Abstract
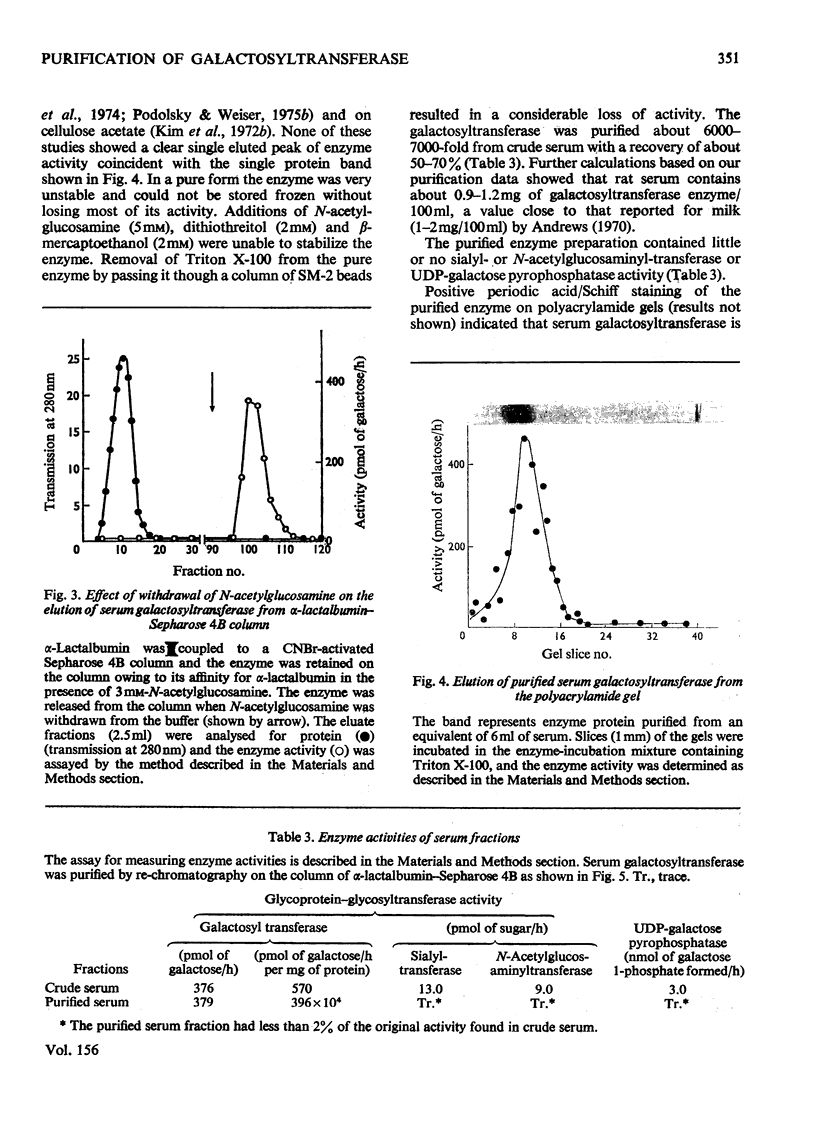
1. Rat liver microsomal preparations incubated with 200mM-NaCl at either 0 or 30 degrees C released about 20-30% of the membrane-bound UDP-galactose-glycoprotein galactosyl-transferase (EC 2.4.1.22) into a 'high-speed' supernatant. The 'high-speed' supernatant was designated the 'saline wash' and the galactosyltransferase released into this fraction required Triton X-100 for activation. It was purified sixfold by chromatography on Sephadex G-200, and appeared to have a higher molecular weight than the soluble serum enzyme. 2. Rat serum galactosyltransferase was purified 6000-7000-fold by an affinity-chromatographic technique using a column of activated Sepharose 4B coupled with alpha-lactalbumin. The purified enzyme ran as a single broad band on polacrylamide gels and contained no sialytransferase, N-acetylglucosaminyltransferase and UDP-galactose pyrophosphatase activities. 3. The highly purified enzyme had properties similar to those of both soluble and membrane-bound galactosyltransferase. It required 0.1% Triton X-100 for stabilization, but lost activity on freezing. The enzyme had an absolute requirement for Mn2+, not replaceable by Ca2+, Mg2+, Zn2+ or Co2+. It was active over a wide pH range (6-8) and had a pH optimum of 6.8. The apparent Km for UDP-galactose was 12.5 x 10(-6) M. Alpha-Lactalbumin had no appreciable effect on UDP-galactose-glycoprotein galactosyltransferase, but it increased the specificity for glucose rather than for N-acetylglucosamine, thus modifying the enzyme to a lactose synthetase. 4. The possibility of a conversion of higher-molecular-weight liver enzyme into soluble serum enzyme is discussed, especially in relation to the elevated activities of this and other glycosyltransferases in patients with liver diseases.
