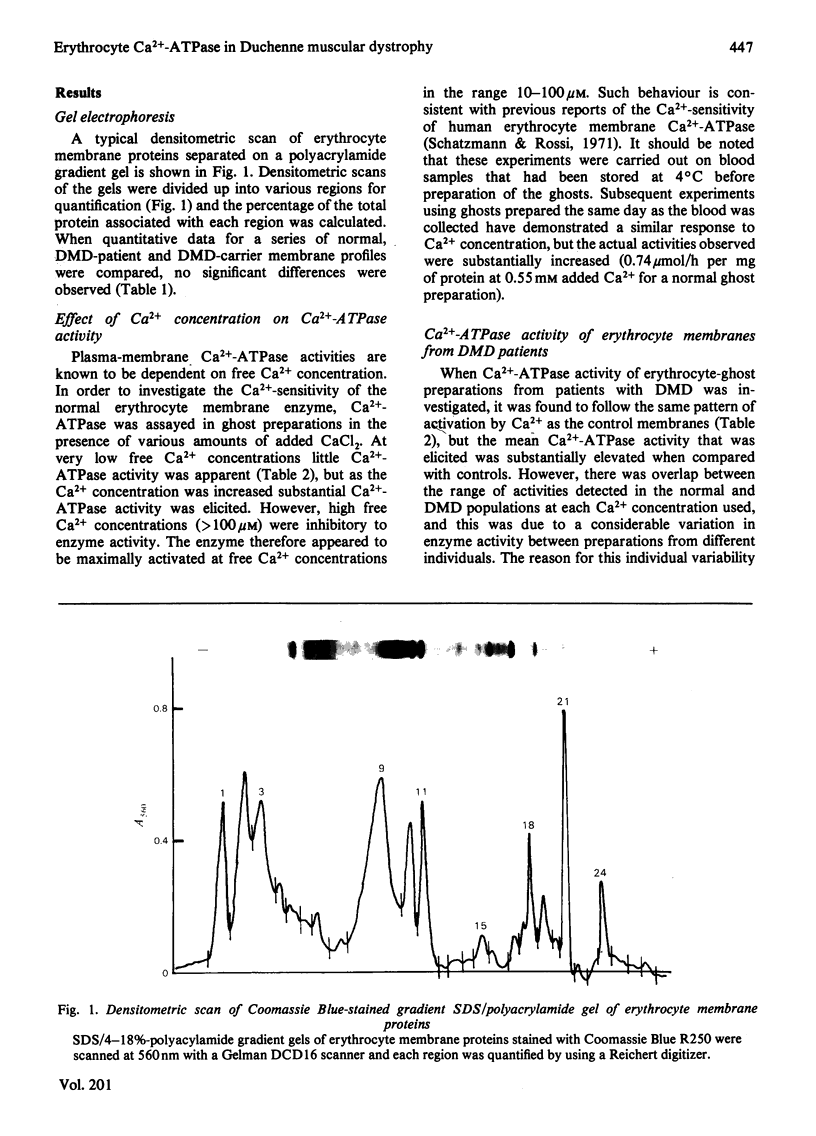
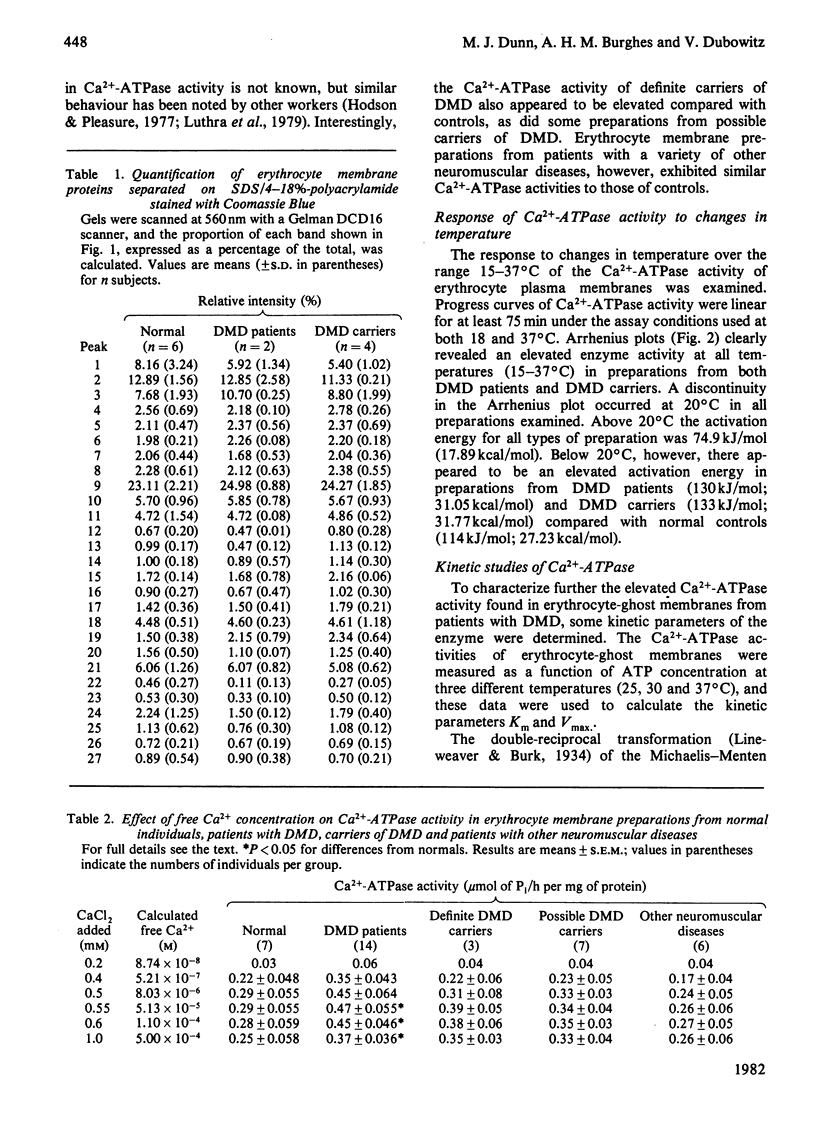
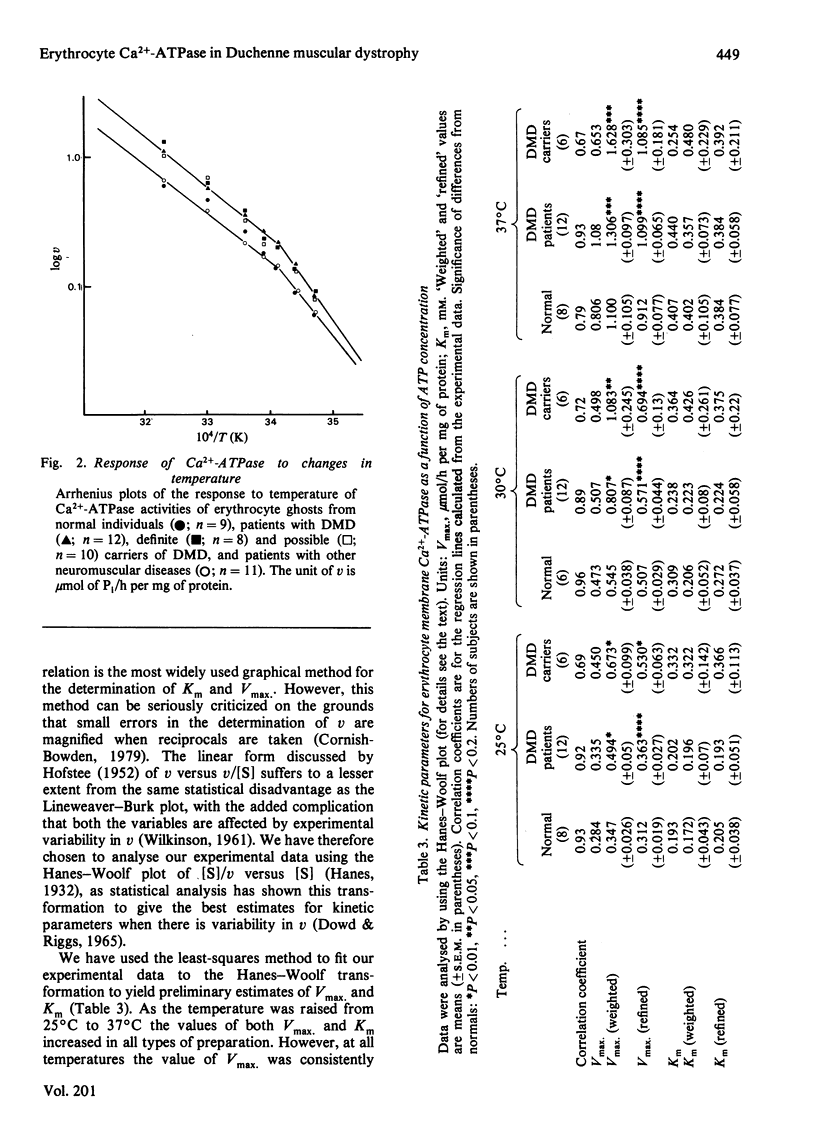
Abstract
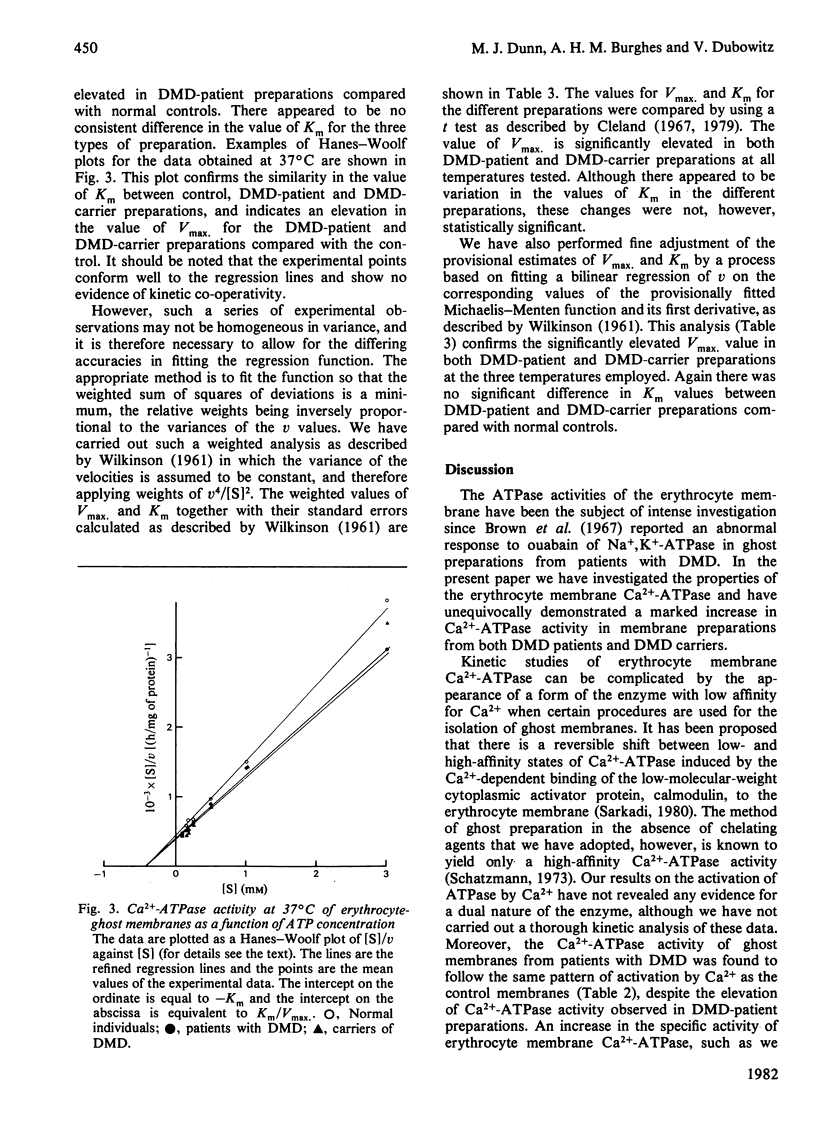
The Ca2+-stimulated Mg2-dependent ATPase activities (Ca2+-ATPase) of erythrocyte-ghost membranes from patients with Duchenne muscular dystrophy (DMD) and carriers of DMD were compared with activities of normal controls. The Ca2+-ATPase activity of DMD-patient ghost preparations was found to follow the same pattern of activation by Ca2+ as the control membranes. However, the Ca2+-ATPase activity in DMD and some DMD-carrier preparations was substantially elevated compared with controls. To characterize further the elevated Ca2+-ATPase activity found in DMD-patient ghost membrane preparations, we estimated kinetic parameters using both fine adjustment and weighting methods to analyse our experimental data. It was established that in both DMD and DMD-carrier preparations the increase in Ca2+-ATPase activity was reflected by a significant increase in Vmax. rather than by any change in Km. The response of the membrane Ca2+-ATPase activity to changes in temperature was also investigated. In all preparations a break in the Arrhenius plot occurred at 20 degrees C, and in DMD and DMD-carrier preparations an elevated Ca2+-ATPase activity was detected at all temperatures. Above 20 degrees C the activation energy for all types of preparation was the same, whereas below this temperature there appeared to be an elevated activation in DMD and DMD-carrier preparations compared with normal controls. The concept that a generalized alteration in the physicochemical nature of the membrane lipid domain may be responsible for the many abnormal membrane properties reported in DMD is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson A., Gatenby A. D., Lowe A. G. The determination of inorganic orthophosphate in biological systems. Biochim Biophys Acta. 1973 Aug 17;320(1):195–204. doi: 10.1016/0304-4165(73)90178-5. [DOI] [PubMed] [Google Scholar]
