detected cerebral microbleeds (CMB) are independently associated with increased risk of symptomatic ICH, they are also associated with risk of recurrent AIS, and the burden of CMB related to ICB remains to be defined.280,292–294 Presence of CMB alone should not per se dictate the decision against anticoagulation.
Due to the rapid onset of action of NOACs as well as an associated risk of bleeding, ‘bridging’ with heparin before (re-)starting a NOAC or treatment with LMWH as an anticoagulant is not recommended.280 If initiation of OAC is delayed in patients with acute ischaemic stroke, aspirin should be administered before initiation according to expert opinion.280 In case of OAC intake peri-onset of stroke, treatment with aspirin should be postponed according to the NOAC half-life and kidney function or should be based on the results of (specific) coagulation tests. Antiplatelets used for secondary stroke prevention in AF patient after AIS should be stopped at the time of (re-) starting a NOAC unless a clear indication exists for concomitant use (e.g. recent coronary- or carotid stenting).
NOAC use at hospital discharge in AF stroke patients was associated with more days spent at home and a lower rate of major adverse cardiovascular events compared to VKA according to a large multicentre cohort study including stroke survivors.295 Of note, appropriate dosing of NOACs and patient adherence is essential to ensure optimal secondary stroke prevention.62,278,295
AF patients with ischaemic stroke and concomitant atherosclerosis
Addition of antiplatelets to a NOAC for a specified period may be necessary or considered in selected AIS patients with AF, if stroke is most probably caused by large-vessel disease [i.e. ‘symptomatic’ (intracranial) stenosis], or the patient has recently undergone a stenting procedure, and bleeding risk is considered to be low. However, evidence for this approach is lacking and further studies are required.296 AF patients with acute ischaemic stroke due to ‘symptomatic’ high-grade carotid stenosis should preferably undergo carotid endarterectomy (CEA), as carotid stenting necessitates (dual) antiplatelet therapy in addition to OAC with a subsequently higher risk of bleeding.296 In AF patients undergoing CEA, aspirin is recommended prior to and for some days after surgery but usually should be stopped on resuming NOAC therapy. AF patients with asymptomatic atherosclerosis or stenosis of the internal carotid and/or intracranial arteries should be treated with a statin and OAC, without the need for additional antiplatelet therapy, similar to the situation in stable coronary artery disease (see ‘Patients with atrial fibrillation and coronary artery disease' section).
AF patients post-intracranial haemorrhage
AF patients post-intracerebral bleeding
In addition to its immediate prognosis, ICB in the setting of AF is also associated with later ischaemic stroke and mortality, partly due to the cessation of anticoagulation after ICB. However, a history of a spontaneous ICB constitutes a contraindication for anticoagulation according to labelling of VKAs and NOACs, unless the cause of the bleeding (like uncontrolled hypertension, aneurysm or arteriovenous malformation, or medical ‘triple’ therapy) has been reversed.
Evidence-based guidelines regarding use of NOACs in AF patients post-ICB are not available but several RCTs are ongoing [PRESTIGE-AF (NCT03996772); APACHE-AF (NCT02565693); NASPAF-ICH (NCT02998905); ASPIRE (NCT03907046); SoSTART (NCT03153150); A3ICH (NCT03243175); ENRICH-AF (NCT03950076)]. Present knowledge is based on observational (mostly retrospective) data with varying proportions of ICB-patients with AF re-starting OAC, predominantly or exclusively with VKA.1,280,297–299 Observational studies including AF patients with a history of ICB showed that restarting OAC with a NOAC vs. VKA was associated with similar to lower rates of ischaemic stroke without difference (or even lower) rates of recurrent ICB.300,301 However, publication and selection bias as well as residual confounding must be taken into account as with all observational non-randomized studies.297 The ESO Karolinska Stroke Update Conference consensus paper states that in selected ICB patients (re-)initiation of OAC compared to no OAC may improve outcomes (Grade C), and that ‘NOACs should preferentially be used over VKA’ (Grade C).293 A recent ESO guideline concludes that ‘restarting oral anticoagulation can be considered after careful weighing of risks and benefits’.280
Therefore, as stated in the 2020 ESC AF guidelines, a case-by-case consideration is needed whether or not to (re-)introduce anticoagulation of any type in patients who have experienced an OAC-related ICB (Figure 22).1 Adequate blood pressure control is of paramount importance in all patients post ICB. Whether genetic polymorphisms, like the apolipoprotein E genotype, or low-density lipoprotein cholesterol levels predict the likelihood of recurrent ICB has to be proven by prospective trials.302–304 Patients with cerebral amyloid angiopathy have a very high risk of recurrent ICB and should not be anticoagulated.305
Figure 22.
(Re-) initiation of anticoagulation post intracranial bleeding. aWithout RCT evidence; ideally include patient in an ongoing trial. bBrain imaging mandatory before (re-)initiation of (N)OAC. NOAC, non-vitamin K antagonist oral anticoagulant; PCI, percutaneous coronary intervention.
Analogous to the management of VKA-related ICB, NOACs may be re-started 4–8 weeks after ICB, if the individual risk of cardio-embolic stroke is high and the risk of recurrent ICB is estimated to be lower.281,297,306
LAA occlusion is a potential alternative strategy to long-term anticoagulation in AF patients post ICB after careful weighing of risks and benefits, as outlined in the 2020 ESC AF guidelines and ESO recommendations.1,280,293 However, this strategy requires a period of antiplatelet or anticoagulant treatment post-deployment, which also carries a risk of recurrent ICB. The safety and effectiveness of shorter duration antiplatelet therapy is unknown. RCT evidence for LAA occlusion after OAC-related ICB is lacking as the number of AF patients with previous ICB in most randomized studies is not reported.307 Patients with AF after ICB in whom LAA occlusion is being considered should ideally be included into an ongoing RCT such as ‘Left Atrial Appendage CLOSURE in Patients With Atrial Fibrillation at High Risk of Stroke and Bleeding Compared to Medical Therapy: a Prospective Randomized Clinical Trial’ (CLOSURE-AF, NCT03463317), ‘Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage’ (STROKECLOSE, NCT02830152), or ‘Comparison of LAA-Closure vs. Oral Anticoagulation in Patients With NVAF and Status Post Intracranial Bleeding’ (CLEARANCE, NCT04298723).
AF patients post-subarachnoid haemorrhage
Incidence of subarachnoid haemorrhage (SAH) was <0.1% per year in AF patients on NOAC treatment in RCTs.170,282,283 There is little evidence to guide the resumption of OAC treatment in patients with AF following SAH.308 Thorough angiographic evaluation, treatment of any underlying aneurysm or arteriovenous malformation and multidisciplinary team (neurological/neurosurgical/neuro-radiological) evaluation of future risk of re-bleeding is needed prior to any consideration to restart OAC in the AF patient after a SAH. When SAH occurs in AF patients taking a NOAC in the absence of a remediable aetiology it seems prudent not to re-initiate OAC treatment. LAA closure may be considered (no RCT data available), ideally in the framework of a randomized trial.
AF patients post-epidural haematoma or subdural haematoma
In RCTs, incidence of subdural and epidural haematoma in AF patients on NOAC treatment was <0.2% and <0.1% per year, respectively.170,282,283 Although there are no specific data, it appears to be safe to start or reinitiate OAC about 4 weeks after (surgical removal of) traumatic epidural or subdural haematoma (SDH), particularly in the absence of drug-/alcohol abuse or a substantial risk of falling (see ‘NOACs in advanced age and frailty' section).308 According to clinical presentation and haematoma extension, brain imaging (using CT or MRI) is recommended before (re-)starting OAC. However, adequately dosed NOAC or no anticoagulation at the time of non-traumatic epidural or SDH does not support (re-) initiation of OAC despite the fact that the risk of ischaemic stroke is increased within 4 weeks after non-traumatic SDH according to a retrospective US cohort study.309
NOACs in advanced age and frailty
NOACs in older populations
The incidence of AF rises steadily with age; by 2050, 4.4% of the world population will be older than 80 years.310,311 Stroke prevention in older AF patients is of great importance as stroke risk rises greatly with age.312 The advent of NOACs has improved prescription rates in older people, but OAC remains underutilized in up to 30% of patients with high stroke risk.313,314
All trials of NOAC treatment in AF included significant populations of older people (defined as ≥75 years) ranging from 31% to 43% in the individual trials, comprising over 27 000 older patients in whom NOACs were studied. Rates of stroke were similarly reduced in older age groups treated with NOAC compared to VKA. Importantly, the higher absolute risk resulted in a larger absolute risk reduction by using NOACs instead of VKA in these older patients, resulting in a lower number needed to treat compared to younger patients.69,315–317 While intracranial bleeding remains lower with all NOACs compared to VKA, a significant effect of age on increased extracranial major bleeding was observed on the higher dose of dabigatran.170,318 Conversely no age interaction on rates of extracranial major bleeding was seen with apixaban, edoxaban or rivaroxaban compared to the overall trial results. In addition major bleeding appeared lower with apixaban and edoxaban compared to VKA even in older age groups.47,69,316 Observational registries in older cohorts indicate that the risk of bleeding with age appears largely consistent with trial findings to date.318–322
Older patients with AF have more favourable outcomes on OAC than without, and on NOACs than on VKA.56,323–326 Therefore, NOACs are preferred in this cohort, consistent with current ESC guidelines.1,327,328 The net clinical benefit for OAC declines with advanced age due to competing risks for bleeding and death but is maintained longer with NOACs than VKA.329 While frailty and cognitive impairment syndromes are associated with greater mortality and underuse of OAC, the benefits of OAC are maintained in these cohorts.330 Better predictive tools may help identify those least likely to benefit due to early mortality,331 but robust evidence for reliably identifying individuals which should a priori not receive OAC are currently missing.
The ELDERCARE-AF trial represents the only placebo-controlled trial investigating a NOAC (very low-dose edoxaban, 15 mg QD) in elderly AF patients deemed unsuitable for standard OAC therapy. In this trial (conducted in Japan and confined to Japanese patients) the use of Edoxaban 15 mg QD resulted in a 4.4%/year absolute risk reduction in stroke (P < 0.001) at the cost of a non-significant absolute increase in 1.5%/year of major bleeding.102,332 It is currently unclear whether these findings translate to non-Japanese populations. If confirmed in other ethnicities, such a strategy could constitute an alternative in older patients deemed unsuitable for or higher risk with approved, full dose NOAC therapy. It would be desirable that such confirmatory evidence is sought as very old age remains a clinical conundrum. As discussed above, use of the lower-dose (30 mg/15 mg) vs. higher-dose edoxaban regimen (60 mg/30 mg) in the ENGAGE AF-TIMI 48 trial resulted in a 43% higher ischaemic stroke risk, while the risk of disabling or fatal strokes was similar between the two dosing regimens and the risk of major bleeding or of having a pre-defined primary net outcome event (stroke, systemic embolism, major bleeding, or death) was lower with the lower-dose edoxaban regimen. These results were consistent (and possibly even more pronounced for the primary net outcome; P interaction = 0.077) in patients ≥ 75 years vs. <75 years.100
In older patients the incidence of cerebral amyloid angiopathy and CMBs are more prevalent and their presence increases the risk of intracerebral haemorrhage (see ‘AF patients presenting with acute stroke while on NOACs' section).333 CMBs are markers of cerebral small vessel disease and can be identified in hemosiderin sensitive brain MRI sequences. An MRI may be helpful in assessing the risk of intracranial bleeding in older people especially with previous history of ICH.334,335 Although the prevalence of CMBs is similar, a significantly higher burden of CMBs in VKA-treated patients compared to NOAC exposure has been reported.336 As indicated in the 2020 ESC AF guidelines, anticoagulation should not be withheld purely based on the presence of CMBs.1
Frailty and falls
Frailty
Frailty is commonly defined as a rules-based distinct phenotype and by clinical judgement of function-deficits in a frailty scale (Table 13).337–339 Both models identify patients at risk of or with established poor physiological reserve, high risk of falls, depression and dementia, poor physical functioning and increased mortality. Frailty and pre-frail states are common with advancing age and raise specific considerations regarding the risk-benefit of OAC. Expert consensus advocates comprehensive geriatric assessment in all older patients with frailty.340 Frailty is associated with weight loss and a risk for deterioration in renal function. As a result, patients need to be weighed and their renal function monitored regularly (see ‘NOACs in patients with chronic kidney disease or advanced liver disease' section) to ensure safe NOAC dosing. There may be no benefit to OAC in states of severe frailty or where life expectancy is likely to be limited (Table 13).
Table 13.
NOAC use in frail patients

|
The ‘Canadian Study of Health and Aging’ (CHSA) Clinical Frailty Scale, based on comprehensive geriatric assessment including structured interview (http://www.csha.ca and Ref.338).
The decision to anticoagulate frail patients depends on multiple aspects (see text for details). While fit or mild frailty per se generally does not pose a problem (green), severe frailty and terminal illness typically indicate a contraindication to anticoagulation (red).
Risk of falling
The risk of falling can be estimated using simple or more sophisticated tools (Table 14). Older patients are more likely to fall. The annual prevalence of all-cause falls and non-accidental falls in community dwelling individuals >75 years of age may be as high as 25% and 8% respectively.341 The rate of falls increases with polypharmacy and institutional care.342 Falls have often been considered a contraindication to OAC due to risk of ICH.343 A Markov decision analytic model published in 1999 demonstrated a patient would have to fall 295 times in order for the risk of a SDH to outweigh the benefit of anticoagulation with VKA.344 These overview calculations come with relevant limitations and it is uncertain if they translate into the current day situation. Nevertheless, given the even lower risk of intracranial bleeding compared with VKA, the ‘number needed to fall’ would be even higher with the use of NOACs.
Table 14.
Examples of falls risk assessment
| (A) High risk of fallsa | |
| Presence of one or more of | |
|
|
| (B) Probability of falls assessmentb | |
| 1 point for each ‘yes’ | |
| Previous falls | Yes/no |
| Medications | |
| >4 | Yes/no |
| Psychotropics | Yes/no |
| Low visual acuity | Yes/no |
| Diminished sensation | Yes/no |
| Near tandem stand 10 s | Yes/no |
| Alternate step test 10 s | Yes/no |
| Sit to stand 12 s | Yes/no |
| Score | 0–1 | 2–3 | 4–5 | 6+ |
| Probability of fall per year | 7% | 13% | 27% | 49% |
The issue of falls in NOAC-treated patients was specifically analysed in subanalyses of two phase III trials. In the ENGAGE-AF TIMI 48 trial patients were prospectively classified as ‘high-‘ or ‘low falls risk’ by the presence of known risk factors and co-morbidities.70 Patients at increased risk of falling were more likely to experience a bone fracture, major bleeding or life threatening bleeding, and death. Edoxaban was associated with reduced risk of severe bleeding, intracranial haemorrhage and the most severe net clinical benefit outcomes (secondary and tertiary net clinical outcome) compared to VKA in both patient categories, and the absolute risk reduction was greater with edoxaban in patients at increased risk of falling.70
In the ARISTOTLE trial patients with a history of falling were older and more likely to have dementia and cerebrovascular disease. These individuals had an increased risk of major bleeding and intracranial bleeding as well as death, but the safety and efficacy of apixaban over warfarin was not affected by falling status.345 Among patients with a history of falls no subdural bleeding was recorded on apixaban.
This is also reflected in observational data indicating better outcomes on NOACs vs. VKA in patients at risk of falling.346–348 Caution is prudent, however, as more delayed intracranial haemorrhage in patients with a fall on NOACs has also been reported.349
In summary, falling per se is not a contraindication to NOAC use (Table 14), but precautions should be taken and modifiable bleeding risk factors assessed (including, importantly, co-use of antiplatelet agents; see ‘Practical considerations for initiation and follow-up' section). In addition, referral to a specialized falls assessment and intervention service should be offered to all patients to reduce risk of further falls.350
Cognitive impairment and dementia
Mild cognitive impairment as well as dementia (cognitive impairment severe enough to compromise social and/or occupational functioning) is common in older age groups.351,352 AF itself is a risk factor for dementia and conversely, encouraging evidence indicates that OAC use may be associated with a reduced risk of dementia.353–357 This risk reduction may be similar with VKA and NOAC; however, low time in therapeutic range has been associated with dementia in VKA-treated patients.357–359
Stroke as well as intracerebral haemorrhage are significant events for patients with dementia with a greater risk of cognitive and functional decline, loss of independence and institutionalization compared to non-dementia patients.360,361 AF in patients with dementia therefore requires similarly rigorous assessment for stroke prevention.
Dementia does pose unique considerations of adherence and safety when considering OAC. All patients with dementia should have a careful assessment of their ability to understand and make a treatment decision regarding OAC in AF, with indicative risks of stroke and bleeding provided. Where capacity is lacking, it may be reasonable for the physician to recommend treatment on the basis of the ‘best medical interest’ principle. This should be documented and explanation given to both patient and next of kin/legal attorney with assent/consent sought as relevant.
Adherence to OAC intake is of crucial importance. Both dementia and twice daily dosing has been shown to affect adherence with NOACs362; as such, once daily medications, weekly tablet boxes, reminders or blister packing may be helpful (see ‘Practical considerations for initiation and follow-up' section). Paradoxically, the fact that others may be supervising medication with dementia patients may guarantee higher adherence.363 Telemedicine to enhance treatment adherence in dementia and other assistive technologies may be useful in this population.364 It is advisable to re-assess cognitive function in older AF patients on a regular basis particularly considering and assessing their ability to adhere to the prescribed anticoagulation regimen.
