Abstract
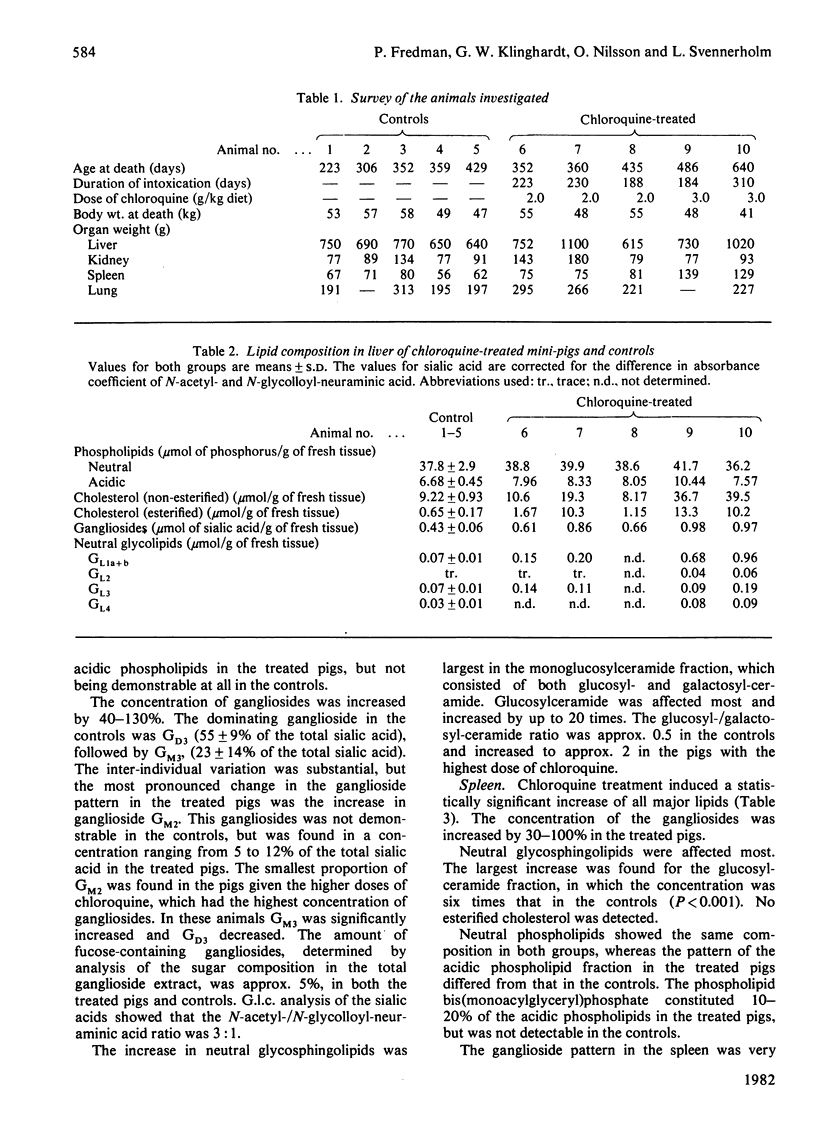
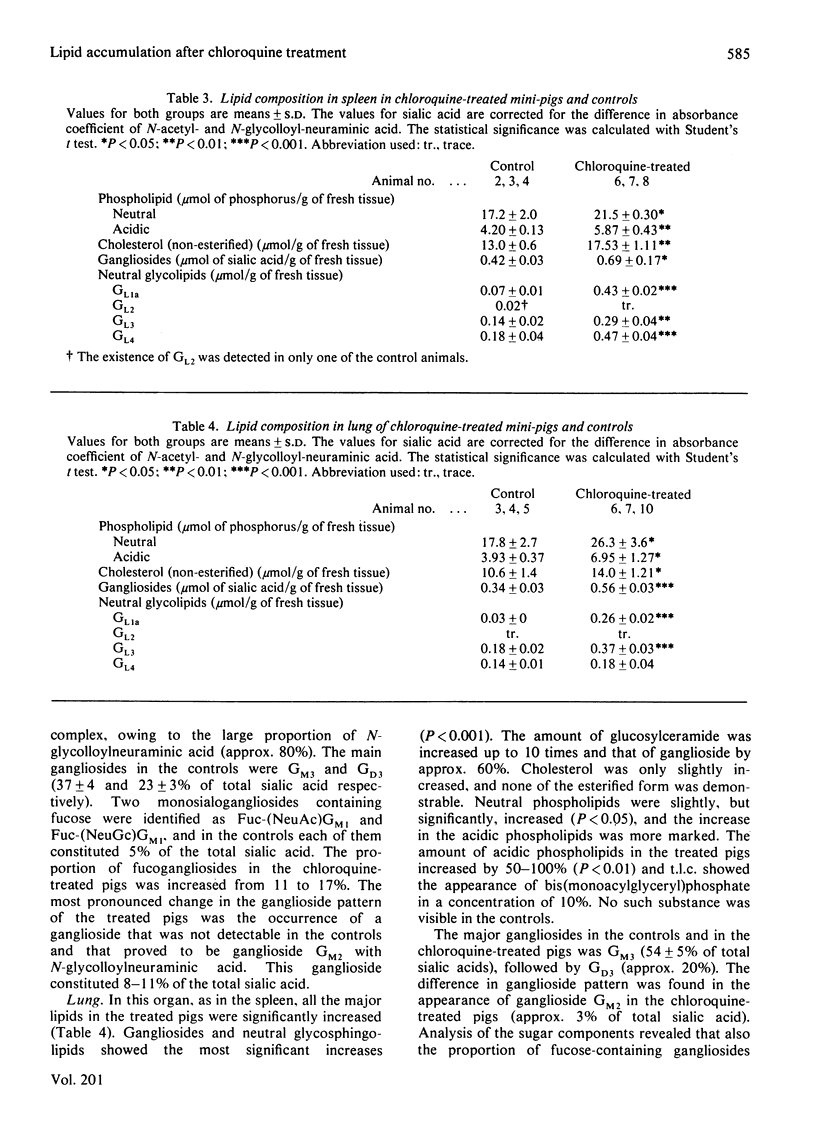
Chronic chloroquine treatment of type-Göttingen miniature-pigs induced lipid accumulation in the liver, spleen, lungs and kidneys. The lipid analyses showed marked quantitative and qualitative differences between the organs. In the liver the lipids affected most were cholesteryl esters and glucosylceramides, which were increased at the most 20 times. Cholesterol and ganglioside concentrations were also increased, though less markedly. The concentration of acidic phospholipids was slightly increased but that of the neutral phospholipids was unaffected. There was a considerable inter-individual variation in the lipid changes. Spleen and lung showed significant increases of all the major lipids. Glucosylceramide was increased more than the other lipids, namely 6-fold in the spleen and 10-fold in the lung. The concentration of acidic phospholipids as well as that of gangliosides was increased by 50% in the spleen and by 100% in the lung. The organ affected least was the kidney, in which only the glycolipids, both acidic and neutral, were significantly increased. Common to all the organs of the chloroquine-treated pigs was the large increase of glucosylceramide, ganglioside CM2 and bis(monoacylglyceryl)phosphate. The ganglioside increase affected all the individual gangliosides and, except for the increased proportion of ganglioside GM2, there were not remarkable changes in the ganglioside pattern in any of the organs.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi S., Matsuzawa Y., Yokomura T., Ishikawa K., Uhara S. Studies on drug-induced lipodosis. V. Changes in the lipid composition of rat liver and spleen following the administration of 4,4'-diethylaminoethoxyhexestrol. Lipids. 1972 Jan;7(1):1–7. doi: 10.1007/BF02531262. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Doyle A. E., Gannon B. J., Gerkens J. F., Iwayama T., Mashford M. L. Prolonged hypotension and ultrastructural changes in sympathetic neurones following guanacline treatment. Eur J Pharmacol. 1971 Jan;13(2):175–187. doi: 10.1016/0014-2999(71)90148-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD N. An improved method for the determination of free and total cholesterol using the ferric chloride reaction. Clin Chim Acta. 1958 Jul;3(4):357–367. doi: 10.1016/0009-8981(58)90025-1. [DOI] [PubMed] [Google Scholar]
- Fewster M. E., Burns B. J., Mead J. F. Quantitative densitometric thin-layer chromatography of lipids using copper acetate reagent. J Chromatogr. 1969 Aug 5;43(1):120–126. doi: 10.1016/s0021-9673(00)99173-8. [DOI] [PubMed] [Google Scholar]
- Fredman P., Nilsson O., Tayot J. L., Svennerholm L. Separation of gangliosides on a new type of anion-exchange resin. Biochim Biophys Acta. 1980 Apr 18;618(1):42–52. doi: 10.1016/0005-2760(80)90052-1. [DOI] [PubMed] [Google Scholar]