- Bodensteiner J. B., Engel A. G. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978 May;28(5):439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Bond G. H., Clough D. L. A soluble protein activator of (Mg2+ plus Ca2+)-dependent ATPase in human red cell membranes. Biochim Biophys Acta. 1973 Nov 16;323(4):592–599. doi: 10.1016/0005-2736(73)90167-3. [DOI] [PubMed] [Google Scholar]
- Brown H. D., Chattopadhyay S. K., Patel A. B. Erythrocyte abnormality in human myopathy. Science. 1967 Sep 29;157(3796):1577–1578. doi: 10.1126/science.157.3796.1577. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Dise C. A., Goodman D. B., Lake W. C., Hodson A., Rasmussen H. Enhanced sensitivity to calcium in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1286–1292. doi: 10.1016/0006-291x(77)91145-7. [DOI] [PubMed] [Google Scholar]
- Dunn M. J., Burghes A. H., Dubowitz V. Erythrocyte ghost Na+,K+-adenosine triphosphatase in Duchenne muscular dystrophy. J Neurol Sci. 1980 May;46(2):209–220. doi: 10.1016/0022-510x(80)90079-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gopinath R. M., Vincenzi F. F. Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1203–1209. doi: 10.1016/s0006-291x(77)80107-1. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson A., Pleasure D. Erythrocyte cation-activated adenosine triphosphatases in Duchenne muscular dystrophy. J Neurol Sci. 1977 Jul;32(3):361–369. doi: 10.1016/0022-510x(77)90019-3. [DOI] [PubMed] [Google Scholar]