NOACs in high- and low body weights
Weight and body mass index (BMI) are important variables in drug distribution and plasma concentration levels. Concerns exist in the absence of readily available measurements of anticoagulant effect that NOACs may not be as effective or safe at extremes of weight with a potential for both over- and underdosing. Weight or BMI was not an exclusion factor in the randomized NOAC-trials in AF (or VTE), although dose reductions for lower body weight (≤60 kg) were mandated for both apixaban (if also age ≥80 years and/or creatinine ≥1.5 mg/dL), and edoxaban.46–49
NOACs in patients with high body weights
Effect of obesity on NOAC plasma levels
Since 1975, obesity has tripled and the WHO now considers it an epidemic. In 2016, 1.3 billion adults were overweight (BMI of greater than 25 kg/m2) of which 650 million were obese (BMI greater 30 kg/m2).365 Obesity increases both the risk of AF (possibly due to electro-modulation of the atrium) and risk of recurrent AF after successful ablation.366–369 Weight loss is an integral part of the multidisciplinary approach to prevention and treatment of patients with AF and obesity.370
Obesity affects the pharmacokinetics of drugs, including the volume of distribution (of lipophilic drugs in particular) as well as drug clearance.371 Renal blood flow and CrCl have been shown to be increased in obesity and could increase elimination of OACs.372 A number of studies of VKA have indicated that obese patients require greater doses and longer lead-in periods for achieving therapeutic INR values.373
Initial studies of dabigatran reported no effect of weight on pharmacokinetic variables although analyses in older healthy individuals did not include very obese patients.374–376 In the RE-LY trial, however, patients with a body weight >100 kg had 21% lower dose-normalized trough concentrations than patients with 50–100 kg body weight.97 The primary efficacy and safety outcomes were similar in patients with weight ≥100 kg vs. 50–99 kg vs. <50 kg (Ezekowitz et al., presented at ESC 2014).48,170
Pharmacokinetic data on both rivaroxaban and apixaban initially reported weight-dependent changes on volume distribution and half-life across a range of weights; however, these were felt unlikely to be clinically significant.377–380 In the ENGAGE AF-TIMI 48 trial, no changes in plasma concentrations of edoxaban or its pharmacodynamic effect on FXa were observed between obese and normal weight patients.381,382
Efficacy and safety of NOACs in obese patients
Concerns have been expressed about the reliability of the anticoagulant effect of NOACs in obese patients.383,384 In the RE-LY trial, no differences in the occurrence of stroke or systemic embolism were observed with dabigatran vs. warfarin in obese (≥100 kg) vs. non-obese patients.48,385 However, case reports of treatment 'failure' with low plasma levels of dabigatran have been reported in cases of severe obesity (BMI ≥ 40 kg/m2).386,387
Similarly, no differences were observed with apixaban vs. warfarin in obese patients (both as defined by BMI > 40 kg/m2 or 120 kg),388,389 rivaroxaban vs. warfarin (obesity defined as BMI ≥ 35 kg/m2),390 and edoxaban vs. warfarin (BMI > 40 kg/m2).381 However, only 620 patients from the ROCKET-AF trial had a very high BMI (≥40 kg/m2), and data from the RE-LY trial for dabigatran were not reported for this range.385,390 In contrast, 1003 and 1149 patients with a BMI ≥40 kg/m2 were included in ARISTOTLE and ENGAGE AF-TIMI 48, respectively.
No difference in the occurrence of major bleeds were observed for dabigatran vs. warfarin, rivaroxaban vs. warfarin and edoxaban vs. warfarin in obese vs. non-obese patients.381,385,390 Relatively more major bleeds were observed with apixaban vs. warfarin in patients with a BMI ≥30 kg/m2 vs. lower BMIs as well as >120 kg vs. <120 kg, although the incidence was still lower with apixaban vs. VKA even in obese patients.388,389
Several studies from daily clinical practice indicated no substantially higher incidence in endpoints in obese vs. non-obese patients on NOACs.391 A systematic review and meta-analysis of the impact of weight on efficacy and safety of NOACs compared to VKA found overall better efficacy across all body weights (low, normal, overweight, obese) with no increased bleeding noted in low or obese categories, although the analysis had no additional high quality data other than the original four pivotal trials.392 Two small retrospective comparative studies found similar efficacy and safety in the NOAC group compared to VKA in the extreme obesity cohort; most data were available for apixaban and rivaroxaban, one reported numerically higher numbers of TIA and stroke with dabigatran and neither study included data on edoxaban.393,394
Based on the pharmacokinetic properties and the available evidence the use of all NOACs appears to be safe and effective up to a BMI of 40 kg/m2 (barring other clinically relevant factors). At BMI ≥40 kg/m2 data are less robust.381,385,388–390
At a BMI ≥50 kg/m2 plasma level measurements with any of the NOACs (including the inherent associated limitations, see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section) or conversion to VKA therapy may be reasonable (Figure 23). Whether trough or peak plasma levels are preferable is a topic of further research; due to better reproducibility and correlation with clinical outcomes we generally advise for trough level measurement with peak level assessment only in selected cases.
Figure 23.
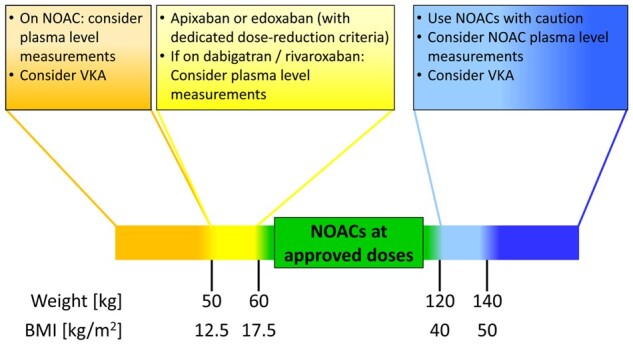
NOACs in under- and overweight patients. See text for details. BMI, body mass index; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; RCT, randomized clinical trial.
NOACs after gastric bypass surgery
Treatment of obesity with bariatric surgery may have important effects on drug levels due to effects of surgery on the site and surface area of absorption, pH, blood flow, intestinal transit time, as well as the effect of post-operative restrictive diets.395 The location of the (presumed) major absorption site varies by anticoagulant but is thought to occur mainly in the proximal small intestine and, to a lower extend, in the distal stomach.396,397 The nature of gastric bypass surgery is also relevant whereby a concomitant bypass of the proximal small intestine may result in delivery of drugs to more P-gp rich distal segments and reduce overall absorption.398 VKA weekly dose-requirements are variable post bariatric surgery with most reports describing an initial decrease but subsequent steady rise in the post-acute phase of surgery.399–401 While cases of warfarin resistance post gastric-bypass procedure have been described,402 even large GI resections usually do not have a major lasting effect on warfarin anticoagulation.395
Absorption of dabigatran may be affected (reduced) by higher pH and use of antacids (Table 4).403,404 While this is not considered relevant under normal circumstance it may play a role in patients after gastric bypass surgery. Bioavailability of rivaroxaban as used for stroke prevention in AF (20 mg, 15 mg) is increased by food, likely due to its lipophilicity and limited aqueous solubility, and administration of rivaroxaban distal to the stomach may lead to reduced absorption and rivaroxaban plasma levels.105,405 Hence, rivaroxaban (in the stroke prevention dose) may not be a preferred primary choice after gastric bypass surgery due to potentially relevant reductions in rivaroxaban exposure.398 One small study showed expected levels for dabigatran and apixaban but below-expected ranges for five of seven patients on rivaroxaban (including all four who had a gastric sleeve procedure).406 Edoxaban is highly and slightly soluble at acidic and neutral pH, respectively, and mainly absorbed in the proximal intestine. One study indicated that delivery directly to the distal intestine reduced both peak (Cmax) and total plasma levels (AUC).407
Ultimately, the choice of anticoagulant post-bariatric surgery is a case by case consideration as strong clinical evidence is lacking, particularly for NOACs. As VKA appear least affected by gastric bypass surgery and target INR ranges are well-established, reverting to a VKA may represent a valid alternative. If use of a NOACs is considered necessary assessment of plasma levels (trough as well as peak levels) seems advisable (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section). This should be performed in the setting of a multidisciplinary team and at a centre with ample experience; in addition, several physiologic parameters are volatile after gastric bypass surgery such that repeated measurements over time may be required.
NOACs in patients with low body weight
There is no universal definition of low body weight although a BMI <18.5 kg/m2 is considered by many western agencies as indicative of being underweight.408 Low body weight may increase exposure to any NOAC and as such increase the risk of bleeding compared to normal weight patients.409,410 Bleeding may also be increased with VKA therapy in underweight patients.410,411 Importantly, patients with low body weight frequently present with other conditions and co-morbidities which may increase the risk of stroke as well as bleeding, including old age, frailty, cancer, and CKD. Of note, renal function may be overestimated in underweight patients due to their reduced muscle mass (especially with the MDRD formula).
Special care is needed when anticoagulating low weight patients (Figure 23). Body weight ≤60 kg requires dose reduction of apixaban [in patients with age ≥80 years and/or serum Creatinine ≥133 µmol/(1.5 mg/dl)] as well as for edoxaban (see ‘NOAC eligibility and dosing' section, Table 2), whereas it is in itself not a factor for dose reduction of rivaroxaban or use of lower dose dabigatran.
Both apixaban and edoxaban showed consistent efficacy and safety compared to warfarin in underweight patients when compared with the overall study population.98,381,389 Drug concentrations and inhibition of Factor Xa did not differ in patients with low body weight (range 30–55 kg) from patients with middle body weight in an analysis from ENGAGE AF-TIMI 48.382 As such, both drugs may be a preferred choice for patients ≤60 kg.
Dabigatran was studied post hoc in patients with low body weight (<50 kg) with consistent efficacy compared with the remainder of the study cohort but a signal for increased bleeding events in patients with a lower BMI (particularly <20 kg/m2; Ezekowitz et al., presented at ESC 2014).48 Observational studies have equally suggested that low BMI may be an independent predictor of bleeding events with dabigatran and a trend to greater bleeding was noted with high dose dabigatran in a meta-analysis of low weight patients.392,412 Frequently co-existing CKD may also make it a less preferable option for underweight patients.
Rivaroxaban showed similar efficacy and safety in an exploratory analysis of the ROCKET-AF trial for lower body weight, but only patients ≤70 kg were compared with those >70 kg.46 No specific outcome data was available for patients with <60 kg or <50 kg in patients on the full AF dose of rivaroxaban. Subsequent meta-analyses and observational data are reassuring with regard to safety in low and severely underweight patients (<50 kg), but limitations (residual confounding in particular) persist.392,413
If therapy with a NOAC is warranted in low and very low weight individuals, measurement of trough levels may be considered to check for accumulation of the drug.414 However, no evidence-based recommendations can be given regarding (further) dose reduction in cases where trough levels are above the expected range (see ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section).
NOACs in other special populations
Special considerations for the use of NOACs in athletes and women of reproductive age are discussed in the Supplementary material online.
Epilepsy and NOACs
Scope of the problem
Epilepsy can have both genetic and acquired causes, the latter including brain trauma, stroke, tumours and brain infections. Epilepsy after a stroke is not an uncommon finding.415 Risk of seizures is reported between 7% and 11.5% overall post-stroke and in 3–6% of cardioembolic stroke.416–420 Incidence of recurrent unprovoked seizure post-stroke may be as high as 71% and prevention of such events using antiepileptic drugs (AEDs) is desirable especially when patients are on OAC.421–423 Many features of AF-associated stroke such as cortical involvement, cerebral artery territory, multiple infarcts, severe deficit and haemorrhagic transformation are also predictive of developing post-stroke epilepsy.424,425
OAC poses a special risk for patients with epilepsy. While most seizures in older people and post-stroke are focal in onset, patients who suffer seizures without aura or rare atonic seizures are particularly vulnerable to head trauma. Tongue biting is a risk in the tonic component of generalized seizures.
Potential drug–drug interactions
Many AEDs relevantly induce hepatic enzymes (e.g. ethosuximide carbamazepine, phenobarbital, phenytoin, primidone) or are mild inducers (e.g. oxcarbazepine, lamotrigine, tiagabine) thereby potentially reducing the efficacy of VKAs as well as certain NOACs (Table 7). Other AEDs inhibit hepatic metabolism (felbamate, topiramate, valproate, vigabatrin) and can increase the risk of bleeding with VKAs. Valproate may have unpredictable effects on CYP3A4.426 Conversely, animal and/or human studies have indicated that carbamazepine, levetiracetam, phenobarbital, phenytoin and valproic acid may decrease the effect of NOACs by inducing P-gp activity. Newer third generation AEDs such as brivaracetam, lacosamide and eslicarbazepine may have less potential for DDI.427 In addition, AEDs can have an indirect effect on the coagulation system, e.g. by causing thrombocytopenia or platelet dysfunction.428
Sporadic case reports exist about DDIs between NOACs and AEDs (Table 7).429,430 The majority of DDIs to date have cited reduced efficacy of NOACs due to these mechanisms.431 One series reported an increased bleeding risk with phenytoin.432 Another retrospective cohort of patients from Taiwan on NOACs and 11 different AEDs reported increased association of bleeding with concomitant prescription of phenytoin, valproic acid or levetiracetam but this may not be generalizable to other populations.433 After inquiry also with the drug manufacturer there is unfortunately no study which reliably investigated the effect of levetiracetam on NOAC plasma levels and clinical events in a sufficiently large ‘real world’ cohort of concomitantly treated patients. We strongly advise such studies should be conducted (not only with levetiracetam, but also with other AEDs) in order to better enable clinical decision-making in this difficult to treat patient population.
Practical advice
Robust evidence is lacking for DDI with NOACs and AEDs and there is poor concordance in international drug compendia on the subject.434 Where AED therapy is desirable in AF patients with epilepsy treated with a NOAC vigilance for potential DDI is warranted (see ‘Pharmacokinetics and drug–drug interactions of NOACs' section) and regular interdisciplinary review with the treating cardiologist, neurologist, primary care physician, and clinical pharmacist is crucial. Especially in the context of comedication with anti-seizure drugs, NOAC plasma level measurements are frequently proposed, similar to plasma-level guided dosing of anticonvulsants.435–438 However, as indicated and discussed in the ‘NOAC plasma level measurements: technical approach, indications, pitfalls' section—and in contrast to the situation with anti-epileptic drug level measurements—such an approach is without any endpoint-derived clinical trial evidence, especially with respect to dosing NOACs according to their measured levels.437,438 Therefore, such patients should be treated at expert centres with extensive experience in the measurements of NOAC plasma levels and their interpretation.
NOACs in Asians and other non-Caucasian ethnicities
In the past, ethnicity has been shown to be a factor in VKA underuse, poor INR control, and increased stroke- and death rates in non-White vs. White populations.439–442 Differences in body mass, genetic polymorphisms of the cytochrome P450 system affecting drug metabolism have been suggested as relevant factors for this difference impacting on efficacy and safety of stroke prevention in AF. Environmental factors around diet and lifestyle, socioeconomic and educational status are important confounders which are not always easy to separate from biological effects.443–445 Concerns are nonetheless frequently raised that the outcomes observed in the large NOAC trials might not be generalizable to all ethnicities encountered in daily clinical practice.
All four phase III trials of dabigatran, rivaroxaban, apixaban, and edoxaban in AF included a predominantly white population, i.e. 70%, 82.9%, 62.7%, and 76.5%, respectively. While the number of Asian patients who were enrolled was relatively large (16%, 12.7%, 14.5%, and 13.6% in RE-LY, ROCKET-AF, ARISTOTLE, and ENGAGE AF-TIMI 48, respectively) a relatively lower number of Hispanic (6.9%, not reported in ROCKET-AF, 19.8% and 12.4%, respectively) and a much lower percentage of Black patients (1%, 1.3%, 1.2%; not reported in ENGAGE AF-TIMI 48) was included.46–49
NOACs in Asians
Overall, Asians are a very diverse ethnic group. Asian patients are at an increased risk for both stroke and bleeding. Indeed, recent data suggests that the risk of stroke may rise from age 50–55 years upwards and that a modified CHA2DS2-VASc score may need to be used in Asian patients.1,446–448 In VKA users, efficacy for the prevention of ischaemic strokes was shown to be lower and the risk of intracerebral haemorrhage higher in Asian- vs. non-Asian patients,445,449,450 possibly linked to a lower TTR combined with more frequent non-cardioembolic stroke sources. Asian ethnicity may also have an impact on metabolism and clearance of NOACs, trough concentrations and anti-FXa activity due to lower body weight and increased rates of renal disease thereby potentially limiting the ability to simply extrapolate data from Caucasians.451,452
Across the four phase III NOAC trials >8600 Asian patients were included. As in previous studies, rates of intracranial haemorrhage as well as ischaemic stroke were higher in Asians as compared to non-Asians.452–455 The reduction in major (especially intracranial) bleeding was at least as pronounced if not greater with NOACs vs. VKA in Asians indicating a possibly even greater safety advantage as compared to non-Asian patients.450,452–455 In addition, and importantly, there were no signs for a reduced efficacy in the prevention of stroke and systemic embolism across the approved NOAC regimens. These findings were largely confirmed in observational registries.55,456,457
Taken together, these data indicate that NOACs may represent a preferred option for anticoagulation also in Asian patients,450,452 which may also extend to Asian patients with low body weight.413
Black, Hispanic, and other ethnicities
Black patients have been shown to have a lower incidence of AF but appear to be at higher risk of stroke.458–460 The rate of stroke in AF equally appears higher and outcomes may be worse in Hispanics vs. non-Hispanic patients.461,462 As such, also these patients would be of particular interest regarding their outcome on NOACs, yet (as indicated above) the number of Black and Hispanic patients included into the four landmark NOAC trials was relatively low.
Subanalyses for ethnicities showed
-
Dabigatran (RE-LY):
-
Rivaroxaban (ROCKET-AF):
-
Apixaban (ARISTOTLE):
-
Edoxaban (ENGAGE AF-TIMI 48):
In totality, these data hence indicate that NOACs should also be the preferred therapy for Black or Hispanic patients, particularly due to the oftentimes difficult and suboptimal alternative of VKA therapy (which may at least in part be due to confounding, as indicated above). However, and similar to all other settings (see ‘Practical considerations for initiation and follow-up' section), measures to improve care including an increase in the awareness of the disease and its consequences, optimal control of comorbidities (particularly blood pressure, diabetes, etc.), frequent medication review and careful assessment for dose reduction criteria are crucial to realize the advantages in daily clinical care. In addition, these findings also indicate the clear necessity for more high-quality data to better understand the efficacy and safety profile of NOACs in diverse ethnic populations.
Patients with thrombocytopenia
NOAC therapy in thrombocytopenia
Platelet count <100 × 103/µL was an exclusion criterion in the RE-LY (dabigatran vs. VKA) and ENGAGE AF-TIMI 48 trials (edoxaban vs. VKA) and a count <90 × 103/µL in the ROCKET-AF trial (rivaroxaban vs. VKA) in AF.46,48,49 Thrombocytopenia was not a listed exclusion factor in the ARISTOTLE trial of apixaban vs. VKA in AF.47 Patients with platelet counts as low as 50 × 103 µL were included in trials of edoxaban and rivaroxaban,466,467 and 75 × 103 µL for apixaban in treatment of cancer-related VTE.468
Observational data indicate that NOACs are associated with a similar rate of ischaemic stroke and systemic embolism and a lower incidence of bleeding than VKA in thrombocytopenic AF-patients.469 A small prospective study looking at patients with AF and mild thrombocytopenia (50–100 × 103/µL) on reduced dose dabigatran (110 mg BID), apixaban (2.5 mg BID), and rivaroxaban (15 mg QD) found no difference in the rates of major bleeding or ischaemic stroke compared to patients with normal thrombocyte count on the recommended doses of those agents.470
There is no ‘safe’ cut-off above which NOAC therapy is without risk in patients with thrombocytopenia. In addition to the absolute number of platelets the dynamics of the platelet count, the underlying reason for thrombocytopenia, and special risk factors (including the likelihood of dysfunctional platelets as well as other coagulation abnormalities) need to be considered.471 Our general advice is summarized in Figure 24. Given the lack of a large evidence base for guidance the decision for NOAC treatment needs to follow an individualized, team-based approach including the patient and his/her needs and expectations (shared decision-making).