Full text
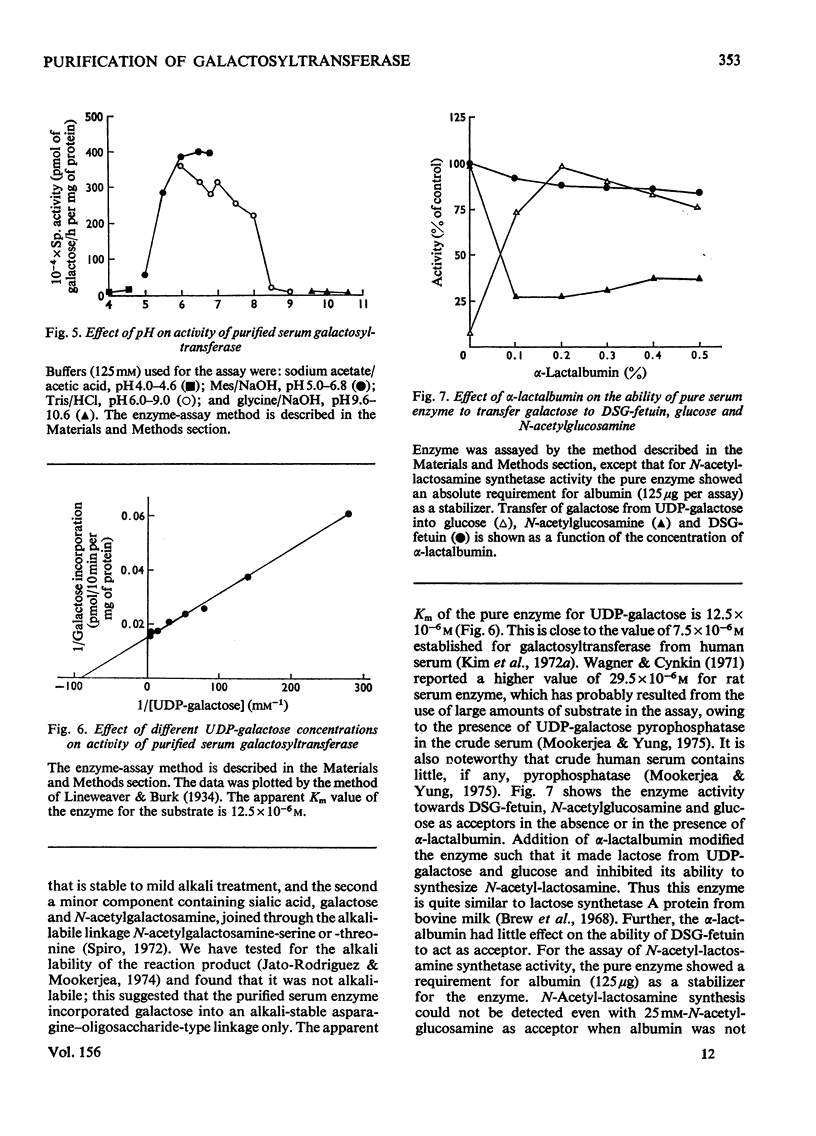
PDF


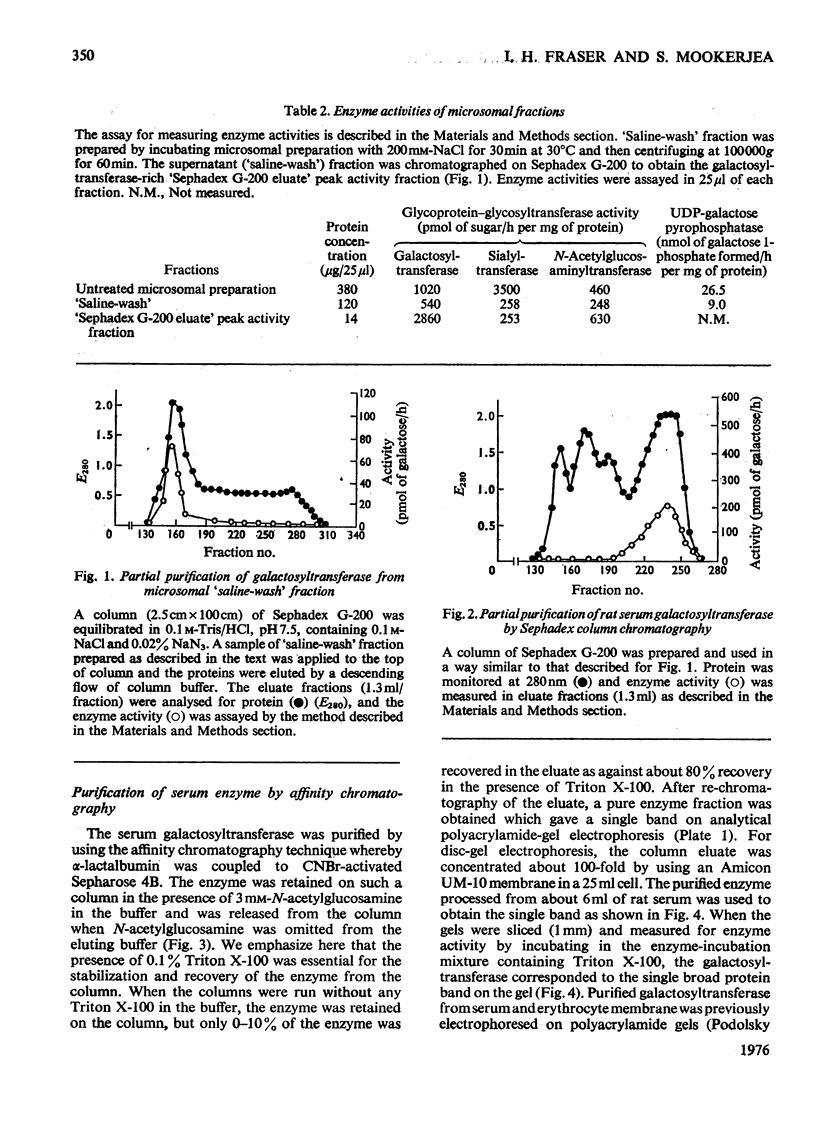
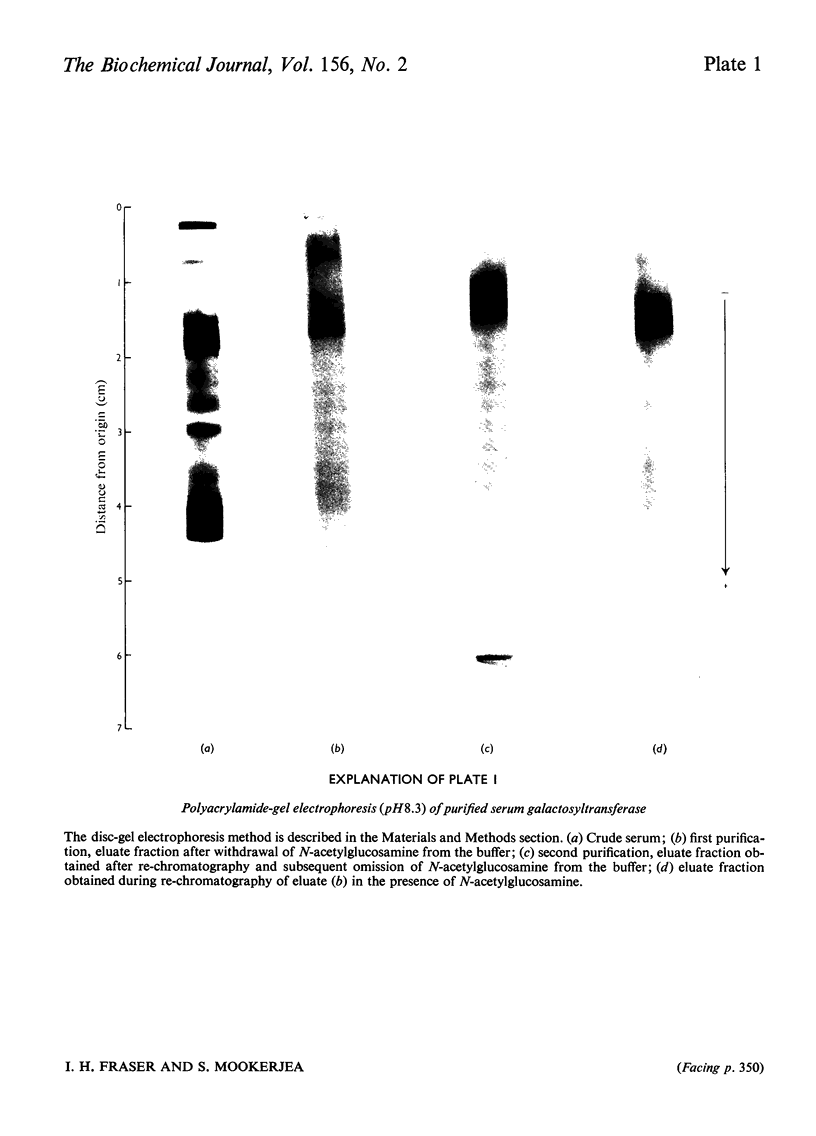



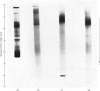
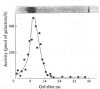
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agopian A., Eylar E. H. Glycoprotein biosynthesis: the solubilization of glycosyl transferases from membranes of HeLa cells and bovine submaxillary glycoproteins. Arch Biochem Biophys. 1969 Feb;129(2):447–455. doi: 10.1016/0003-9861(69)90201-x. [DOI] [PubMed] [Google Scholar]
- Andrews P. Purification of lactose synthetase a protein from human milk and demonstration of its interaction with alpha-lactalbumin. FEBS Lett. 1970 Aug 31;9(5):297–300. doi: 10.1016/0014-5793(70)80382-9. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Schachter H. Porcine sugar nucleotide: glycoprotein glycosyltransferases. II. Blood serum and liver galactosyltransferase. Can J Biochem. 1971 Jul;49(7):838–846. doi: 10.1139/o71-118. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Mookerjea S. UDP-galactose:glycoprotein galactosyltransferase activity in tissues of developing rat. Arch Biochem Biophys. 1974 May;162(1):281–292. doi: 10.1016/0003-9861(74)90127-1. [DOI] [PubMed] [Google Scholar]
- Judah J. D., Gamble M., Steadman J. H. Biosynthesis of serum albumin in rat liver. Evidence for the existence of 'proalbumin'. Biochem J. 1973 Aug;134(4):1083–1091. doi: 10.1042/bj1341083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLANDER J. SEPARATION OF HUMAN HEME- AND HEMOGLOBIN-BINDING PLASMA PROTEINS, CERULOPLASMIN AND ALBUMIN BY GEL FILTRATION. Biochim Biophys Acta. 1964 Oct 9;93:1–14. doi: 10.1016/0304-4165(64)90254-5. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Glycosyltransferases: do they exist on the surface membrane of mammalian cells? FEBS Lett. 1975 Jul 15;55(1):8–13. doi: 10.1016/0014-5793(75)80944-6. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Whitehead J. S., Curtis K. J. Glycosyltransferases in human blood. II. Study of serum galactosyltransferase and N-acetylgalactosaminyltransferase in patients with liver diseases. J Clin Invest. 1972 Aug;51(8):2033–2039. doi: 10.1172/JCI107009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Whitehead J. S. Glycosyltransferases in human blood.I. Galactosyltransferase in human serum and erythrocyte membranes. J Clin Invest. 1972 Aug;51(8):2024–2032. doi: 10.1172/JCI107008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman E. D., Hudson B. G., Ebner K. E. Studies on the carbohydrate structure of bovine milk galactosyltransferase. FEBS Lett. 1975 Jun 1;54(1):65–69. doi: 10.1016/0014-5793(75)81069-6. [DOI] [PubMed] [Google Scholar]
- Mawal R., Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Detection of enzyme-reactant complexes by means of affinity chromatography. J Biol Chem. 1971 Dec 10;246(23):7106–7109. [PubMed] [Google Scholar]
- Mookerjea S., Chow A., Hudgin R. L. Occurrence of UDP-N-acetylglucosamine: glycoprotein N-acetylglucosaminyltransferase activity in human and rat sera. Can J Biochem. 1971 Mar;49(3):297–299. doi: 10.1139/o71-042. [DOI] [PubMed] [Google Scholar]