- Howland J. L., Iyer S. L. Erythrocyte lipids in heterozygous carriers of duchenne muscular dystrophy. Science. 1977 Oct 21;198(4314):309–310. doi: 10.1126/science.910129. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Penniston J. T. Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1210–1216. doi: 10.1016/s0006-291x(77)80108-3. [DOI] [PubMed] [Google Scholar]
- Kalofoutis A., Jullien G., Spanos V. Erythrocyte phospholipids in Duchenne muscular dystrophy. Clin Chim Acta. 1977 Jan 3;74(1):85–87. doi: 10.1016/0009-8981(77)90391-6. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K. Alterations in phospholipid-dependent (Na+ +K+)-ATPase activity due to lipid fluidity. Effects of cholesterol and Mg2+. Biochim Biophys Acta. 1975 Nov 17;413(1):143–156. doi: 10.1016/0005-2736(75)90065-6. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Kumamoto J., Raison J. K., Lyons J. M. Temperature "breaks" in Arrhenius plots: a thermodynamic consequence of a phase change. J Theor Biol. 1971 Apr;31(1):47–51. doi: 10.1016/0022-5193(71)90120-2. [DOI] [PubMed] [Google Scholar]
- Kunze D., Reichmann G., Egger E., Leuschner G., Eckhardt H. Erythrozytenlipide bei Progressiver Muskeldystrophie. Clin Chim Acta. 1973 Feb 12;43(3):333–341. doi: 10.1016/0009-8981(73)90471-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luthra M. G., Kim H. D. Effects of calcium and soluble cytoplasmic activator protein (calmodulin) on various states of (Ca2+ + Mg2+)-ATPase activity in isolated membranes of human red cells. Biochim Biophys Acta. 1980 Aug 4;600(2):467–479. doi: 10.1016/0005-2736(80)90449-6. [DOI] [PubMed] [Google Scholar]
- Luthra M. G., Stern L. Z., Kim H. D. (Ca++ + Mg++)-ATPase of red cells in Duchenne and myotonic dystrophy: effect of soluble cytoplasmic activator. Neurology. 1979 Jun;29(6):835–841. doi: 10.1212/wnl.29.6.835. [DOI] [PubMed] [Google Scholar]