Figure 24.
NOACs in patients with thrombocytopenia. NOAC, non-vitamin K antagonist oral anticoagulant.
NOACs and heparin-induced thrombocytopenia
Thrombocytopenia is listed in the individual SmPCs as ‘uncommon’ (≥1/1000 to <1/100 patients) as a side effect of NOACs,403,405,472,473 but isolated cases have been reported.474–479 In heparin-induced thrombocytopenia ± thrombosis (HIT/HITT) there is growing evidence that NOACs are not recognized by pre-existing HIT antibodies, do not complex with platelet factor 4 and do not cause platelet aggregation.480–482 NOAC therapy may hence constitute a viable less expensive and easier to administer alternative to parenteral heparin substitutes (e.g. argatroban, fondaparinux) especially if the latter are not available or are deemed unsuitable.483,484 Further research is required in this field to confirm and strengthen these first positive experiences.
NOACs in patients with atrial fibrillation and malignancy
The scope of the problem
Cancers are not infrequent in older patients, similar to AF.485 Cancer and cancer therapy may in turn precipitate AF, while both age and malignancy are independent risk factors for thrombosis and bleeding. The scope of the problem of AF and malignancy is outlined in detail in the Supplementary material online.
Anticoagulant therapy in patients with malignancy
In the phase III VTE trials specifically targeting cancer patients, edoxaban (Hokusai Cancer),466 rivaroxaban (Select-D),467 and apixaban (Caravaggio)486 were non-inferior to dalteparin in the prevention of recurrent VTE. While there was a signal of improved efficacy with both edoxaban and rivaroxaban vs. dalteparin, bleeding tended to be higher with the two NOACs as compared to dalteparin, which was driven mainly by patients with GI cancers. For apixaban, efficacy and safety were broadly similar between the NOAC and LMWH.
Concerning the prevention of stroke and systemic embolism in AF patients with cancer, available evidence is less strong, as active malignancy was an exclusion criterion in most NOAC AF Phase III trials. In a recent meta-analysis487 of five studies (post hoc analyses of the ROCKET AF,488 ENGAGE AF-TIMI 48,489 and ARISTOTLE490 trials, and two retrospective population-based cohorts),491,492 the use of NOACs compared to warfarin was associated with a significantly reduced risk of stroke, systemic embolism, and VTE, a strong trend towards fewer ischaemic strokes (P = 0.05) and a numerically lower incidence of MI, all-cause mortality and cardiovascular death. There was a strong trend towards fewer major bleedings (P = 0.05), significantly fewer intracranial or GI bleedings, and a comparable number of clinically relevant major or non-major bleeds with NOACs. Pooling the three post hoc studies showed similar rates of efficacy and safety outcomes with NOACs vs. warfarin in AF patients with and without cancer.
A large registry using a prescription-based analysis for AF patients on VKA or NOAC with and without cancer reported equivalence for bleeding and thromboembolic incidence and cancer status, although the rates of both were lower in the NOAC population.493 However, much is still unknown about DDIs between NOACs and specific chemotherapeutic agents, urging further caution (Table 6).494
Overall, anticoagulation with NOACs may appear as a valid option in patients with AF and malignancy based on the few available data from RCTs as well as using extrapolations from cancer-related VTE treatment. Antithrombotic therapy in patients with AF suffering from a malignancy needs a dedicated interdisciplinary team approach (Figure 25).495 Especially when myelosuppressive chemotherapy or radiation therapy is planned, temporary dose reduction or cessation of NOAC therapy needs to be evaluated, taking into account full blood counts including platelets, renal/liver function, and physical signs of bleeding. Gastric protection with PPI or H2 blockers should be considered in all such patients.
Figure 25.
Important aspects in the management of AF patients with malignancies. AF, atrial fibrillation; LMWH, low molecular weight heparin; NOAC, non-vitamin K antagonist oral anticoagulant; PPI, proton pump inhibitor; VKA, vitamin K antagonist.
Optimizing dose adjustments of vitamin-K antagonists
Specific considerations for optimizing dose adjustments of VKA are discussed in the Supplementary material online. One algorithm to optimize VKA dosing is presented in Table 15, derived from the warfarin arm of the RE-LY trial.556
Table 15.
Maintenance warfarin dosing for out-of-therapeutic-range international normalized ratio
| INR | Dose adjustment per week |
|---|---|
| ≤1.5 | ↑ by 15%/week |
| 1.6–1.9 | ↑ by 10%/week |
| 2–2.9 | Unchanged |
| 3–3.9 | ↓ by 10%/week |
| 4–4.9 | Hold 1 dose, then restart with dose ↓ by 10%/week |
| ≥5 | Hold until INR is 2–3, then restart with dose ↓ by 15%/week |
Suggested dose adjustment in case of out-of-therapeutic-range INR.556 Importantly, dosing is optimized not using daily dose adjustments but adjustments based on the weekly intake in warfarin.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors thank the EHRA Scientific Document Committee: Dr Nikolaos Dagres, Prof. Thomas Deneke, Prof. Arthur Wilde, Prof. Frank R. Heinzel, Prof. Christian Meyer, Prof. Lucas Boersma, Prof. Radoslaw Lenarczyk, Prof. Luigi Di Biase, Dr Elena Arbelo, Dr Avi Sabbag, Prof. Pierre Jais, Prof. Milos Taborsky, and Associate Prof. Markus Stühlinger.
Funding
This article and derived educational materials (slide set, website, booklet, and NOAC card) were produced by and under the sole responsibility of the European Heart Rhythm Association, and supported by Bayer Pharma AG, Bristol-Myers Squibb and Pfizer Alliance, and Daiichi-Sankyo Europe GmbH in the form of an Unrestricted Educational Grant.
Conflict of interest: JS has received consultant and / or speaker fees from Abbott, Amgen, Astra-Zeneca, Bayer, Berlin-Chemie, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Portola, Roche Diagnostics, Pfizer, Portola, Saja, Servier, and WebMD. He reports ownership of CorXL. JS has received grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic. RC has received speakers honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer. MA reports personal fees from Bayer, Biosense Webster, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer; and fees to his institution from Abbott, Bayer, Berlin-Chemie, Biosense Webster, Biotronik, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Medtronic, Pfizer and Sanofi Aventis. KGH reports a study grant by Bayer, lecture fees/advisory board fees from Abbott, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol-Myers-Squibb, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Pfizer, Premier Research, and W. L. Gore & Associates. JO reports fees to his institution from Alexion, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Roche Diagnostics, and Sanofi. HR reports personal fees from Daiichi, Pfizer, MedUpdate, DiaPlan, NeoVasc, Pluristem, NovoNordisk and Corvia. Furthermore, grants were received from Pluristem, BMS, Pfizer, Bard and Biotronik. NR reports personal fees from Bristol-Myers Squibb and Pfizer. PS reports small institutional speaker s/advisory fees from Daiichi Sankyo, Boehringer Ing, BMS/Pfizer, Bayer, AstraZeneca. He is co-recipient of a named chair funded by Bayer to the University of Leuven. TV has received honoraria for participation in advisory boards, consultancy services, and/or participation as a speaker from Bayer, Boehringer Ingelheim, BMS/Pfizer, Daiichi Sankyo, Sanofi Aventis, and Leo Pharma. TP reports consultancy for Bayer and Pfizer (no fees). AJC reports personal fees from Bayer, Daiichi Sankyo and Pfizer/BMS. HH reports minor personal honoraria from BMS, Pfizer, Biotronik and Daiichi-Sankyo and received unconditional research support through the University of Antwerp or University of Hasselt from Bayer, Daiichi-Sankyo, Boehringer-Ingelheim, Bracco Imaging Europe, Medtronic, Boston-Scientific, Biotronik, and St. Jude Medical. PC, LD and VRS declare that they have nothing to disclose.
Contributor Information
Jan Steffel, Department of Cardiology, Division of Electrophysiology, University Heart Center Zurich, Switzerland.
Ronan Collins, Age-Related Health Care, Tallaght University Hospital / Department of Gerontology Trinity College, Dublin, Ireland.
Matthias Antz, Department of Electrophysiology, Hospital Braunschweig, Braunschweig, Germany.
Pieter Cornu, Faculty of Medicine and Pharmacy, Research Group Clinical Pharmacology and Clinical Pharmacy, Vrije Universiteit Brussel, Brussels, Belgium.
Lien Desteghe, Cardiology, Antwerp University and University Hospital, Antwerp, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium.
Karl Georg Haeusler, Department of Neurology, Universitätsklinikum Würzburg, Würzburg, Germany.
Jonas Oldgren, Uppsala Clinical Research Center and Department of Medical Sciences, Uppsala University, Uppsala, Sweden.
Holger Reinecke, Department of Cardiology I - Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Münster, Münster, Germany.
Vanessa Roldan-Schilling, University of Murcia, Murcia, Spain.
Nigel Rowell, Middlesbrough, UK.
Peter Sinnaeve, Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Thomas Vanassche, Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium.
Tatjana Potpara, School of Medicine, Belgrade University, Belgrade, Serbia.
A John Camm, Cardiology Clinical Academic Group, Molecular & Clinical Sciences Institute, St George’s University, London, UK.
Hein Heidbüchel, Cardiology, Antwerp University and University Hospital, Antwerp, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium.
External reviewers:
Gregory Y H Lip, Thomas Deneke, Nikolaos Dagres, Giuseppe Boriani, Tze-Fan Chao, Eue-Keun Choi, Mellanie True Hills, Itamar de Souza Santos, Deirdre A Lane, Dan Atar, Boyoung Joung, Oana Maria Cole, and Mark Field
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]
- 3. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C et al. 2018 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371–92. [DOI] [PubMed] [Google Scholar]
- 4. Chiang CE, Okumura K, Zhang S, Chao TF, Siu CW, Wei Lim T et al. 2017 consensus of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm 2017;33:345–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes GD, Ageno W, Ansell J, Kaatz S; Subcommittee on the Control of Anticoagulation of the International Society on Thrombosis and Haemostasis. Recommendation on the nomenclature for oral anticoagulants: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:1154–6. [DOI] [PubMed] [Google Scholar]
- 6. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 7. Steffel J, Braunwald E. Novel oral anticoagulants: focus on stroke prevention and treatment of venous thrombo-embolism. Eur Heart J 2011;32:1968–76. [DOI] [PubMed] [Google Scholar]
- 8. Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J et al. ; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51. [DOI] [PubMed] [Google Scholar]
- 9. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- 10. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L et al. ; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–93. [DOI] [PubMed] [Google Scholar]
- 10a. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace 2018;20:1231–1242. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. ; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609––78.. [DOI] [PubMed] [Google Scholar]
- 12. Lip GYH, Collet JP, Caterina R, Fauchier L, Lane DA, Larsen TB et al. ; ESC Scientific Document Group. Antithrombotic therapy in atrial fibrillation associated with valvular heart disease: a joint consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology Working Group on Thrombosis, endorsed by the ESC Working Group on Valvular Heart Disease, Cardiac Arrhythmia Society of Southern Africa (CASSA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), South African Heart (SA Heart) Association and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017;19:1757–8. [DOI] [PubMed] [Google Scholar]
- 13. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al. ; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 14. De Caterina R, Camm AJ. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation accompanying mitral stenosis: the concept for a trial. Europace 2016;18:6–11. [DOI] [PubMed] [Google Scholar]
- 15. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ et al. ; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206–14. [DOI] [PubMed] [Google Scholar]
- 16. Duraes AR, de Souza Lima Bitar Y, Schonhofen IS, Travassos KSO, Pereira LV, Filho JAL et al. Rivaroxaban versus warfarin in patients with mechanical heart valves: open-label, proof-of-concept trial—the RIWA study. Am J Cardiovasc Drugs. 2020 Nov 5. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 17. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M et al. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation 2015;132:624–32. [DOI] [PubMed] [Google Scholar]
- 18. Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M et al. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulant Therapy). Circulation 2016;134:589–98. [DOI] [PubMed] [Google Scholar]
- 19. Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Stevens SR et al. ; ROCKET AF Steering Committee & Investigators. Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J 2014;35:3377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Caterina R, Renda G, Carnicelli AP, Nordio F, Trevisan M, Mercuri MF et al. Valvular heart disease patients on edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol 2017;69:1372–82. [DOI] [PubMed] [Google Scholar]
- 21. Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. 2017;6:e005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renda G, Ricci F, Giugliano RP, De Caterina R. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and valvular heart disease. J Am Coll Cardiol 2017;69:1363–71. [DOI] [PubMed] [Google Scholar]
- 23. Noseworthy PA, Yao X, Shah ND, Gersh BJ. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease. Int J Cardiol 2016;209:181–3. [DOI] [PubMed] [Google Scholar]
- 24. Guimaraes HP, Lopes RD, de Barros E, Liporace IL, Sampaio RO, Tarasoutchi F et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med 2020;383:2117–26. [DOI] [PubMed] [Google Scholar]
- 25. Nijenhuis VJ, Brouwer J, Delewi R, Hermanides RS, Holvoet W, Dubois CLF et al. Anticoagulation with or without clopidogrel after transcatheter aortic-valve implantation. N Engl J Med 2020;382:1696–707. [DOI] [PubMed] [Google Scholar]
- 26. Seeger J, Gonska B, Rodewald C, Rottbauer W, Wohrle J. Apixaban in patients with atrial fibrillation after transfemoral aortic valve replacement. JACC Cardiovasc Interv 2017;10:66–74. [DOI] [PubMed] [Google Scholar]
- 27. Kawashima H, Watanabe Y, Hioki H, Kozuma K, Kataoka A, Nakashima M et al. ; OCEAN-TAVI Investigator. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation after TAVR. JACC Cardiovasc Interv 2020;13:2587–97. [DOI] [PubMed] [Google Scholar]
- 28. Collet JP, Berti S, Cequier A, Van Belle E, Lefevre T, Leprince P et al. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: the randomized ATLANTIS trial. Am Heart J 2018;200:44–50. [DOI] [PubMed] [Google Scholar]
- 29. Van Mieghem NM, Unverdorben M, Valgimigli M, Mehran R, Boersma E, Baber U et al. Edoxaban Versus standard of care and their effects on clinical outcomes in patients having undergone Transcatheter Aortic Valve Implantation in Atrial Fibrillation-Rationale and design of the ENVISAGE-TAVI AF trial. Am Heart J 2018;205:63–9. [DOI] [PubMed] [Google Scholar]
- 30. Dangas GD, Tijssen JGP, Wohrle J, Sondergaard L, Gilard M, Mollmann H et al. ; GALILEO Investigators. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med 2020;382:120–9. [DOI] [PubMed] [Google Scholar]
- 31. Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W et al. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation 2017;136:2420–36. [DOI] [PubMed] [Google Scholar]
- 32. Nasser MF, Gandhi S, Siegel RJ, Rader F. Anticoagulation for stroke prevention in patients with hypertrophic cardiomyopathy and atrial fibrillation: a review. Heart Rhythm 2021;18:297–302. [DOI] [PubMed] [Google Scholar]
- 33. Noseworthy PA, Yao X, Shah ND, Gersh BJ. Stroke and bleeding risks in NOAC- and warfarin-treated patients with hypertrophic cardiomyopathy and atrial fibrillation. J Am Coll Cardiol 2016;67:3020–1. [DOI] [PubMed] [Google Scholar]
- 34. Dominguez F, Climent V, Zorio E, Ripoll-Vera T, Salazar-Mendiguchia J, Garcia-Pinilla JM et al. Direct oral anticoagulants in patients with hypertrophic cardiomyopathy and atrial fibrillation. Int J Cardiol 2017;248:232–8. [DOI] [PubMed] [Google Scholar]
- 35. Jung H, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest 2019;155:354–63. [DOI] [PubMed] [Google Scholar]
- 36. Lee HJ, Kim HK, Jung JH, Han KD, Lee H, Park JB et al. Novel oral anticoagulants for primary stroke prevention in hypertrophic cardiomyopathy patients with atrial fibrillation. Stroke 2019;50:2582–6. [DOI] [PubMed] [Google Scholar]
- 37. van Diepen S, Hellkamp AS, Patel MR, Becker RC, Breithardt G, Hacke W et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail 2013;6:740–7. [DOI] [PubMed] [Google Scholar]
- 38. McMurray JJ, Ezekowitz JA, Lewis BS, Gersh BJ, van Diepen S, Amerena J et al. ; for the ARISTOTLE Committees and Investigators. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail 2013;6:451–60. [DOI] [PubMed] [Google Scholar]
- 39. Magnani G, Giugliano RP, Ruff CT, Murphy SA, Nordio F, Metra M et al. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48. Eur J Heart Fail 2016;18:1153–61. [DOI] [PubMed] [Google Scholar]
- 40. Male C, Lensing AWA, Palumbo JS, Kumar R, Nurmeev I, Hege K et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol 2020;7:e18–27. [DOI] [PubMed] [Google Scholar]
- 41. Brandao LR, Albisetti M, Halton J, Bomgaars L, Chalmers E, Mitchell LG et al. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood 2020;135:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018;132:1365–71. [DOI] [PubMed] [Google Scholar]
- 43. Chan YH, Chao TF, Lee HF, Yeh YH, Yeh CH, Huang YC et al. Impacts of different renal function estimation formulas on dosing of DOACs and clinical outcomes. J Am Coll Cardiol 2020;76:1808–10. [DOI] [PubMed] [Google Scholar]
- 44. Kirchhof P, Haas S, Amarenco P, Hess S, Lambelet M, van Eickels M et al. ; XANTUS Investigators. Impact of modifiable bleeding risk factors on major bleeding in patients with atrial fibrillation anticoagulated with rivaroxaban. J Am Heart Assoc 2020;9:e009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guo Y, Lane DA, Chen Y, Lip GYH; mAF-App II Trial investigators. Regular bleeding risk assessment associated with reduction in bleeding outcomes: the mAFA-II randomized trial. Am J Med 2020; 133: 1195–202.e2. [DOI] [PubMed] [Google Scholar]
- 46. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W et al. ; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 47. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 48. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 49. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 50. Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. 2016;5:e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ et al. ; ORBIT-AF Investigators. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J 2017;194:132–40. [DOI] [PubMed] [Google Scholar]
- 52. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale CP. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J 2018;39:2975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Forslund T, Komen JJ, Andersen M, Wettermark B, von Euler M, Mantel-Teeuwisse AK et al. Improved stroke prevention in atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants. Stroke 2018;49:2122–8. [DOI] [PubMed] [Google Scholar]
- 54. Lee SR, Choi EK, Kwon S, Jung JH, Han KD, Cha MJ et al. Effectiveness and safety of direct oral anticoagulants in relation to temporal changes in their use. Circ Cardiovasc Qual Outcomes 2020;13:e005894. [DOI] [PubMed] [Google Scholar]
- 55. Lee SR, Choi EK, Kwon S, Han KD, Jung JH, Cha MJ et al. Effectiveness and safety of contemporary oral anticoagulants among Asians with nonvalvular atrial fibrillation. Stroke 2019;50:2245–9. [DOI] [PubMed] [Google Scholar]
- 56. Haas S, Camm AJ, Bassand JP, Angchaisuksiri P, Cools F, Corbalan R et al. ; GARFIELD-AF Investigators. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: results from GARFIELD-AF. Am Heart J 2019;213:35–46. [DOI] [PubMed] [Google Scholar]
- 57. Maura G, Billionnet C, Drouin J, Weill A, Neumann A, Pariente A. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non-vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011-2016. BMJ Open 2019;9:e026645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mazurek M, Halperin JL, Huisman MV, Diener H-C, Dubner SJ, Ma CS et al. Antithrombotic treatment for newly diagnosed atrial fibrillation in relation to patient age: the GLORIA-AF registry programme. Europace 2019;22:47–57. [DOI] [PubMed] [Google Scholar]
- 59. Lip GYH, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K et al. Effectiveness and safety of oral anticoagulants among nonvalvular atrial fibrillation patients. Stroke 2018;49:2933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hess PL, Kim S, Fonarow GC, Thomas L, Singer DE, Freeman JV et al. ; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Patients and Investigators. Absence of oral anticoagulation and subsequent outcomes among outpatients with atrial fibrillation. Am J Med 2017;130:449–56. [DOI] [PubMed] [Google Scholar]
- 61. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ et al. Off-Label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol 2016;68:2597–604. [DOI] [PubMed] [Google Scholar]
- 62. Steinberg BA, Shrader P, Pieper K, Thomas L, Allen LA, Ansell J et al. ; the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) II Investigators. Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II) 2018;7:e007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xing LY, Barcella CA, Sindet-Pedersen C, Bonde AN, Gislason GH, Olesen JB. Dose reduction of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: a Danish nationwide cohort study. Thromb Res 2019;178:101–9. [DOI] [PubMed] [Google Scholar]