- GOLDMAN L., PRESTON R. H. Reactions to chloroquine observed during the treatment of various dermatologic disorders. Am J Trop Med Hyg. 1957 Jul;6(4):654–657. doi: 10.4269/ajtmh.1957.6.654. [DOI] [PubMed] [Google Scholar]
- Gleiser C. A., Bay W. W., Dukes T. W., Brown R. S., Read W. K., Pierce K. R. Study of chloroquine toxicity and a drug-induced cerebrospinal lipodystrophy in swine. Am J Pathol. 1968 Jul;53(1):27–45. [PMC free article] [PubMed] [Google Scholar]
- HARING F., GRUHN R., SMIDT D., SCHEVEN B. ZUECHTUNG EINES MINIATURSCHWEINES ALS VERSUCHS- UND LABORATORIUMSTIER. Zentralbl Bakteriol Orig. 1963 Aug;189:521–537. [PubMed] [Google Scholar]
- HOBBS H. E., SORSBY A., FREEDMAN A. Retinopathy following chloroquine therapy. Lancet. 1959 Oct 3;2(7101):478–480. doi: 10.1016/s0140-6736(59)90604-x. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R., Wassermann O. Lipidosis induced by amphiphilic cationic drugs. Biochem Pharmacol. 1978;27(8):1103–1108. doi: 10.1016/0006-2952(78)90435-5. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Degradation of bis(monoacylglycero)phosphate by an acid phosphodiesterase in rat liver lysosomes. J Biol Chem. 1979 Jul 10;254(13):5997–6001. [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Studies on drug-induced lipidosis: subcellular localization of phospholipid and cholesterol in the liver of rats treated with chloroquine or 4,4'-bis (diethylaminoethoxy)alpha, beta-diethyldiphenylethane. J Lipid Res. 1980 Feb;21(2):202–214. [PubMed] [Google Scholar]
- Matsuzawa Y., Poorthuis B. J., Hostetler K. Y. Mechanism of phosphatidylinostiol stimulation of lysosomal bis (monoacylglyceryl)phosphate synthesis. J Biol Chem. 1978 Oct 10;253(19):6650–6653. [PubMed] [Google Scholar]
- Matsuzawa Y., Yamamoto A., Adachi S., Nishikawa M. Studies on drug-induced lipidosis. VIII. Correlation between drug accumulation and acidic phospholipids. J Biochem. 1977 Nov;82(5):1369–1377. doi: 10.1093/oxfordjournals.jbchem.a131824. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Fredman P., Klinghardt G. W., Dreyfus H., Svennerholm L. Chloroquine-induced accumulation of gangliosides and phospholipids in skeletal muscles. Quantitative determination and characterization of stored lipids. Eur J Biochem. 1981 Jun 1;116(3):565–571. doi: 10.1111/j.1432-1033.1981.tb05373.x. [DOI] [PubMed] [Google Scholar]
- ORCHARD B. Rapid estimation of amino-acids. Nature. 1963 May 18;198:688–688. doi: 10.1038/198688a0. [DOI] [PubMed] [Google Scholar]