- Mollman J. E., Cardenas J. C., Pleasure D. E. Alteration of calcium transport in Duchenne erythrocytes. Neurology. 1980 Nov;30(11):1236–1239. doi: 10.1212/wnl.30.11.1236. [DOI] [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Regulatory interaction between calmodulin and ATP on the red cell Ca2+ pump. Biochim Biophys Acta. 1980 Apr 24;597(3):631–636. doi: 10.1016/0005-2736(80)90235-7. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Penniston J. T., Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981 Jan 10;256(1):395–401. [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. A high affinity calcium-stimulated magnesium-dependent adenosine triphosphatase in rat adipocyte plasma membranes. J Biol Chem. 1980 May 10;255(9):4087–4093. [PubMed] [Google Scholar]
- Peter J. B., Worsfold M., Pearson C. M. Erythrocyte ghost adenosine triphosphatase (ATPase) in Duchenne dystrophy. J Lab Clin Med. 1969 Jul;74(1):103–108. [PubMed] [Google Scholar]
- Raison J. K. The influence of temperature-induced phase changes on the kinetics of respiratory and other membrane-associated enzyme systems. J Bioenerg. 1973 Jan;4(1):285–309. doi: 10.1007/BF01516063. [DOI] [PubMed] [Google Scholar]
- Richards D. E., Rega A. F., Garrahan P. J. Two classes of site for ATP in the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1978 Aug 4;511(2):194–201. doi: 10.1016/0005-2736(78)90313-9. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Hartwig G. B., Mabry M., Nagano Y., Miller S. E. Red blood cell and fibroblast membranes in Duchenne and myotonic muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):36–54. doi: 10.1002/mus.880030106. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Ruitenbeek W. Membrane-bound enzymes of erythrocytes in human muscular dystrophy: (Na+ + K+-ATPase, Ca2+-ATPase, K+- and Ca2+-p-nitrophenylphosphatase. J Neurol Sci. 1979 Mar;41(1):71–80. doi: 10.1016/0022-510x(79)90141-2. [DOI] [PubMed] [Google Scholar]