- 64. Amarenco P, Haas S, Hess S, Kirchhof P, Lambelet M, Bach M et al. Outcomes associated with non-recommended dosing of rivaroxaban: results from the XANTUS study. Eur Heart J Cardiovasc Pharmacother 2019;5:70–9. [DOI] [PubMed] [Google Scholar]
- 65. García Rodríguez LA, Martín-Pérez M, Vora P, Roberts L, Balabanova Y, Brobert G et al. Appropriateness of initial dose of non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation in the UK. BMJ Open 2019;9:e031341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chan YH, Chao TF, Chen SW, Lee HF, Yeh YH, Huang YC et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and clinical outcomes in Asian patients with atrial fibrillation. Heart Rhythm 2020;17:2102–10. [DOI] [PubMed] [Google Scholar]
- 67. Yu HT, Yang PS, Jang E, Kim TH, Uhm JS, Kim JY et al. Label adherence of direct oral anticoagulants dosing and clinical outcomes in patients with atrial fibrillation. J Am Heart Assoc 2020;9:e014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee SR, Lee YS, Park JS, Cha MJ, Kim TH, Park J et al. Label adherence for non-vitamin K antagonist oral anticoagulants in a prospective cohort of Asian patients with atrial fibrillation. Yonsei Med J 2019;60:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. 2016;5:e003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF-TIMI 48 analysis. J Am Coll Cardiol 2016;68:1169–78. [DOI] [PubMed] [Google Scholar]
- 71. Chao TF, Chiang CE, Lin YJ, Chang SL, Lo LW, Hu YF et al. Evolving changes of the use of oral anticoagulants and outcomes in patients with newly diagnosed atrial fibrillation in Taiwan. Circulation 2018;138:1485–7. [DOI] [PubMed] [Google Scholar]
- 72. Moudallel S, van den Bemt BJF, Zwikker H, de Veer A, Rydant S, Dijk LV et al. Association of conflicting information from healthcare providers and poor shared decision making with suboptimal adherence in direct oral anticoagulant treatment: a cross-sectional study in patients with atrial fibrillation. Patient Educ Couns 2021;104:155–62. [DOI] [PubMed] [Google Scholar]
- 73. Rush KL, Burton L, Schaab K, Lukey A. The impact of nurse-led atrial fibrillation clinics on patient and healthcare outcomes: a systematic mixed studies review. Eur J Cardiovasc Nurs 2019;18:526–33. [DOI] [PubMed] [Google Scholar]
- 74. Lane DA, Meyerhoff J, Rohner U, Lip GYH. Atrial fibrillation patient preferences for oral anticoagulation and stroke knowledge: Results of a conjoint analysis. Clin Cardiol 2018;41:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:123–260. [Google Scholar]
- 76. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al. ; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- 77. Undas A, Drabik L, Potpara T. Bleeding in anticoagulated patients with atrial fibrillation: practical considerations. Pol Arch Intern Med 2020;130:47–58. [DOI] [PubMed] [Google Scholar]
- 78. Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, et al. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double-blind, placebo-controlled trial. Gastroenterology 2019;157:403–12.e5. [DOI] [PubMed] [Google Scholar]
- 79. Chan EW, Lau WC, Leung WK, Mok MT, He Y, Tong TS, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology 2015; 149: 586–95.e3. [DOI] [PubMed] [Google Scholar]
- 80. Ray WA, Chung CP, Murray KT, Smalley WE, Daugherty JR, Dupont WD et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA 2018;320:2221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Scarpignato C, Gatta L, Zullo A, Blandizzi C; for the SIF-AIGO-FIMMG Group. Effective and safe proton pump inhibitor therapy in acid-related diseases—a position paper addressing benefits and potential harms of acid suppression. BMC Med 2016;14:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ray WA, Chung CP, Murray KT, Smalley WE, Daugherty JR, Dupont WD et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology 2016; 151: 1105–12.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Di Minno A, Spadarella G, Spadarella E, Tremoli E, Di Minno G. Gastrointestinal bleeding in patients receiving oral anticoagulation: current treatment and pharmacological perspectives. Thromb Res 2015;136:1074–81. [DOI] [PubMed] [Google Scholar]
- 84. Shields AM, Lip GY. Choosing the right drug to fit the patient when selecting oral anticoagulation for stroke prevention in atrial fibrillation. J Intern Med 2015;278:1–18. [DOI] [PubMed] [Google Scholar]
- 85. Okumura K, Hori M, Tanahashi N, John Camm A. Special considerations for therapeutic choice of non-vitamin K antagonist oral anticoagulants for Japanese patients with nonvalvular atrial fibrillation. Clin Cardiol 2017;40:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Diener HC, Aisenberg J, Ansell J, Atar D, Breithardt G, Eikelboom J et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 1. Eur Heart J 2017; 38:852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Diener HC, Aisenberg J, Ansell J, Atar D, Breithardt G, Eikelboom J et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Eur Heart J 2017; 38:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sanchis-Gomar F, Perez-Quilis C, Lavie CJ. Should atrial fibrillation be considered a cardiovascular risk factor for a worse prognosis in COVID-19 patients? Eur Heart J 2020;41:3092–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bae S, Kim SR, Kim MN, Shim WJ, Park SM. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart 2021;107:373–80. [DOI] [PubMed] [Google Scholar]
- 90. Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Atrial fibrillation and the risk of 30-day incident thromboembolic events, and mortality in adults ≥ 50 years with COVID-19. J Arrhythm 2021;37:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance (30 August 2020, date last accessed).
- 93. Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther 2011;338:372–80. [DOI] [PubMed] [Google Scholar]
- 94. Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013;76:455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang L, Zhang D, Raghavan N, Yao M, Ma L, Frost CE et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab Dispos 2010;38:448–58. [DOI] [PubMed] [Google Scholar]
- 96. LaHaye SA, Gibbens SL, Ball DG, Day AG, Olesen JB, Skanes AC. A clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. Eur Heart J 2012;33:2163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321–8. [DOI] [PubMed] [Google Scholar]
- 98. Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF et al. Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 2015;385:2288–95. [DOI] [PubMed] [Google Scholar]
- 99. Lip GY, Clemens A, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost 2014;111:933–42. [DOI] [PubMed] [Google Scholar]
- 100. Steffel J, Ruff CT, Yin O, Braunwald E, Park J-G, Murphy SA et al. Randomized, double-blind comparison of half-dose versus full-dose edoxaban in 14,014 patients with atrial fibrillation. J Am Coll Cardiol 2021;77:1197–207. [DOI] [PubMed] [Google Scholar]
- 101. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S et al. ; on behalf of the J-ROCKET AF study investigators. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J 2012;76:2104–11. [DOI] [PubMed] [Google Scholar]
- 102. Okumura K, Akao M, Yoshida T, Kawata M, Okazaki O, Akashi S et al. Low-dose edoxaban in very elderly patients with atrial fibrillation. N Engl J Med 2020;383:1735–45. [DOI] [PubMed] [Google Scholar]
- 103. Alexander JH, Andersson U, Lopes RD, Hijazi Z, Hohnloser SH, Ezekowitz JA et al. ; Apixaban for Reduction of Stroke and Other Thromboembolic Complications in Atrial Fibrillation (ARISTOTLE) Investigators. Apixaban 5 mg twice daily and clinical outcomes in patients with atrial fibrillation and advanced age, low body weight, or high creatinine: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:673–81. [DOI] [PubMed] [Google Scholar]
- 104. Rottenstreich A, Zacks N, Kleinstern G, Raccah BH, Roth B, Da’as N et al. Direct-acting oral anticoagulant drug level monitoring in clinical patient management. J Thromb Thrombolysis 2018;45:543–9. [DOI] [PubMed] [Google Scholar]
- 105. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol 2006;46:549–58. [DOI] [PubMed] [Google Scholar]
- 106. Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008;36:386–99. [DOI] [PubMed] [Google Scholar]
- 107. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285–95. [DOI] [PubMed] [Google Scholar]
- 108. Mendell J, Tachibana M, Shi M, Kunitada S. Effects of food on the pharmacokinetics of edoxaban, an oral direct factor Xa inhibitor, in healthy volunteers. J Clin Pharmacol 2011;51:687–94. [DOI] [PubMed] [Google Scholar]
- 109. Upreti VV, Song Y, Wang J, Byon W, Boyd RA, Pursley JM et al. Effect of famotidine on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Clin Pharmacol 2013;5:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song Y, Chang M, Suzuki A, Frost RJ, Kelly A, LaCreta F et al. Evaluation of crushed tablet for oral administration and the effect of food on apixaban pharmacokinetics in healthy adults. Clin Ther 2016; 38: 1674–85.e1. [DOI] [PubMed] [Google Scholar]
- 111. Duchin K, Duggal A, Atiee GJ, Kidokoro M, Takatani T, Shipitofsky NL et al. An open-label crossover study of the pharmacokinetics of the 60-mg edoxaban tablet crushed and administered either by a nasogastric tube or in apple puree in healthy adults. Clin Pharmacokinet 2018;57:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Moore KT, Krook MA, Vaidyanathan S, Sarich TC, Damaraju CV, Fields LE. Rivaroxaban crushed tablet suspension characteristics and relative bioavailability in healthy adults when administered orally or via nasogastric tube. Clin Pharmacol Drug Dev 2014;3:321–7. [DOI] [PubMed] [Google Scholar]
- 113. Odell K, Costello J. Safety of apixaban administered via nasogastric tube. Cardiology 2019;142:39. [DOI] [PubMed] [Google Scholar]
- 114. Dans AL, Connolly SJ, Wallentin L, Yang S, Nakamya J, Brueckmann M et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013;127:634–40. [DOI] [PubMed] [Google Scholar]
- 115. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 116. Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M et al. ; APPRAISE Steering Committee and Investigators. Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation 2009;119:2877–85. [DOI] [PubMed] [Google Scholar]
- 117. Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R et al. eGFR and albuminuria in relation to risk of incident atrial fibrillation: a meta-analysis of the Jackson Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study. Clin J Am Soc Nephrol 2017;12:1386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Go AS, Fang MC, Udaltsova N, Chang Y, Pomernacki NK, Borowsky L et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 2009;119:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 2010;159:1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J 2009;158:629–36. [DOI] [PubMed] [Google Scholar]
- 121. Reinecke H, Brand E, Mesters R, Schabitz WR, Fisher M, Pavenstadt H et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009;20:705–11. [DOI] [PubMed] [Google Scholar]
- 122. Steffel J, Hindricks G. Apixaban in renal insufficiency: successful navigation between the Scylla and Charybdis. Eur Heart J 2012;33:2766–8. [DOI] [PubMed] [Google Scholar]
- 123. Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation 2020;141:1384–92. [DOI] [PubMed] [Google Scholar]
- 124. Weir MR, Ashton V, Moore KT, Shrivastava S, Peterson ED, Ammann EM. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J 2020;223:3–11. [DOI] [PubMed] [Google Scholar]
- 125. Hanni C, Petrovitch E, Ali M, Gibson W, Giuliano C, Holzhausen J et al. Outcomes associated with apixaban vs warfarin in patients with renal dysfunction. Blood Adv 2020;4:2366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Fazio G, Dentamaro I, Gambacurta R, Alcamo P, Colonna P. Safety of edoxaban 30 mg in elderly patients with severe renal impairment. Clin Drug Investig 2018;38:1023–30. [DOI] [PubMed] [Google Scholar]
- 127. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012;33:2821–30. [DOI] [PubMed] [Google Scholar]
- 128. Fanaroff AC, Steffel J, Alexander JH, Lip GYH, Califf RM, Lopes RD. Stroke prevention in atrial fibrillation: re-defining ‘real-world data’ within the broader data universe. Eur Heart J 2018;39:2932–41. [DOI] [PubMed] [Google Scholar]
- 129. Olesen JB, Lip GY, Kamper AL, Hommel K, Kober L, Lane DA et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- 130. Bonde AN, Lip GY, Kamper AL, Hansen PR, Lamberts M, Hommel K et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014;64:2471–82. [DOI] [PubMed] [Google Scholar]
- 131. Friberg L, Benson L, Lip GY. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the Swedish Atrial Fibrillation Cohort study. Eur Heart J 2015;36:297–306. [DOI] [PubMed] [Google Scholar]
- 132. Shah M, Avgil Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014;129:1196–203. [DOI] [PubMed] [Google Scholar]
- 133. Pokorney SD, Black-Maier E, Hellkamp AS, Friedman DJ, Vemulapalli S, Granger CB et al. Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J Am Coll Cardiol 2020;75:1299–308. [DOI] [PubMed] [Google Scholar]
- 134. Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol 2020;75:273–85. [DOI] [PubMed] [Google Scholar]
- 135. Galloway PA, El-Damanawi R, Bardsley V, Pritchard NR, Fry AC, Ojha SK et al. Vitamin K antagonists predispose to calciphylaxis in patients with end-stage renal disease. Nephron 2015;129:197–201. [DOI] [PubMed] [Google Scholar]
- 136. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. JASN 2017;28:2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Koretsune Y, Yamashita T, Kimura T, Fukuzawa M, Abe K, Yasaka M. Short-term safety and plasma concentrations of edoxaban in Japanese patients with non-valvular atrial fibrillation and severe renal impairment. Circ J 2015;79:1486–95. [DOI] [PubMed] [Google Scholar]
- 138. De Vriese AS, Caluwe R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis 2015;66:91–8. [DOI] [PubMed] [Google Scholar]
- 139. Dias C, Moore KT, Murphy J, Ariyawansa J, Smith W, Mills RM et al. Pharmacokinetics, pharmacodynamics, and safety of single-dose rivaroxaban in chronic hemodialysis. Am J Nephrol 2016;43:229–36. [DOI] [PubMed] [Google Scholar]
- 140. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 2015;131:972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and efficacy of apixaban versus warfarin in patients with advanced chronic kidney disease. Ann Pharmacother 2018;52:1078–84. [DOI] [PubMed] [Google Scholar]
- 142. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation 2018;138:1519–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol 2020;104:328–35. [DOI] [PubMed] [Google Scholar]
- 144. Reinecke H, Jurgensmeyer S, Engelbertz C, Gerss J, Kirchhof P, Breithardt G et al. Design and rationale of a randomised controlled trial comparing apixaban to phenprocoumon in patients with atrial fibrillation on chronic haemodialysis: the AXADIA-AFNET 8 study. BMJ Open 2018;8:e022690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Chan KE, Giugliano RP, Patel MR, Abramson S, Jardine M, Zhao S et al. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J Am Coll Cardiol 2016;67:2888–99. [DOI] [PubMed] [Google Scholar]
- 146. Reinecke H, Engelbertz C, Schabitz WR. Preventing stroke in patients with chronic kidney disease and atrial fibrillation: benefit and risks of old and new oral anticoagulants. Stroke 2013;44:2935–41. [DOI] [PubMed] [Google Scholar]
- 147. Douxfils J, Ageno W, Samama CM, Lessire S, Ten Cate H, Verhamme P et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost 2018;16:209–19. [DOI] [PubMed] [Google Scholar]
- 148. Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogne JM. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res 2012;130:956–66. [DOI] [PubMed] [Google Scholar]
- 149. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost 2012;107:985–97. [DOI] [PubMed] [Google Scholar]
- 150. van Ryn J, Baruch L, Clemens A. Interpretation of point-of-care INR results in patients treated with dabigatran. Am J Med 2012;125:417–20. [DOI] [PubMed] [Google Scholar]
- 151. Harenberg J, Schreiner R, Hetjens S, Weiss C. Detecting anti-IIa and anti-Xa direct oral anticoagulant (DOAC) agents in urine using a DOAC dipstick. Semin Thromb Hemost 2019;45:275–84. [DOI] [PubMed] [Google Scholar]
- 152. Salmonson T, Dogne JM, Janssen H, Garcia Burgos J, Blake P. Non-vitamin-K oral anticoagulants and laboratory testing: now and in the future: views from a workshop at the European Medicines Agency (EMA). Eur Heart J Cardiovasc Pharmacother 2017;3:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Altman R, Gonzalez CD. Simple and rapid assay for effect of the new oral anticoagulant (NOAC) rivaroxaban: preliminary results support further tests with all NOACs. Thromb J 2014;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014;64:1128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013;11:756–60. [DOI] [PubMed] [Google Scholar]
- 156. Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost 2018;118:437–50. [DOI] [PubMed] [Google Scholar]
- 157. Douxfils J, Chatelain C, Chatelain B, Dogne JM, Mullier F. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost 2013;110:283–94. [DOI] [PubMed] [Google Scholar]
- 158. Patel JP, Byrne RA, Patel RK, Arya R. Progress in the monitoring of direct oral anticoagulant therapy. Br J Haematol 2019;184:912–24. [DOI] [PubMed] [Google Scholar]
- 159. Favaloro EJ, Lippi G. Interference of direct oral anticoagulants in haemostasis assays: high potential for diagnostic false positives and false negatives. Blood Transfus 2017;15:491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jacquemin M, Toelen J, Feyen L, Schoeters J, Van Horenbeeck I, Vanlinthout I et al. The adsorption of dabigatran is as efficient as addition of idarucizumab to neutralize the drug in routine coagulation assays. Int J Lab Hematol 2018;40:442–7. [DOI] [PubMed] [Google Scholar]
- 161. Favresse J, Lardinois B, Sabor L, Devalet B, Vandepapeliere J, Braibant M et al. Evaluation of the DOAC-Stop(R) procedure to overcome the effect of DOACs on several thrombophilia screening tests. TH Open 2018;02:e202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Godier A, Dincq AS, Martin AC, Radu A, Leblanc I, Antona M et al. Predictors of pre-procedural concentrations of direct oral anticoagulants: a prospective multicentre study. Eur Heart J 2017;38:2431–9. [DOI] [PubMed] [Google Scholar]
- 163. Suzuki S, Yamashita T, Akao M, Okumura K; J-ELD AF investigators. Clinical implications of assessment of apixaban levels in elderly atrial fibrillation patients: J-ELD AF registry sub-cohort analysis. Eur J Clin Pharmacol 2020;76:1111–24. [DOI] [PubMed] [Google Scholar]
- 164. Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non-vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol 2017;2:566–74. [DOI] [PubMed] [Google Scholar]
- 165. Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: the ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol 2014;63:2141–7. [DOI] [PubMed] [Google Scholar]
- 166. Piccini JP, Garg J, Patel MR, Lokhnygina Y, Goodman SG, Becker RC et al. ; on behalf of the ROCKET AF Investigators. Management of major bleeding events in patients treated with rivaroxaban vs. warfarin: results from the ROCKET AF trial. Eur Heart J 2014;35:1873–80. [DOI] [PubMed] [Google Scholar]
- 167. Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 2013;128:2325–32. [DOI] [PubMed] [Google Scholar]
- 168. Giugliano RP, Ruff CT, Wiviott SD, Nordio F, Murphy SA, Kappelhof JA et al. Mortality in patients with atrial fibrillation randomized to edoxaban or warfarin: insights from the ENGAGE AF-TIMI 48 trial. Am J Med 2016;129:850–7.e2. [DOI] [PubMed] [Google Scholar]
- 169. Kawabori M, Niiya Y, Iwasaki M, Mabuchi S, Ozaki H, Matsubara K et al. Characteristics of symptomatic intracerebral hemorrhage in patient receiving direct oral anticoagulants: comparison with warfarin. J Stroke Cerebrovasc Dis 2018;27:1338–42. [DOI] [PubMed] [Google Scholar]
- 170. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363–72. [DOI] [PubMed] [Google Scholar]
- 171. Bovill EG, Terrin ML, Stump DC, Berke AD, Frederick M, Collen D et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI), Phase II Trial. Ann Intern Med 1991;115:256–65. [DOI] [PubMed] [Google Scholar]
- 172. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 173.GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993;329:673–82. [DOI] [PubMed] [Google Scholar]
- 174. Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW et al. 2020 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:594–622. [DOI] [PubMed] [Google Scholar]
- 175. Lee SB, Manno EM, Layton KF, Wijdicks EF. Progression of warfarin-associated intracerebral hemorrhage after INR normalization with FFP. Neurology 2006;67:1272–4. [DOI] [PubMed] [Google Scholar]
- 176. Dowlatshahi D, Butcher KS, Asdaghi N, Nahirniak S, Bernbaum ML, Giulivi A et al. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke 2012;43:1812–7. [DOI] [PubMed] [Google Scholar]
- 177. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 178. Cuker A, Siegal D. Monitoring and reversal of direct oral anticoagulants. Hematology Am Soc Hematol Educ Program 2015;2015:117–24. [DOI] [PubMed] [Google Scholar]
- 179. Connolly SJ, Milling TJ, Eikelboom JW, Gibson CM, Curnutte JT, Gold A et al. ; ANNEXA-4 Investigators. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med 2016;375:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Enriquez A, Lip GY, Baranchuk A. Anticoagulation reversal in the era of the non-vitamin K oral anticoagulants. Europace 2016;18:955–64. [DOI] [PubMed] [Google Scholar]
- 181. Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA et al. Idarucizumab for dabigatran reversal—full cohort analysis. N Engl J Med 2017;377:431–41. [DOI] [PubMed] [Google Scholar]
- 182. Fanikos J, Murwin D, Gruenenfelder F, Tartakovsky I, Franca LR, Reilly PA et al. Global use of idarucizumab in clinical practice: outcomes of the RE-VECTO Surveillance Program. Thromb Haemost 2020;120:27–35. [DOI] [PubMed] [Google Scholar]
- 183.European Medicines Agency ISoPC. https://www.ema.europa.eu/en/documents/product-information/praxbind-epar-product-information_en.pdf (31 October 2020, date last accessed).