- Rouser G., Kritchevsky G., Knudson A. G., Jr, Simon G. Accumulation of a glycerolphospholipid in classical niemann-pick disease. Lipids. 1968 May;3(3):287–290. doi: 10.1007/BF02531203. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. On the isolation and characterization of N-acetyl-sialic acid. Acta Soc Med Ups. 1956 Jun 30;61(1-2):74–85. [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Seiler K. U., Wassermann O. Drug-induced phospholipidosis. II. Alterations in the phospholipid pattern of organs from mice, rats and guinea-pigs after chronic treatment with chlorphentermine. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(2-3):261–268. doi: 10.1007/BF00500531. [DOI] [PubMed] [Google Scholar]
- Stein Y., Ebin V., Bar-On H., Stein O. Chloroquine-induced interference with degradation of serum lipoproteins in rat liver, studied in vivo and in vitro. Biochim Biophys Acta. 1977 Feb 23;486(2):286–297. doi: 10.1016/0005-2760(77)90024-8. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Vanier M. T. The distribution of lipids in the human nervous system. II. Lipid composition of human fetal and infant brain. Brain Res. 1972 Dec 12;47(2):457–468. doi: 10.1016/0006-8993(72)90652-x. [DOI] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- WHISNANT J. P., ESPINOSA R. E., KIERLAND R. R., LAMBERT E. H. CHLOROQUINE NEUROMYOPATHY. Proc Staff Meet Mayo Clin. 1963 Nov 6;38:501–513. [PubMed] [Google Scholar]
- Wherrett J. R., Huterer S. Enrichment of bis-(monoacylglyceryl) phosphate in lysosomes from rat liver. J Biol Chem. 1972 Jul 10;247(13):4114–4120. [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Ishibe T., Shinji Y., Kaki-Uchi Y., Seki K. I., Kitani T. Accumulation of acidic phospholipids in a case of hyperlipidemia with hepatosplenomegaly. Lipids. 1970 Jun;5(6):566–571. doi: 10.1007/BF02532747. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Ishikawa K., Yokomura T., Kitani T. Studies on drug-induced lipidosis. 3. Lipid composition of the liver and some other tissues in clinical cases of "Niemann-Pick-like syndrome" induced by 4,4'-diethylaminoethoxyhexestrol. J Biochem. 1971 Nov;70(5):775–784. doi: 10.1093/oxfordjournals.jbchem.a129695. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Kitani T., Shinji Y., Seki K. Drug-induced lipidosis in human cases and in animal experiments. Accumulation of an acidic glycerophospholipid. J Biochem. 1971 Mar;69(3):613–615. [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Matsuzawa Y., Kitani T., Hiraoka A., Seki K. Studies on drug-induced lipidosis: VII. Effects of bis-beta-diethyl-aminoethylether of hexestrol, chloroquine, homochlorocyclizine, prenylamine, and diazacholesterol on the lipid composition of rat liver and kidney. Lipids. 1976 Aug;11(8):616–622. doi: 10.1007/BF02532875. [DOI] [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gas--liquid chromatographic assay of lipid-bound sialic acids: measurement of gangliosides in brain of several species. J Lipid Res. 1970 Nov;11(6):506–516. [PubMed] [Google Scholar]