- Sarkadi B. Active calcium transport in human red cells. Biochim Biophys Acta. 1980 Sep 30;604(2):159–190. doi: 10.1016/0005-2736(80)90573-8. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Reversible shift between two states of Ca2+-ATPase in human erythrocytes mediated by Ca2+ and a membrane-bound activator. Biochim Biophys Acta. 1978 May 4;509(1):67–77. doi: 10.1016/0005-2736(78)90008-1. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Rossi G. L. (Ca 2+ + Mg 2+ )-activated membrane ATPases in human red cells and their possible relations to cation transport. Biochim Biophys Acta. 1971 Aug 13;241(2):379–392. doi: 10.1016/0005-2736(71)90037-x. [DOI] [PubMed] [Google Scholar]
- Siddiqui P. Q., Pennington R. J. Effect of ouabain upon erythrocyte membrane adenosine triphosphatase in Duchenne muscular dystrophy. J Neurol Sci. 1977 Dec;34(3):365–372. doi: 10.1016/0022-510x(77)90153-8. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., Read B. D., McElhaney R. N. Membrane enzymes: artifacts in Arrhenius plots due to temperature dependence of substrate-binding affinity. Science. 1978 Feb 24;199(4331):902–904. doi: 10.1126/science.146257. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruyff B., van Dijck P. W., Godlbach R. W., Demel R. A., van Deenen L. L. Influence of fatty acid and sterol composition on the lipid phase transition and activity of membrane-bound enzymes in Acholeplasma laidlawii. Biochim Biophys Acta. 1973 Dec 22;330(3):269–282. doi: 10.1016/0005-2736(73)90232-0. [DOI] [PubMed] [Google Scholar]