- 184. Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010;49:259–68. [DOI] [PubMed] [Google Scholar]
- 185. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH et al. ; ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019;380:1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Beyer-Westendorf J, Forster K, Pannach S, Ebertz F, Gelbricht V, Thieme C et al. Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood 2014;124:955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012;126:343–8. [DOI] [PubMed] [Google Scholar]
- 188. Zhou W, Schwarting S, Illanes S, Liesz A, Middelhoff M, Zorn M et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 2011;42:3594–9. [DOI] [PubMed] [Google Scholar]
- 189. Pragst I, Zeitler SH, Doerr B, Kaspereit FJ, Herzog E, Dickneite G et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost 2012;10:1841–8. [DOI] [PubMed] [Google Scholar]
- 190. Godier A, Miclot A, Le Bonniec B, Durand M, Fischer AM, Emmerich J et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology 2012;116:94–102. [DOI] [PubMed] [Google Scholar]
- 191. Zahir H, Brown KS, Vandell AG, Desai M, Maa JF, Dishy V et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation 2015;131:82–90. [DOI] [PubMed] [Google Scholar]
- 192. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9. [DOI] [PubMed] [Google Scholar]
- 193. Song Y, Wang Z, Perlstein I, Wang J, LaCreta F, Frost RJA et al. Reversal of apixaban anticoagulation by four-factor prothrombin complex concentrates in healthy subjects: a randomized three-period crossover study. J Thromb Haemost 2017;15:2125–37. [DOI] [PubMed] [Google Scholar]
- 194. Levi M, Moore KT, Castillejos CF, Kubitza D, Berkowitz SD, Goldhaber SZ et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost 2014;12:1428–36. [DOI] [PubMed] [Google Scholar]
- 195. Albaladejo P, Samama CM, Sie P, Kauffmann S, Memier V, Suchon P et al. ; GIHP-NACO Study Group. Management of severe bleeding in patients treated with direct oral anticoagulants: an observational registry analysis. Anesthesiology 2017;127:111–20. [DOI] [PubMed] [Google Scholar]
- 196. Majeed A, Agren A, Holmstrom M, Bruzelius M, Chaireti R, Odeberg J et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood 2017;130:1706–12. [DOI] [PubMed] [Google Scholar]
- 197. Schulman S, Gross PL, Ritchie B, Nahirniak S, Lin Y, Lieberman L et al. ; Study Investigators. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost 2018;118:842–51. [DOI] [PubMed] [Google Scholar]
- 198. Xu Y, Schulman S, Dowlatshahi D, Holbrook AM, Simpson CS, Shepherd LE et al. Direct oral anticoagulant- or warfarin-related major bleeding: characteristics, reversal strategies, and outcomes from a multicenter observational study. Chest 2017;152:81–91. [DOI] [PubMed] [Google Scholar]
- 199. Zada I, Wang S, Akerman M, Hanna A. Four-factor prothrombin complex concentrate for the reversal of direct oral anticoagulants. J Intensive Care Med 2021;36:58–62. [DOI] [PubMed] [Google Scholar]
- 200. Gerner ST, Kuramatsu JB, Sembill JA, Sprugel MI, Endres M, Haeusler KG et al. ; for the RETRACE II (German-Wide Multicenter Analysis of Oral Anticoagulation-Associated Intracerebral Hemorrhage II) Investigators. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol 2018;83:186–96. [DOI] [PubMed] [Google Scholar]
- 201. Frontera JA, Bhatt P, Lalchan R, Yaghi S, Ahuja T, Papadopoulos J et al. Cost comparison of andexanet versus prothrombin complex concentrates for direct factor Xa inhibitor reversal after hemorrhage. J Thromb Thrombolysis 2020;49:121–31. [DOI] [PubMed] [Google Scholar]
- 202. Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012;119:2172–4. [DOI] [PubMed] [Google Scholar]
- 203. Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW et al. 2017 ACC Expert Consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2017;70:3042–67. [DOI] [PubMed] [Google Scholar]
- 204. Kubitza D, Becka M, Roth A, Mueck W. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008;24:2757–65. [DOI] [PubMed] [Google Scholar]
- 205. Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost 2012;107:838–47. [DOI] [PubMed] [Google Scholar]
- 206. Green R, Grierson R, Sitar DS, Tenenbein M. How long after drug ingestion is activated charcoal still effective? J Toxicol Clin Toxicol 2001;39:601–5. [DOI] [PubMed] [Google Scholar]
- 207.The NCEPOD Classification of Intervention. https://www.ncepod.org.uk/classification.html(last accessed March 25th 2021).
- 208. Douketis JD, Spyropoulos AC, Duncan J, Carrier M, Le Gal G, Tafur AJ et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med 2019;179:1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Colonna P, von Heymann C, Santamaria A, Saxena M, Vanassche T, Wolpert D et al. Routine clinical practice in the periprocedural management of edoxaban therapy is associated with low risk of bleeding and thromboembolic complications: the prospective, observational, and multinational EMIT-AF/VTE study. Clin Cardiol 2020;43:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Tripodi A. To measure or not to measure direct oral anticoagulants before surgery or invasive procedures: reply. J Thromb Haemost 2016;14:2559–61. [DOI] [PubMed] [Google Scholar]
- 211. Shaw JR, Li N, Vanassche T, Coppens M, Spyropoulos AC, Syed S et al. Predictors of preprocedural direct oral anticoagulant levels in patients having an elective surgery or procedure. Blood Adv 2020;4:3520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS et al. ; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015;373:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Garcia D, Alexander JH, Wallentin L, Wojdyla DM, Thomas L, Hanna M et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood 2014;124:3692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Sherwood MW, Douketis JD, Patel MR, Piccini JP, Hellkamp AS, Lokhnygina Y et al. ; ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014;129:1850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Beyer-Westendorf J, Gelbricht V, Forster K, Ebertz F, Kohler C, Werth S et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J 2014;35:1888–96. [DOI] [PubMed] [Google Scholar]
- 216. Douketis JD. Perioperative management of patients who are receiving warfarin therapy: an evidence-based and practical approach. Blood 2011;117:5044–9. [DOI] [PubMed] [Google Scholar]
- 217. Graham MM, Sessler DI, Parlow JL, Biccard BM, Guyatt G, Leslie K et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann Intern Med 2018;168:237–44. [DOI] [PubMed] [Google Scholar]
- 218. Albaladejo P, Marret E, Samama CM, Collet JP, Abhay K, Loutrel O et al. Non-cardiac surgery in patients with coronary stents: the RECO study. Heart 2011;97:1566–72. [DOI] [PubMed] [Google Scholar]
- 219. Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A et al. ; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med 2014;370:1494–503. [DOI] [PubMed] [Google Scholar]
- 220. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Haeusler KG, Kirchhof P, Endres M. Left atrial catheter ablation and ischemic stroke. Stroke 2012;43:265–70. [DOI] [PubMed] [Google Scholar]
- 222. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation 2014;129:2638–44. [DOI] [PubMed] [Google Scholar]
- 223. Kirchhof P, Haeusler KG, Blank B, De Bono J, Callans D, Elvan A et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J 2018;39:2942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med 2017;376:1627–36. [DOI] [PubMed] [Google Scholar]
- 225. Hohnloser SH, Camm J, Cappato R, Diener HC, Heidbuchel H, Mont L et al. Uninterrupted edoxaban vs. vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J 2019;40:3013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Cappato R, Marchlinski FE, Hohnloser SH, Naccarelli GV, Xiang J, Wilber DJ et al. Uninterrupted rivaroxaban vs. uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J 2015;36:1805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Steffel J. Non-vitamin K antagonist oral anticoagulants therapy for atrial fibrillation patients undergoing electrophysiologic procedures. Eur Heart J Suppl 2020;22:I32–I7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ge Z, Faggioni M, Baber U, Sartori S, Sorrentino S, Farhan S et al. Safety and efficacy of nonvitamin K antagonist oral anticoagulants during catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Cardiovasc Ther 2018;36:e12457. [DOI] [PubMed] [Google Scholar]
- 229. Sticherling C, Marin F, Birnie D, Boriani G, Calkins H, Dan GA et al. Antithrombotic management in patients undergoing electrophysiological procedures: a European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Europace 2015;17:1197–214. [DOI] [PubMed] [Google Scholar]
- 230. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace 2018;20:157–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Torn M, Rosendaal FR. Oral anticoagulation in surgical procedures: risks and recommendations. Br J Haematol 2003;123:676–82. [DOI] [PubMed] [Google Scholar]
- 232. Bassiouny M, Saliba W, Rickard J, Shao M, Sey A, Diab M et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Di Biase L, Lakkireddy D, Trivedi C, Deneke T, Martinek M, Mohanty S et al. Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm 2015;12:1162–8. [DOI] [PubMed] [Google Scholar]
- 234. Dincq AS, Lessire S, Chatelain B, Gourdin M, Dogne JM, Mullier F et al. Impact of the direct oral anticoagulants on activated clotting time. J Cardiothorac Vasc Anesth 2017;31:e24–7. [DOI] [PubMed] [Google Scholar]
- 235. Sousa-Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:5–33. [DOI] [PubMed] [Google Scholar]
- 236. Eche IM, Elsamadisi P, Wex N, Wyers MC, Brat GA, Cunningham K et al. Intraoperative unfractionated heparin unresponsiveness during endovascular repair of a ruptured abdominal aortic aneurysm following administration of andexanet alfa for the reversal of rivaroxaban. Pharmacotherapy 2019;39:861–5. [DOI] [PubMed] [Google Scholar]
- 237. Levy JH, Spyropoulos AC, Samama CM, Douketis J. Direct oral anticoagulants: new drugs and new concepts. JACC Cardiovasc Interv 2014;7:1333–51. [DOI] [PubMed] [Google Scholar]
- 238. Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME et al. ; CTSN. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med 2016;374:1911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lin MH, Kamel H, Singer DE, Wu YL, Lee M, Ovbiagele B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke 2019;50:1364–71. [DOI] [PubMed] [Google Scholar]
- 240. Verma S, Goodman SG, Mehta SR, Latter DA, Ruel M, Gupta M et al. Should dual antiplatelet therapy be used in patients following coronary artery bypass surgery? A meta-analysis of randomized controlled trials. BMC Surg 2015;15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Deo SV, Dunlay SM, Shah IK, Altarabsheh SE, Erwin PJ, Boilson BA et al. Dual anti-platelet therapy after coronary artery bypass grafting: is there any benefit? A systematic review and meta-analysis. J Card Surg 2013;28:109–16. [DOI] [PubMed] [Google Scholar]
- 242. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A et al. ; ESC Scientific Document Group. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg 2018;53:34–78. [DOI] [PubMed] [Google Scholar]
- 243. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C et al. ; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. [DOI] [PubMed] [Google Scholar]
- 244. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–24. [DOI] [PubMed] [Google Scholar]
- 245. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019;394:1335–43. [DOI] [PubMed] [Google Scholar]
- 246. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–34. [DOI] [PubMed] [Google Scholar]
- 247. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. [DOI] [PubMed] [Google Scholar]
- 248. Windecker S, Lopes RD, Massaro T, Jones-Burton C, Granger CB, Aronson R et al. ; AUGUSTUS Investigators. Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome treated medically or with percutaneous coronary intervention or undergoing elective percutaneous coronary intervention: insights from the AUGUSTUS Trial. Circulation 2019;140:1921–32. [DOI] [PubMed] [Google Scholar]
- 249. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP et al. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol 2020;5:582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–67. [DOI] [PubMed] [Google Scholar]
- 251. Khan SU, Osman M, Khan MU, Khan MS, Zhao D, Mamas MA et al. Dual versus triple therapy for atrial fibrillation after percutaneous coronary intervention: a systematic review and meta-analysis. Ann Intern Med 2020;172:474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Potpara TS, Mujovic N, Proietti M, Dagres N, Hindricks G, Collet JP et al. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace 2020;22:33–46. [DOI] [PubMed] [Google Scholar]
- 253. Gargiulo G, Cannon CP, Gibson CM, Goette A, Lopes RD, Oldgren J et al. Safety and efficacy of double versus triple antithrombotic therapy in patients with atrial fibrillation with or without acute coronary syndrome undergoing percutaneous coronary intervention: a collaborative meta-analysis of NOAC-based randomized clinical trials. Eur Heart J Cardiovasc Pharmacother. 2020 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020. Online ahead of print [Google Scholar]
- 255. Alexander JH, Wojdyla D, Vora AN, Thomas L, Granger CB, Goodman SG et al. Risk/benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation 2020;141:1618–27. [DOI] [PubMed] [Google Scholar]
- 256. Oldgren J, Steg PG, Hohnloser SH, Lip GYH, Kimura T, Nordaby M et al. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: a subgroup analysis from the RE-DUAL PCI trial. Eur Heart J 2019;40:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T et al. ; TROPICAL-ACS Investigators. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet 2017;390:1747–57. [DOI] [PubMed] [Google Scholar]
- 258. Lee SR, Rhee TM, Kang DY, Choi EK, Oh S, Lip GYH. Meta-analysis of oral anticoagulant monotherapy as an antithrombotic strategy in patients with stable coronary artery disease and nonvalvular atrial fibrillation. Am J Cardiol 2019;124:879–85. [DOI] [PubMed] [Google Scholar]
- 259. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019;381:1103–13. [DOI] [PubMed] [Google Scholar]
- 260. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrie D, Naber C et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015;373:2038–47. [DOI] [PubMed] [Google Scholar]
- 261. Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R et al. ; ONYX ONE Investigators. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med 2020;382:1208–18. [DOI] [PubMed] [Google Scholar]
- 262. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrie D et al. ; SENIOR investigators. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 263. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. [DOI] [PubMed] [Google Scholar]
- 265. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 266. Robinson AA, Trankle CR, Eubanks G, Schumann C, Thompson P, Wallace RL et al. Off-label use of direct oral anticoagulants compared with warfarin for left ventricular thrombi. JAMA Cardiol 2020;5:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kajy M, Shokr M, Ramappa P. Use of direct oral anticoagulants in the treatment of left ventricular thrombus: systematic review of current literature. Am J Ther 2020;27:e584–90. [DOI] [PubMed] [Google Scholar]
- 268. Ali Z, Isom N, Dalia T, Sami F, Mahmood U, Shah Z et al. Direct oral anticoagulant use in left ventricular thrombus. Thromb J 2020;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Dalia T, Lahan S, Ranka S, Goyal A, Zoubek S, Gupta K et al. Warfarin versus direct oral anticoagulants for treating left ventricular thrombus: a systematic review and meta-analysis. Thromb J 2021;19(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Lip GY, Hammerstingl C, Marin F, Cappato R, Meng IL, Kirsch B et al. ; X-TRA study and CLOT-AF registry investigators. Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J 2016;178:126–34. [DOI] [PubMed] [Google Scholar]
- 271. Hao L, Zhong JQ, Zhang W, Rong B, Xie F, Wang JT et al. Uninterrupted dabigatran versus warfarin in the treatment of intracardiac thrombus in patients with non-valvular atrial fibrillation. Int J Cardiol 2015;190:63–6. [DOI] [PubMed] [Google Scholar]
- 272. Ezekowitz MD, Pollack CV Jr, Halperin JL, England RD, VanPelt Nguyen S, Spahr J et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J 2018;39:2959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Lee WC, Fang CY, Chen YL, Fang HY, Chen HC, Liu WH et al. Left Atrial or Left Atrial appendage thrombus resolution after adjustment of oral anticoagulant treatment. J Stroke Cerebrovasc Dis 2019;28:90–6. [DOI] [PubMed] [Google Scholar]
- 274. Niku AD, Shiota T, Siegel RJ, Rader F. Prevalence and resolution of left atrial thrombus in patients with nonvalvular atrial fibrillation and flutter with oral anticoagulation. Am J Cardiol 2019;123:63–8. [DOI] [PubMed] [Google Scholar]
- 275. Yang Y, Du X, Dong J, Ma C. Outcome of anticoagulation therapy of left atrial thrombus or sludge in patients with nonvalvular atrial fibrillation or flutter. Am J Med Sci 2019;358:273–8. [DOI] [PubMed] [Google Scholar]
- 276. Lee OH, Kim JS, Pak HN, Hong GR, Shim CY, Uhm JS et al. Feasibility of left atrial appendage occlusion for left atrial appendage thrombus in patients with persistent atrial fibrillation. Am J Cardiol 2018;121:1534–9. [DOI] [PubMed] [Google Scholar]
- 277. Macha K, Marsch A, Siedler G, Breuer L, Strasser EF, Engelhorn T et al. Cerebral ischemia in patients on direct oral anticoagulants. Stroke 2019;50:873–9. [DOI] [PubMed] [Google Scholar]
- 278. Hellwig S, Grittner U, Audebert H, Endres M, Haeusler KG. Non-vitamin K-dependent oral anticoagulants have a positive impact on ischaemic stroke severity in patients with atrial fibrillation. Europace 2018;20:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Meinel TR, Frey S, Arnold M, Kendroud S, Fischer U, Kaesmacher J et al. Clinical presentation, diagnostic findings and management of cerebral ischemic events in patients on treatment with non-vitamin K antagonist oral anticoagulants—a systematic review. PloS One 2019;14:e0213379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Klijn CJ, Paciaroni M, Berge E, Korompoki E, Korv J, Lal A et al. Antithrombotic treatment for secondary prevention of stroke and other thromboembolic events in patients with stroke or transient ischemic attack and non-valvular atrial fibrillation: a European Stroke Organisation guideline. Eur Stroke J 2019;4:198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Nguyen NY, Frishman WH. Restarting oral anticoagulation in patients with atrial fibrillation after an intracranial hemorrhage. Cardiol Rev 2020;28:190–6. [DOI] [PubMed] [Google Scholar]
- 282. Giugliano RP, Ruff CT, Rost NS, Silverman S, Wiviott SD, Lowe C et al. Cerebrovascular events in 21 105 patients with atrial fibrillation randomized to edoxaban versus warfarin: effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48. Stroke 2014;45:2372–8. [DOI] [PubMed] [Google Scholar]
- 283. Lopes RD, Guimaraes PO, Kolls BJ, Wojdyla DM, Bushnell CD, Hanna M et al. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation therapy. Blood 2017;129:2980–7. [DOI] [PubMed] [Google Scholar]
- 284. Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- 285. Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE et al. Association of intracerebral hemorrhage among patients taking non-vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA 2018;319:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Kurogi R, Nishimura K, Nakai M, Kada A, Kamitani S, Nakagawara J et al. ; J-ASPECT Study Collaborators. Comparing intracerebral hemorrhages associated with direct oral anticoagulants or warfarin. Neurology 2018;90:e1143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Katsi V, Georgiopoulos G, Skafida A, Oikonomou D, Klettas D, Vemmos K et al. Noncardioembolic stroke in patients with atrial fibrillation. Angiology 2019;70:299–304. [DOI] [PubMed] [Google Scholar]
- 288. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the early management of acute ischemic stroke: a guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418. [DOI] [PubMed] [Google Scholar]
- 289. Mizoguchi T, Tanaka K, Toyoda K, Yoshimura S, Itabashi R, Takagi M et al. ; SAMURAI Study Investigators. Early initiation of direct oral anticoagulants after onset of stroke and short- and long-term outcomes of patients with nonvalvular atrial fibrillation. Stroke 2020;51:883–91. [DOI] [PubMed] [Google Scholar]
- 290. Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ et al. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol 2019;18:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Escudero-Martinez I, Mazya M, Teutsch C, Lesko N, Gdovinova Z, Barbarini L et al. ; SITS Investigators. Dabigatran initiation in patients with non-valvular AF and first acute ischaemic stroke: a retrospective observational study from the SITS registry. BMJ Open 2020;10:e037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M et al. ; on behalf of the CROMIS-2, RAF, RAF-DOAC, SAMURAI, NOACISP LONGTERM, Erlangen and Verona registry orators. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol 2019;85:823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Ahmed N, Audebert H, Turc G, Cordonnier C, Christensen H, Sacco S et al. Consensus statements and recommendations from the ESO-Karolinska Stroke Update Conference, Stockholm 11-13 November 2018. Eur Stroke J 2019;4:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol 2018;17:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Xian Y, Xu H, O’Brien EC, Shah S, Thomas L, Pencina MJ et al. Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. JAMA Neurol 2019;76:1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Wang Z, Korantzopoulos P, Liu T. Carotid atherosclerosis in patients with atrial fibrillation. Curr Atheroscler Rep 2019;21:55. [DOI] [PubMed] [Google Scholar]
- 297. Hawkes MA, Rabinstein AA. Anticoagulation for atrial fibrillation after intracranial hemorrhage: a systematic review. Neurol Clin Pract 2018;8:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Chao TF, Liu CJ, Liao JN, Wang KL, Lin YJ, Chang SL et al. Use of oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation 2016;133:1540–7. [DOI] [PubMed] [Google Scholar]
- 299. Lee SR, Choi EK, Kwon S, Jung JH, Han KD, Cha MJ et al. Oral anticoagulation in Asian patients with atrial fibrillation and a history of intracranial hemorrhage. Stroke 2020;51:416–23. [DOI] [PubMed] [Google Scholar]
- 300. Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GYH, Larsen TB. Non-vitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with intracerebral hemorrhage. Stroke 2019;50:939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Tsai CT, Liao JN, Chiang CE, Lin YJ, Chang SL, Lo LW et al. Association of ischemic stroke, major bleeding, and other adverse events with warfarin use vs non-vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation with a history of intracranial hemorrhage. JAMA Netw Open 2020;3:e206424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Biffi A, Urday S, Kubiszewski P, Gilkerson L, Sekar P, Rodriguez-Torres A et al. Combining imaging and genetics to predict recurrence of anticoagulation-associated intracerebral hemorrhage. Stroke 2020;51:2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Chen YC, Chang KH, Chen CM. Genetic polymorphisms associated with spontaneous intracerebral hemorrhage. Int J Mol Sci 2018;19:3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Falcone GJ, Kirsch E, Acosta JN, Noche RB, Leasure A, Marini S et al. ; For the International Stroke Genetics Consortium. Genetically elevated LDL associates with lower risk of intracerebral hemorrhage. Ann Neurol 2020;88:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Cannistraro RJ, Meschia JF. The clinical dilemma of anticoagulation use in patients with cerebral amyloid angiopathy and atrial fibrillation. Curr Cardiol Rep 2018;20:106. [DOI] [PubMed] [Google Scholar]
- 306. Pappas MA, Burke JF. Net clinical benefit of anticoagulation for atrial fibrillation following intracerebral hemorrhage. Vasc Med 2020;25:55–9. [DOI] [PubMed] [Google Scholar]
- 307. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P et al. ; PRAGUE-17 Trial Investigators. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. [DOI] [PubMed] [Google Scholar]
- 308. Lu VM, Phan K, Rao PJ, Sharma SV, Kasper EM. Dabigatran reversal by idarucizumab in the setting of intracranial hemorrhage: a systematic review of the literature. Clin Neurol Neurosurg 2019;181:76–81. [DOI] [PubMed] [Google Scholar]
- 309. Murthy SB, Wu X, Diaz I, Parasram M, Parikh NS, Iadecola C et al. Non-traumatic subdural hemorrhage and risk of arterial ischemic events. Stroke 2020;51:1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J 2008;156:57–64. [DOI] [PubMed] [Google Scholar]
- 311. 2019 UNDoEaSADWpp. https://population.un.org/wpp/Download/Probabilistic/Population/ (29 August 2020, date last accessed).
- 312. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 313. Fohtung RB, Novak E, Rich MW. Effect of new oral anticoagulants on prescribing practices for atrial fibrillation in older adults. J Am Geriatr Soc 2017;65:2405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Henrard S, Vandenabeele C, Marien S, Boland B, Dalleur O. Underuse of anticoagulation in older patients with atrial fibrillation and CHADS2 score >/= 2: are we doing better since the marketing of direct oral anticoagulants? Drugs Aging 2017;34:841–50. [DOI] [PubMed] [Google Scholar]
- 315. Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014;130:138–46. [DOI] [PubMed] [Google Scholar]
- 316. Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Lauw MN, Eikelboom JW, Coppens M, Wallentin L, Yusuf S, Ezekowitz M et al. Effects of dabigatran according to age in atrial fibrillation. Heart 2017;103:1015–23. [DOI] [PubMed] [Google Scholar]
- 318. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–64. [DOI] [PubMed] [Google Scholar]
- 319. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR et al. Stroke, bleeding, and mortality risks in elderly medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med 2016;176:1662–71. [DOI] [PubMed] [Google Scholar]
- 320. de Groot JR, Weiss TW, Kelly P, Monteiro P, Deharo JC, de Asmundis C et al. Edoxaban for stroke prevention in atrial fibrillation in routine clinical care: one year follow up of the prospective observational ETNA-AF-Europe study. Eur Heart J Cardiovasc Pharmacother 2020. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Bassand JP, Accetta G, Al Mahmeed W, Corbalan R, Eikelboom J, Fitzmaurice DA et al. ; GARFIELD-AF Investigators. Risk factors for death, stroke, and bleeding in 28,628 patients from the GARFIELD-AF registry: rationale for comprehensive management of atrial fibrillation. PLoS One 2018;13:e0191592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Kwon S, Lee SR, Choi EK, Choe WS, Lee E, Jung JH et al. Non-vitamin K antagonist oral anticoagulants in very elderly east Asians with atrial fibrillation: a nationwide population-based study. Am Heart J 2020;229:81–91. [DOI] [PubMed] [Google Scholar]
- 323. Bai Y, Guo SD, Deng H, Shantsila A, Fauchier L, Ma CS et al. Effectiveness and safety of oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-regression analysis. Age Ageing 2018;47:9. [DOI] [PubMed] [Google Scholar]
- 324. Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ et al. ; GLORIA-AF Investigators. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF Registry Phase 2. J Am Coll Cardiol 2017;69:777–85. [DOI] [PubMed] [Google Scholar]
- 325. Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation 2018;138:37–47. [DOI] [PubMed] [Google Scholar]
- 326. Chao TF, Chiang CE, Liao JN, Chen TJ, Lip GYH, Chen SA. Comparing the effectiveness and safety of nonvitamin K antagonist oral anticoagulants and warfarin in elderly Asian patients with atrial fibrillation: a nationwide cohort study. Chest 2020;157:1266–77. [DOI] [PubMed] [Google Scholar]
- 327. Fumagalli S, Potpara TS, Bjerregaard Larsen T, Haugaa KH, Dobreanu D, Proclemer A et al. Frailty syndrome: an emerging clinical problem in the everyday management of clinical arrhythmias. The results of the European Heart Rhythm Association survey. Europace 2017;19:1896–902. [DOI] [PubMed] [Google Scholar]
- 328. Pazan F, Collins R, Gil VM, Hanon O, Hardt R, Hoffmeister M et al. A structured literature review and international consensus validation of FORTA labels of oral anticoagulants for long-term treatment of atrial fibrillation in older patients (OAC-FORTA 2019). Drugs Aging 2020;37:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Shah SJ, Singer DE, Fang MC, Reynolds K, Go AS, Eckman MH. Net clinical benefit of oral anticoagulation among older adults with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2019;12:e006212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Madhavan M, Holmes DN, Piccini JP, Ansell JE, Fonarow GC, Hylek EM et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J 2019;211:77–89. [DOI] [PubMed] [Google Scholar]
- 331. Bassand JP, Virdone S, Goldhaber SZ, Camm AJ, Fitzmaurice DA, Fox KAA et al. Early risks of death, stroke/systemic embolism, and major bleeding in patients with newly diagnosed atrial fibrillation. Circulation 2019;139:787–98. [DOI] [PubMed] [Google Scholar]
- 332. Okumura K, Lip GYH, Akao M, Tanizawa K, Fukuzawa M, Abe K et al. Edoxaban for the management of elderly Japanese patients with atrial fibrillation ineligible for standard oral anticoagulant therapies: rationale and design of the ELDERCARE-AF study. Am Heart J 2017;194:99–106. [DOI] [PubMed] [Google Scholar]
- 333. An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 2017;19:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Purrucker JC, Wolf M, Haas K, Siedler T, Rizos T, Khan S et al. ; the RASUNOA Investigators. Microbleeds in ischemic vs hemorrhagic strokes on novel oral anticoagulants. Acta Neurol Scand 2018;138:163–9. [DOI] [PubMed] [Google Scholar]
- 335. Soo Y, Abrigo J, Leung KT, Liu W, Lam B, Tsang SF et al. Correlation of non-vitamin K antagonist oral anticoagulant exposure and cerebral microbleeds in Chinese patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2018;89:680–6. [DOI] [PubMed] [Google Scholar]
- 336. Lioutas VA, Goyal N, Katsanos AH, Krogias C, Zand R, Sharma VK et al. Microbleed prevalence and burden in anticoagulant-associated intracerebral bleed. Ann Clin Transl Neurol 2019;6:1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 338. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet 1999;353:205–6. [DOI] [PubMed] [Google Scholar]
- 339. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al. A global clinical measure of fitness and frailty in elderly people. Cmaj 2005;173:489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Hanon O, Assayag P, Belmin J, Collet JP, Emeriau JP, Fauchier L et al. ; French Society of Cardiology. Expert consensus of the French Society of Geriatrics and Gerontology and the French Society of Cardiology on the management of atrial fibrillation in elderly people. Arch Cardiovasc Dis 2013;106:303–23. [DOI] [PubMed] [Google Scholar]
- 341. Bhangu J, King-Kallimanis BL, Donoghue OA, Carroll L, Kenny RA. Falls, non-accidental falls and syncope in community-dwelling adults aged 50 years and older: implications for cardiovascular assessment. PLoS One 2017;12:e0180997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 2006;35(Suppl 2):ii37–41. [DOI] [PubMed] [Google Scholar]
- 343. Hylek EM, D'Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke 2006;37:1075–80. [DOI] [PubMed] [Google Scholar]
- 344. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999;159:677–85. [DOI] [PubMed] [Google Scholar]
- 345. Rao MP, Vinereanu D, Wojdyla DM, Alexander JH, Atar D, Hylek EM, et al. Clinical outcomes and history of fall in patients with atrial fibrillation treated with oral anticoagulation: insights from the ARISTOTLE trial. Am J Med 2018; 131: 269–75e2. [DOI] [PubMed] [Google Scholar]
- 346. Connolly BJ, Pearce LA, Hart RG. Vitamin K antagonists and risk of subdural hematoma: meta-analysis of randomized clinical trials. Stroke 2014;45:1672–8. [DOI] [PubMed] [Google Scholar]
- 347. Scotti P, Seguin C, Lo BWY, de Guise E, Troquet JM, Marcoux J. Antithrombotic agents and traumatic brain injury in the elderly population: hemorrhage patterns and outcomes. J Neurosurg 2019 Jul 5;1-10. [DOI] [PubMed] [Google Scholar]
- 348. Feeney JM, Santone E, DiFiori M, Kis L, Jayaraman V, Montgomery SC. Compared to warfarin, direct oral anticoagulants are associated with lower mortality in patients with blunt traumatic intracranial hemorrhage: a TQIP study. J Trauma Acute Care Surg 2016;81:843–8. [DOI] [PubMed] [Google Scholar]
- 349. Cocca AT, Privette A, Leon SM, Crookes BA, Hall G, Lena J et al. Delayed intracranial hemorrhage in anticoagulated geriatric patients after ground level falls. J Emerg Med 2019;57:812–6. [DOI] [PubMed] [Google Scholar]
- 350. Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med 1994;331:821–7. [DOI] [PubMed] [Google Scholar]
- 351. Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014;30:421–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Dagres N, Chao TF, Fenelon G, Aguinaga L, Benhayon D, Benjamin EJ et al. ; ESC Scientific Document Group. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: what is the best practice? Europace 2018;20:1399–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Friberg L, Andersson T, Rosenqvist M. Less dementia and stroke in low-risk patients with atrial fibrillation taking oral anticoagulation. Eur Heart J 2019;40:2327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Singh-Manoux A, Fayosse A, Sabia S, Canonico M, Bobak M, Elbaz A et al. Atrial fibrillation as a risk factor for cognitive decline and dementia. Eur Heart J 2017;38:2612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019;40:2313–23. [DOI] [PubMed] [Google Scholar]
- 356. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS et al. Association of anticoagulant therapy with risk of dementia among patients with atrial fibrillation. Europace 2021;23:184–95. [DOI] [PubMed] [Google Scholar]
- 357. Søgaard M, Skjøth F, Jensen M, Kjældgaard JN, Lip GYH, Larsen TB et al. Nonvitamin K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients and risk of dementia: a nationwide propensity-weighted cohort study. J Am Heart Assoc 2019;8:e011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Mongkhon P, Fanning L, Lau WCY, Tse G, Lau KK, Wei L et al. Oral anticoagulant and reduced risk of dementia in patients with atrial fibrillation: a population-based cohort study. Heart Rhythm 2020;17:706–13. [DOI] [PubMed] [Google Scholar]
- 359. Bunch TJ, May HT, Bair TL, Crandall BG, Cutler MJ, Day JD et al. Atrial fibrillation patients treated with long-term warfarin anticoagulation have higher rates of all dementia types compared with patients receiving long-term warfarin for other indications. JAHA 2016;5:e003932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Garcia-Ptacek S, Contreras Escamez B, Zupanic E, Religa D, von Koch L, Johnell K et al. Prestroke mobility and dementia as predictors of stroke outcomes in patients over 65 years of age: a cohort study from the Swedish Dementia and Stroke Registries. J Am Med Dir Assoc 2018;19:154–61. [DOI] [PubMed] [Google Scholar]
- 361. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res 2017;120:573–91. [DOI] [PubMed] [Google Scholar]
- 362. Emren SV, Şenöz O, Bilgin M, Beton O, Aslan A, Taşkin U et al. Drug adherence in patients with nonvalvular atrial fibrillation taking non-vitamin K antagonist oral anticoagulants in Turkey: NOAC-TR. Clin Appl Thromb Hemost 2018;24:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. El-Saifi N, Moyle W, Jones C, Alston-Knox C. Determinants of medication adherence in older people with dementia from the caregivers' perspective. Int Psychogeriatr 2019;31:331–9. [DOI] [PubMed] [Google Scholar]
- 364. Goodman-Casanova JM, Guzman-Parra J, Guerrero G, Vera E, Barnestein-Fonseca P, Cortellessa G et al. TV-based assistive integrated service to support European adults living with mild dementia or mild cognitive impairment (TV-AssistDem): study protocol for a multicentre randomized controlled trial. BMC Geriatr 2019;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.World Health Organization. Obesity and Overweight Fact Sheet. http://www.who.int/mediacentre/factsheets/fs311/en/ (January 2021, date last accessed).
- 366. Wang TJ, Parise H, Levy D, D'Agostino RB Sr., Wolf PA, Vasan RS et al. Obesity and the risk of new-onset atrial fibrillation. JAMA 2004;292:2471–7. [DOI] [PubMed] [Google Scholar]
- 367. Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 368. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol 2017;70:2022–35. [DOI] [PubMed] [Google Scholar]
- 369. Sivasambu B, Balouch MA, Zghaib T, Bajwa RJ, Chrispin J, Berger RD et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol 2018;29:239–45. [DOI] [PubMed] [Google Scholar]
- 370. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. Jama 2013;310:2050–60. [DOI] [PubMed] [Google Scholar]
- 371. Wang SY, Giugliano RP. Non-vitamin K antagonist oral anticoagulant for atrial fibrillation in obese patients. Am J Cardiol 2020;127:176–83. [DOI] [PubMed] [Google Scholar]
- 372. Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol 2000;278:F817–22. [DOI] [PubMed] [Google Scholar]
- 373. Wallace JL, Reaves AB, Tolley EA, Oliphant CS, Hutchison L, Alabdan NA et al. Comparison of initial warfarin response in obese patients versus non-obese patients. J Thromb Thrombolysis 2013;36:96–101. [DOI] [PubMed] [Google Scholar]
- 374. Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet 2008;47:47–59. [DOI] [PubMed] [Google Scholar]
- 375. Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost 2011;9:2168–75. [DOI] [PubMed] [Google Scholar]
- 376. Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet 2011;50:675–86. [DOI] [PubMed] [Google Scholar]
- 378. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol 2007;47:218–26. [DOI] [PubMed] [Google Scholar]
- 379. Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol 2013;76:908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Barsam SJ, Patel JP, Roberts LN, Kavarthapu V, Patel RK, Green B et al. The impact of body weight on rivaroxaban pharmacokinetics. Res Pract Thromb Haemost 2017;1:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Boriani G, Ruff CT, Kuder JF, Shi M, Lanz HJ, Rutman H et al. Relationship between body mass index and outcomes in patients with atrial fibrillation treated with edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. Eur Heart J 2019;40:1541–50. [DOI] [PubMed] [Google Scholar]
- 382. Boriani G, Ruff CT, Kuder JF, Shi M, Lanz HJ, Antman EM et al. Edoxaban versus warfarin in patients with atrial fibrillation at the extremes of body weight: an analysis from the ENGAGE AF-TIMI 48 trial. Thromb Haemost 2021;121:140–9. [DOI] [PubMed] [Google Scholar]
- 383. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost 2016;14:1308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis 2016;41:206–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Proietti M, Guiducci E, Cheli P, Lip GY. Is there an obesity paradox for outcomes in atrial fibrillation? A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant trials. Stroke 2017;48:857–66. [DOI] [PubMed] [Google Scholar]
- 386. Breuer L, Ringwald J, Schwab S, Kohrmann M. Ischemic stroke in an obese patient receiving dabigatran. N Engl J Med 2013;368:2440–2. [DOI] [PubMed] [Google Scholar]
- 387. Safouris A, Demulder A, Triantafyllou N, Tsivgoulis G. Rivaroxaban presents a better pharmacokinetic profile than dabigatran in an obese non-diabetic stroke patient. J Neurol Sci 2014;346:366–7. [DOI] [PubMed] [Google Scholar]
- 388. Sandhu RK, Ezekowitz J, Andersson U, Alexander JH, Granger CB, Halvorsen S et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J 2016;37:2869–78. [DOI] [PubMed] [Google Scholar]
- 389. Hohnloser SH, Fudim M, Alexander JH, Wojdyla DM, Ezekowitz JA, Hanna M et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight. Circulation 2019;139:2292–300. [DOI] [PubMed] [Google Scholar]
- 390. Balla SR, Cyr DD, Lokhnygina Y, Becker RC, Berkowitz SD, Breithardt G et al. Relation of risk of stroke in patients with atrial fibrillation to body mass index (from patients treated with rivaroxaban and warfarin in the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation trial). Am J Cardiol 2017;119:1989–96. [DOI] [PubMed] [Google Scholar]
- 391. Tittl L, Endig S, Marten S, Reitter A, Beyer-Westendorf I, Beyer-Westendorf J. Impact of BMI on clinical outcomes of NOAC therapy in daily care—results of the prospective Dresden NOAC Registry (NCT01588119). Int J Cardiol 2018;262:85–91. [DOI] [PubMed] [Google Scholar]
- 392. Malik AH, Yandrapalli S, Shetty S, Aronow WS, Jain D, Frishman WH et al. ; MAGIC (Meta-analysis And oriGinal Investigations in Cardiology) Investigators. Impact of weight on the efficacy and safety of direct-acting oral anticoagulants in patients with non-valvular atrial fibrillation: a meta-analysis. Europace 2020;22:361–7. [DOI] [PubMed] [Google Scholar]
- 393. Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother 2019;53:165–70. [DOI] [PubMed] [Google Scholar]
- 394. Kushnir M, Choi Y, Eisenberg R, Rao D, Tolu S, Gao J et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single-centre, retrospective analysis of chart data. Lancet Haematol 2019;6:e359–65. [DOI] [PubMed] [Google Scholar]
- 395. Martin KA, Lee CR, Farrell TM, Moll S. Oral anticoagulant use after bariatric surgery: a literature review and clinical guidance. Am J Med 2017;130:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 2009;48:1–22. [DOI] [PubMed] [Google Scholar]
- 397. Byon W, Nepal S, Schuster AE, Shenker A, Frost CE. Regional gastrointestinal absorption of apixaban in healthy subjects. J Clin Pharmacol 2018;58:965–71. [DOI] [PubMed] [Google Scholar]
- 398. Hakeam HA, Al-Sanea N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (DOACs). J Thromb Thrombolysis 2017;43:343–51. [DOI] [PubMed] [Google Scholar]
- 399. Chan LN. Warfarin dosing changes after bariatric surgery: implications on the mechanism for altered dose requirements and safety concerns–an alternative viewpoint. Pharmacotherapy 2014;34:e26–8. [DOI] [PubMed] [Google Scholar]
- 400. Bechtel P, Boorse R, Rovito P, Harrison TD, Hong J. Warfarin users prone to coagulopathy in first 30 days after hospital discharge from gastric bypass. Obes Surg 2013;23:1515–9. [DOI] [PubMed] [Google Scholar]
- 401. Schullo-Feulner AM, Stoecker Z, Brown GA, Schneider J, Jones TA, Burnett B. Warfarin dosing after bariatric surgery: a retrospective study of 10 patients previously stable on chronic warfarin therapy. Clin Obes 2014;4:108–15. [DOI] [PubMed] [Google Scholar]
- 402. Sobieraj DM, Wang F, Kirton OC. Warfarin resistance after total gastrectomy and Roux-en-Y esophagojejunostomy. Pharmacotherapy 2008;28:1537–41. [DOI] [PubMed] [Google Scholar]
- 403.European Medicines Agency DSoPC. https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf (26 August 2020, date last accessed).
- 404. Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H et al. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol 2005;45:555–63. [DOI] [PubMed] [Google Scholar]
- 405.European Medicines Agency RSoPC. https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-product-information_en.pdf (26 August 2020, date last accessed).
- 406. Rottenstreich A, Barkai A, Arad A, Raccah BH, Kalish Y. The effect of bariatric surgery on direct-acting oral anticoagulant drug levels. Thromb Res 2018;163:190–5. [DOI] [PubMed] [Google Scholar]
- 407. Parasrampuria DA, Kanamaru T, Connor A, Wilding I, Ogata K, Shimoto Y et al. Evaluation of regional gastrointestinal absorption of edoxaban using the enterion capsule. J Clin Pharmacol 2015;55:1286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.World Health Organization. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (10 August 2020, date last accessed).
- 409. Braekkan SK, van der Graaf Y, Visseren FL, Algra A. Obesity and risk of bleeding: the SMART study. J Thromb Haemost 2016;14:65–72. [DOI] [PubMed] [Google Scholar]
- 410. Park CS, Choi EK, Kim HM, Lee SR, Cha MJ, Oh S. Increased risk of major bleeding in underweight patients with atrial fibrillation who were prescribed non-vitamin K antagonist oral anticoagulants. Heart Rhythm 2017;14:501–7. [DOI] [PubMed] [Google Scholar]
- 411. Barba R, Marco J, Martin-Alvarez H, Rondon P, Fernandez-Capitan C, Garcia-Bragado F et al. ; for the RIETE Investigators. The influence of extreme body weight on clinical outcome of patients with venous thromboembolism: findings from a prospective registry (RIETE). J Thromb Haemost 2005;3:856–62. [DOI] [PubMed] [Google Scholar]
- 412. Lee CH, Lin TY, Chang SH, Chen CH, Hsu YJ, Hung KC et al. Body mass index is an independent predictor of major bleeding in non-valvular atrial fibrillation patients taking dabigatran. Int J Cardiol 2017;228:771–8. [DOI] [PubMed] [Google Scholar]
- 413. Lee SR, Choi EK, Park CS, Han KD, Jung JH, Oh S et al. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am Coll Cardiol 2019;73:919–31. [DOI] [PubMed] [Google Scholar]
- 414. De Caterina R, Lip GYH. The non-vitamin K antagonist oral anticoagulants (NOACs) and extremes of body weight-a systematic literature review. Clin Res Cardiol 2017;106:565–72. [DOI] [PubMed] [Google Scholar]
- 415. Myint PK, Staufenberg EF, Sabanathan K. Post-stroke seizure and post-stroke epilepsy. Postgrad Med J 2006;82:568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. BMJ 1997;315:1582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Zou S, Wu X, Zhu B, Yu J, Yang B, Shi J. The pooled incidence of post-stroke seizure in 102 008 patients. Top Stroke Rehabil 2015;22:460–7. [DOI] [PubMed] [Google Scholar]
- 418. Beghi E, D'Alessandro R, Beretta S, Consoli D, Crespi V, Delaj L et al. ; Epistroke Group. Incidence and predictors of acute symptomatic seizures after stroke. Neurology 2011;77:1785–93. [DOI] [PubMed] [Google Scholar]
- 419. Stefanidou M, Das RR, Beiser AS, Sundar B, Kelly-Hayes M, Kase CS et al. Incidence of seizures following initial ischemic stroke in a community-based cohort: the Framingham Heart Study. Seizure 2017;47:105–10. [DOI] [PubMed] [Google Scholar]
- 420. Wang JZ, Vyas MV, Saposnik G, Burneo JG. Incidence and management of seizures after ischemic stroke: systematic review and meta-analysis. Neurology 2017;89:1220–8. [DOI] [PubMed] [Google Scholar]
- 421. Holtkamp M, Beghi E, Benninger F, Kalviainen R, Rocamora R, Christensen H; European Stroke Organisation. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur Stroke J 2017;2:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. van Tuijl JH, van Raak EP, de Krom MC, Lodder J, Aldenkamp AP. Early treatment after stroke for the prevention of late epileptic seizures: a report on the problems performing a randomised placebo-controlled double-blind trial aimed at anti-epileptogenesis. Seizure 2011;20:285–91. [DOI] [PubMed] [Google Scholar]
- 423. Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia 2009;50:1102–8. [DOI] [PubMed] [Google Scholar]
- 424. Galovic M, Dohler N, Erdelyi-Canavese B, Felbecker A, Siebel P, Conrad J et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol 2018;17:143–52. [DOI] [PubMed] [Google Scholar]
- 425. Leung T, Leung H, Soo YO, Mok VC, Wong KS. The prognosis of acute symptomatic seizures after ischaemic stroke. J Neurol Neurosurg Psychiatry 2017;88:86–94. [DOI] [PubMed] [Google Scholar]
- 426. Stollberger C, Finsterer J. Interactions between non-vitamin K oral anticoagulants and antiepileptic drugs. Epilepsy Res 2016;126:98–101. [DOI] [PubMed] [Google Scholar]
- 427. de Biase S, Nilo A, Bernardini A, Gigli GL, Valente M, Merlino G. Timing use of novel anti-epileptic drugs: is earlier better? Expert Rev Neurother 2019;19:945–54. [DOI] [PubMed] [Google Scholar]
- 428. Manohar C, Avitsian R, Lozano S, Gonzalez-Martinez J, Cata JP. The effect of antiepileptic drugs on coagulation and bleeding in the perioperative period of epilepsy surgery: the Cleveland Clinic experience. J Clin Neurosci 2011;18:1180–4. [DOI] [PubMed] [Google Scholar]
- 429. Di Gennaro L, Lancellotti S, De Cristofaro R, De Candia E. Carbamazepine interaction with direct oral anticoagulants: help from the laboratory for the personalized management of oral anticoagulant therapy. J Thromb Thrombolysis 2019;48:528–31. [DOI] [PubMed] [Google Scholar]
- 430. Langenbruch L, Meuth SG, Wiendl H, Mesters R, Moddel G. Clinically relevant interaction of rivaroxaban and valproic acid—a case report. Seizure 2020;80:46–7. [DOI] [PubMed] [Google Scholar]
- 431. Taha M, Li W, Schmidt CM, Gonzalez-Castellon M, Taraschenko O. The interactions between anticonvulsants and non-vitamin K antagonist oral anticoagulant agents: a systematic review. Epilepsy Res 2020;162:106304. [DOI] [PubMed] [Google Scholar]
- 432. Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017;318:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Wang CL, Wu VC, Chang KH, Tu HT, Kuo CF, Huang YT et al. Assessing major bleeding risk in atrial fibrillation patients concurrently taking non-vitamin K antagonist oral anticoagulants and antiepileptic drugs. Eur Heart J Cardiovasc Pharmacother 2020;6:147–54. [DOI] [PubMed] [Google Scholar]
- 434. Acton EK, Willis AW, Gelfand MA, Kasner SE. Poor concordance among drug compendia for proposed interactions between enzyme-inducing antiepileptic drugs and direct oral anticoagulants. Pharmacoepidemiol Drug Saf 2019;28:1534–8. [DOI] [PubMed] [Google Scholar]
- 435. von Oertzen TJ, Trinka E, Bornstein NM. Levetiracetam and non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and epilepsy: a reasonable combination. Eur Heart J 2019;40:3800–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Mathy FX, Dohin E, Bonfitto F, Pelgrims B. Drug-drug interaction between levetiracetam and non-vitamin K antagonist anticoagulants. Eur Heart J 2019;40:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Potpara T, Steffel J. Strategies in patients with atrial fibrillation taking non-vitamin K antagonist oral anticoagulants (NOACs) and co-medications with possible drug-drug interaction. Eur Heart J 2019;40:3802. [DOI] [PubMed] [Google Scholar]
- 438. Steffel J, Potpara TS. Challenges in clinical decision-making on concomitant drug therapies in patients with atrial fibrillation taking oral anticoagulants. Eur Heart J 2019;40:1569–70. [DOI] [PubMed] [Google Scholar]
- 439. Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson ED et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol 2009;54:1280–9. [DOI] [PubMed] [Google Scholar]
- 440. Thomas KL, Piccini JP, Liang L, Fonarow GC, Yancy CW, Peterson ED et al. ; Tthe Get With the Guidelines Steering Committee and Hospitals. Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. JAHA 2013;2:e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT2R2 score. Chest 2013;144:1555–63. [DOI] [PubMed] [Google Scholar]
- 442. Corbalan R, Conejeros C, Rey C, Stockins B, Eggers G, Astudillo C et al. [Features, management and prognosis of Chilean patients with non valvular atrial fibrillation: GARFIELD AF registry]. Rev Med Chil 2017;145:963–71. [DOI] [PubMed] [Google Scholar]
- 443. Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke 2006;37:1070–4. [DOI] [PubMed] [Google Scholar]
- 444. Sholzberg M, Gomes T, Juurlink DN, Yao Z, Mamdani MM, Laupacis A. The influence of socioeconomic status on selection of anticoagulation for atrial fibrillation. PLoS One 2016;11:e0149142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/Ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke 2008;39:2736–43. [DOI] [PubMed] [Google Scholar]
- 446. Chao TF, Wang KL, Liu CJ, Lin YJ, Chang SL, Lo LW et al. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol 2015;66:1339–47. [DOI] [PubMed] [Google Scholar]
- 447. Chao TF, Lip GY, Liu CJ, Tuan TC, Chen SJ, Wang KL et al. Validation of a modified CHA2DS2-VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke 2016;47:2462–9. [DOI] [PubMed] [Google Scholar]
- 448. Kim TH, Yang PS, Yu HT, Jang E, Uhm JS, Kim JY et al. Age threshold for ischemic stroke risk in atrial fibrillation. Stroke 2018;49:1872–9. [DOI] [PubMed] [Google Scholar]
- 449. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50:309–15. [DOI] [PubMed] [Google Scholar]
- 450. Steffel J, Eikelboom JW. Stroke prevention in AF: of Asians and non-Asians. Eur Heart J 2019;40:1528–30. [DOI] [PubMed] [Google Scholar]
- 451. Byon W, Sweeney K, Frost C, Boyd RA. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism. CPT Pharmacometrics Syst Pharmacol 2017;6:340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Chao TF, Chen SA, Ruff CT, Hamershock RA, Mercuri MF, Antman EM et al. Clinical outcomes, edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur Heart J 2019;40:1518–27. [DOI] [PubMed] [Google Scholar]
- 453. Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P et al. ; RE-LY Investigators. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013;44:1891–6. [DOI] [PubMed] [Google Scholar]
- 454. Wong KS, Hu DY, Oomman A, Tan RS, Patel MR, Singer DE et al. ; Executive Steering Committee and the ROCKET AF Study Investigators. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke 2014;45:1739–47. [DOI] [PubMed] [Google Scholar]
- 455. Goto S, Zhu J, Liu L, Oh BH, Wojdyla DM, Aylward P et al. ; ARISTOTLE Investigators. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am Heart J 2014;168:303–9. [DOI] [PubMed] [Google Scholar]
- 456. Cha MJ, Choi EK, Han KD, Lee SR, Lim WH, Oh S et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2017;48:3040–8. [DOI] [PubMed] [Google Scholar]
- 457. Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Edoxaban in Asian patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol 2018;72:838–53. [DOI] [PubMed] [Google Scholar]
- 458. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR et al. Racial differences in atrial fibrillation-related cardiovascular disease and mortality: the Atherosclerosis Risk in Communities (ARIC) study. JAMA Cardiol 2016;1:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460. Ross JS, Halm EA, Bravata DM. Use of stroke secondary prevention services: are there disparities in care? Stroke 2009;40:1811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Simpson JR, Zahuranec DB, Lisabeth LD, Sanchez BN, Skolarus LE, Mendizabal JE et al. Mexican Americans with atrial fibrillation have more recurrent strokes than do non-Hispanic whites. Stroke 2010;41:2132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 2016;388:1161–9. [DOI] [PubMed] [Google Scholar]
- 463. Avezum A, Oliveira GBF, Diaz R, Hermosillo JAG, Oldgren J, Ripoll EF et al. Efficacy and safety of dabigatran versus warfarin from the RE-LY trial. Open Heart 2018;5:e000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464. Hankey GJ, Stevens SR, Piccini JP, Lokhnygina Y, Mahaffey KW, Halperin JL et al. ; ROCKET AF Steering Committee and Investigators. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke 2014;45:1304–12. [DOI] [PubMed] [Google Scholar]
- 465. Corbalan R, Nicolau JC, Lopez-Sendon J, Garcia-Castillo A, Botero R, Sotomora G et al. Edoxaban versus warfarin in Latin American patients with atrial fibrillation: the ENGAGE AF-TIMI 48 trial. J Am Coll Cardiol 2018;72:1466–75. [DOI] [PubMed] [Google Scholar]
- 466. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D et al. ; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378:615–24. [DOI] [PubMed] [Google Scholar]
- 467. Young AM, Marshall A, Thirlwall J, Chapman O, Lokare A, Hill C et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36:2017–23. [DOI] [PubMed] [Google Scholar]
- 468. Agnelli G, Becattini C, Bauersachs R, Brenner B, Campanini M, Cohen A et al. ; Caravaggio Study Investigators. Apixaban versus dalteparin for the treatment of acute venous thromboembolism in patients with cancer: the Caravaggio study. Thromb Haemost 2018;118:1668–78. [DOI] [PubMed] [Google Scholar]
- 469. Wang CL, Wu VC, Lee CH, Kuo CF, Chen YL, Chu PH et al. Effectiveness and safety of non-vitamin-K antagonist oral anticoagulants versus warfarin in atrial fibrillation patients with thrombocytopenia. J Thromb Thrombolysis 2019;47:512–9. [DOI] [PubMed] [Google Scholar]
- 470. Janion-Sadowska A, Papuga-Szela E, Lukaszuk R, Chrapek M, Undas A. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation and thrombocytopenia. J Cardiovasc Pharmacol 2018;72:153–60. [DOI] [PubMed] [Google Scholar]
- 471. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 472.European Medicines Agency ASoPC. https://www.ema.europa.eu/en/documents/product-information/eliquis-epar-product-information_en.pdf (26 August 2020, date last accessed).
- 473.European Medicines Agency ESoPC. https://www.ema.europa.eu/en/documents/product-information/lixiana-epar-product-information_en.pdf (26 August 2020, date last accessed).
- 474. Kang HG, Lee SJ, Chung JY, Cheong JS. Thrombocytopenia induced by dabigatran: two case reports. BMC Neurol 2017;17:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. He XY, Bai Y. Acute thrombocytopenia after anticoagulation with rivaroxaban: a case report. World J Clin Cases 2020;8:928–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Pop MK, Farokhi F, Iduna L. Drug-induced thrombocytopenia after anticoagulation with rivaroxaban. Am J Emerg Med 2018;36:531.e1–2. [DOI] [PubMed] [Google Scholar]
- 477. Mima Y, Sangatsuda Y, Yasaka M, Wakugawa Y, Nagata S, Okada Y. Acute thrombocytopenia after initiating anticoagulation with rivaroxaban. Intern Med 2014;53:2523–7. [DOI] [PubMed] [Google Scholar]
- 478. Snellgrove O. Case report: apixaban-induced thrombocytopenia. Clin Case Rep 2017;5:268–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479. Sadaka F. Thrombocytopenia: a possible side effect of apixaban. Clin Case Rep 2019;7:2543–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Krauel K, Hackbarth C, Furll B, Greinacher A. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood 2012;119:1248–55. [DOI] [PubMed] [Google Scholar]
- 481. Walenga JM, Prechel M, Hoppensteadt D, Escalante V, Chaudhry T, Jeske WP et al. Apixaban as an alternate oral anticoagulant for the management of patients with heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2013;19:482–7. [DOI] [PubMed] [Google Scholar]
- 482. Walenga JM, Prechel M, Jeske WP, Hoppensteadt D, Maddineni J, Iqbal O et al. Rivaroxaban—an oral, direct Factor Xa inhibitor—has potential for the management of patients with heparin-induced thrombocytopenia. Br J Haematol 2008;143:92–9. [DOI] [PubMed] [Google Scholar]
- 483. Tran PN, Tran MH. Emerging role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia. Clin Appl Thromb Hemost 2018;24:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood 2017;130:1104–13. [DOI] [PubMed] [Google Scholar]
- 485. Hu YF, Liu CJ, Chang PM, Tsao HM, Lin YJ, Chang SL et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol 2013;165:355–7. [DOI] [PubMed] [Google Scholar]
- 486. Agnelli G, Becattini C, Meyer G, Munoz A, Huisman MV, Connors JM et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med 2020;382:1599–607. [DOI] [PubMed] [Google Scholar]
- 487. Deng Y, Tong Y, Deng Y, Zou L, Li S, Chen H. Non-vitamin K antagonist oral anticoagulants versus warfarin in patients with cancer and atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2019;8:e012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Chen ST, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Fox KAA et al. Efficacy and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and a history of cancer: observations from ROCKET AF. Eur Heart J Qual Care Clin Outcomes 2019;5:145–52. [DOI] [PubMed] [Google Scholar]
- 489. Fanola CL, Ruff CT, Murphy SA, Jin J, Duggal A, Babilonia NA et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF-TIMI 48 trial. J Am Heart Assoc 2018;7:e008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Melloni C, Dunning A, Granger CB, Thomas L, Khouri MG, Garcia DA et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the ARISTOTLE trial. Am J Med 2017;130:1440–8. [DOI] [PubMed] [Google Scholar]
- 491. Shah S, Norby FL, Datta YH, Lutsey PL, MacLehose RF, Chen LY et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv 2018;2:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492. Kim K, Lee YJ, Kim TH, Uhm JS, Pak HN, Lee MH et al. Effect of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with newly diagnosed cancer. Korean Circ J 2018;48:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Ording AG, Horváth-Puhó E, Adelborg K, Pedersen L, Prandoni P, Sørensen HT. Thromboembolic and bleeding complications during oral anticoagulation therapy in cancer patients with atrial fibrillation: a Danish nationwide population-based cohort study. Cancer Med 2017;6:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist 2014;19:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M et al. ; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–801. [DOI] [PubMed] [Google Scholar]
- 496.European Medicines Agency AaSoPC. https://www.ema.europa.eu/en/documents/product-information/ondexxya-epar-product-information_en.pdf (12 September 2020, date last accessed).
- 497. Drouet L, Bal Dit Sollier C, Steiner T, Purrucker J. Measuring non-vitamin K antagonist oral anticoagulant levels: when is it appropriate and which methods should be used? Int J Stroke 2016;11:748–58. [DOI] [PubMed] [Google Scholar]
- 498. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 499. Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 500. Buller HR, Decousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15. [DOI] [PubMed] [Google Scholar]
- 501. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 502. Büller, HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 503. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 504. Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18. [DOI] [PubMed] [Google Scholar]
- 505. Raskob G, Ageno W, Cohen AT, Brekelmans MP, Grosso MA, Segers A et al. Extended duration of anticoagulation with edoxaban in patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol 2016;3:e228–36. [DOI] [PubMed] [Google Scholar]
- 506. Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med 2017;376:1211–22. [DOI] [PubMed] [Google Scholar]
- 507. Huang J, Cao Y, Liao C, Wu L, Gao F. Apixaban versus enoxaparin in patients with total knee arthroplasty. A meta-analysis of randomised trials. Thromb Haemost 2011;105:245–53. [DOI] [PubMed] [Google Scholar]
- 508. Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K et al. ; for the RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II). A randomised, double-blind, non-inferiority trial. Thromb Haemost 2011;105:721–9. [DOI] [PubMed] [Google Scholar]
- 509. Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP et al. ; RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85. [DOI] [PubMed] [Google Scholar]
- 510. Fuji T, Wang CJ, Fujita S, Kawai Y, Nakamura M, Kimura T et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res 2014;134:1198–204. [DOI] [PubMed] [Google Scholar]
- 511. Fuji T, Fujita S, Kawai Y, Nakamura M, Kimura T, Fukuzawa M et al. Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS J-V. Thromb J 2015;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75. [DOI] [PubMed] [Google Scholar]
- 513. Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31–9. [DOI] [PubMed] [Google Scholar]
- 514. Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86. [DOI] [PubMed] [Google Scholar]
- 515. Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM et al. ; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673–80. [DOI] [PubMed] [Google Scholar]
- 516. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. [DOI] [PubMed] [Google Scholar]
- 517. Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009;37:74–81. [DOI] [PubMed] [Google Scholar]
- 518. Ogata K, Mendell-Harary J, Tachibana M, Masumoto H, Oguma T, Kojima M et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol 2010;50:743–53. [DOI] [PubMed] [Google Scholar]
- 519. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005;78:412–21. [DOI] [PubMed] [Google Scholar]
- 521. Salazar DE, Mendell J, Kastrissios H, Green M, Carrothers TJ, Song S et al. Modelling and simulation of edoxaban exposure and response relationships in patients with atrial fibrillation. Thromb Haemost 2012;107:925–36. [DOI] [PubMed] [Google Scholar]
- 522. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Atar D, Heidbuchel H et al. Edoxaban vs. warfarin in patients with atrial fibrillation on amiodarone: a subgroup analysis of the ENGAGE AF-TIMI 48 trial. Eur Heart J 2015;36:2239–45. [DOI] [PubMed] [Google Scholar]
- 523. Mendell J, Zahir H, Matsushima N, Noveck R, Lee F, Chen S et al. Drug-drug interaction studies of cardiovascular drugs involving P-glycoprotein, an efflux transporter, on the pharmacokinetics of edoxaban, an oral factor Xa inhibitor. Am J Cardiovasc Drugs 2013;13:331–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Frost C, Song Y, Yu Z, Wang J, Lee LS, Schuster A et al. The effect of apixaban on the pharmacokinetics of digoxin and atenolol in healthy subjects. Clin Pharmacol 2017;9:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. No interaction between the novel, oral direct factor Xa inhibitor BAY 59-7939 and digoxin. J Clin Pharmacol 2006;46:702. [DOI] [PubMed] [Google Scholar]
- 526. Frost CE, Byon W, Song Y, Wang J, Schuster AE, Boyd RA et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol 2015;79:838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Greenblatt DJ, Patel M, Harmatz JS, Nicholson WT, Rubino CM, Chow CR. Impaired rivaroxaban clearance in mild renal insufficiency with verapamil coadministration: potential implications for bleeding risk and dose selection. J Clin Pharmacol 2018;58:533–40. [DOI] [PubMed] [Google Scholar]
- 528. Pham P, Schmidt S, Lesko L, Lip GYH, Brown JD. Association of oral anticoagulants and verapamil or diltiazem with adverse bleeding events in patients with nonvalvular atrial fibrillation and normal kidney function. JAMA Netw Open 2020;3:e203593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529. Stangier J, Rathgen K, Stahle H, Reseski K, Kornicke T, Roth W. Coadministration of dabigatran etexilate and atorvastatin: assessment of potential impact on pharmacokinetics and pharmacodynamics. Am J Cardiovasc Drugs 2009;9:59–68. [DOI] [PubMed] [Google Scholar]
- 530. Kubitza D, Becka M, Roth A, Mueck W. Absence of clinically relevant interactions between rivaroxaban—an oral, direct Factor Xa inhibitor–and digoxin or atorvastatin in healthy subjects. J Int Med Res 2012;40:1688–707. [DOI] [PubMed] [Google Scholar]
- 531. Parasrampuria DA, Mendell J, Shi M, Matsushima N, Zahir H, Truitt K. Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol 2016;82:1591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Mendell J, Chen S, He L, Desai M, Parasramupria DA. The effect of rifampin on the pharmacokinetics of edoxaban in healthy adults. Clin Drug Investig 2015;35:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Kumar P, Gordon LA, Brooks KM, George JM, Kellogg A, McManus M et al. Differential influence of the antiretroviral pharmacokinetic enhancers ritonavir and cobicistat on intestinal P-glycoprotein transport and the pharmacokinetic/pharmacodynamic disposition of dabigatran. Antimicrob Agents Chemother 2017;61:e01201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534. Gordon LA, Kumar P, Brooks KM, Kellogg A, McManus M, Alfaro RM et al. Antiretroviral boosting agent cobicistat increases the pharmacokinetic exposure and anticoagulant effect of dabigatran in HIV-negative healthy volunteers. Circulation 2016;134:1909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535. Frost C, Shenker A, Gandhi MD, Pursley J, Barrett YC, Wang J et al. Evaluation of the effect of naproxen on the pharmacokinetics and pharmacodynamics of apixaban. Br J Clin Pharmacol 2014;78:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Mendell J, Lee F, Chen S, Worland V, Shi M, Samama MM. The effects of the antiplatelet agents, aspirin and naproxen, on pharmacokinetics and pharmacodynamics of the anticoagulant edoxaban, a direct factor Xa inhibitor. J Cardiovasc Pharmacol 2013;62:212–21. [DOI] [PubMed] [Google Scholar]
- 537. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Rivaroxaban (BAY 59-7939)—an oral, direct Factor Xa inhibitor–has no clinically relevant interaction with naproxen. Br J Clin Pharmacol 2007;63:469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Moore KT, Plotnikov AN, Thyssen A, Vaccaro N, Ariyawansa J, Burton PB. Effect of multiple doses of omeprazole on the pharmacokinetics, pharmacodynamics, and safety of a single dose of rivaroxaban. J Cardiovasc Pharmacol 2011;58:581–8. [DOI] [PubMed] [Google Scholar]
- 539. Zhang C, Kwan P, Zuo Z, Baum L. The transport of antiepileptic drugs by P-glycoprotein. Adv Drug Deliv Rev 2012;64:930–42. [DOI] [PubMed] [Google Scholar]
- 540. Schelleman H, Pollard JR, Newcomb C, Markowitz CE, Bilker WB, Leonard MB et al. Exposure to CYP3A4-inducing and CYP3A4-non-inducing antiepileptic agents and the risk of fractures. Pharmacoepidemiol Drug Saf 2011;20:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541. Galgani A, Palleria C, Iannone LF, De Sarro G, Giorgi FS, Maschio M et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol 2018;9:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542. Lutz JD, Kirby BJ, Wang L, Song Q, Ling J, Massetto B et al. Cytochrome P450 3A induction predicts P-glycoprotein Induction; Part 2: prediction of decreased substrate exposure after rifabutin or carbamazepine. Clin Pharmacol Ther 2018;104:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Wiggins BS, Northup A, Johnson D, Senfield J. Reduced anticoagulant effect of dabigatran in a patient receiving concomitant phenytoin. Pharmacotherapy 2016;36:e5–7. [DOI] [PubMed] [Google Scholar]
- 544. Stollberger C, Finsterer J. Prolonged anticoagulant activity of rivaroxaban in a polymorbid elderly female with non-convulsive epileptic state. Heart Lung 2014;43:262–3. [DOI] [PubMed] [Google Scholar]
- 545. Di Minno A, Frigerio B, Spadarella G, Ravani A, Sansaro D, Amato M et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev 2017;31:193–203. [DOI] [PubMed] [Google Scholar]
- 546. Mousa SA. Antithrombotic effects of naturally derived products on coagulation and platelet function. Methods Mol Biol 2010;663:229–40. [DOI] [PubMed] [Google Scholar]
- 547. Tsai HH, Lin HW, Lu YH, Chen YL, Mahady GB. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One 2013;8:e64255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100:1419–26. [DOI] [PubMed] [Google Scholar]
- 549. van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010;103:1116–27. [DOI] [PubMed] [Google Scholar]
- 550. Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol 2013;76:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Girgis IG, Patel MR, Peters GR, Moore KT, Mahaffey KW, Nessel CC et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol 2014;54:917–27. [DOI] [PubMed] [Google Scholar]
- 552. Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit 2010;32:673–9. [DOI] [PubMed] [Google Scholar]
- 553. Mani H, Herth N, Kasper A, Wendt T, Schuettfort G, Weil Y et al. Point-of-care coagulation testing for assessment of the pharmacodynamic anticoagulant effect of direct oral anticoagulant. Ther Drug Monit 2014;36:624–31. [DOI] [PubMed] [Google Scholar]
- 554. Kaess BM, Ammar S, Reents T, Dillier R, Lennerz C, Semmler V et al. Comparison of safety of left atrial catheter ablation procedures for atrial arrhythmias under continuous anticoagulation with apixaban versus phenprocoumon. Am J Cardiol 2015;115:47–51. [DOI] [PubMed] [Google Scholar]
- 555. Tiedemann A, Lord SR, Sherrington C. The development and validation of a brief performance-based fall risk assessment tool for use in primary care. J Gerontol A Biol Sci Med Sci 2010;65:896–903. [DOI] [PubMed] [Google Scholar]
- 556. Van Spall HG, Wallentin L, Yusuf S, Eikelboom JW, Nieuwlaat R, Yang S et al. Variation in warfarin dose adjustment practice is responsible for differences in the quality of anticoagulation control between centers and countries: an analysis of patients receiving warfarin in the randomized evaluation of long-term anticoagulation therapy (RE-LY) trial. Circulation 2012;126:2309–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.