- Mookerjea S. Glycoprotein biosynthesis: stimulation of N-acetylglucosaminyltransferase activity by cytidine 5'-diphosphocholine. Can J Biochem. 1972 Oct;50(10):1082–1093. doi: 10.1139/o72-149. [DOI] [PubMed] [Google Scholar]
- Mookerjea S., Michaels M. A., Hudgin R. L., Moscarello M. A., Chow A., Schachter H. The levels of nucleotide-sugar: glycoprotein sialyl- and N-acetylglucosaminyltransferases in normal and pathological human sera. Can J Biochem. 1972 Jul;50(7):738–740. doi: 10.1139/o72-102. [DOI] [PubMed] [Google Scholar]
- Mookerjea S., Yung J. W. A study on the effect of lysolecithin and phospholipase A on membrane-bound galactosyltransferase. Can J Biochem. 1974 Nov;52(11):1053–1066. doi: 10.1139/o74-147. [DOI] [PubMed] [Google Scholar]
- Mookerjea S., Yung J. W. Studies on uridine diphosphate-galactose pyrophosphatase and uridine diphosphate-galactose: glycoprotein galactosyltransferase activities in microsomal membranes. Arch Biochem Biophys. 1975 Jan;166(1):223–226. doi: 10.1016/0003-9861(75)90383-5. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M. Galactosyltransferase activities in human sera: detection of a cancer-associated isoenzyme. Biochem Biophys Res Commun. 1975 Jul 22;65(2):545–551. doi: 10.1016/s0006-291x(75)80181-1. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M., La Mont J. T., Isselbacher K. J. Galactosyltransferase and concanavalin A agglutination of cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):904–908. doi: 10.1073/pnas.71.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Weiser M. M. Role of cell membrane galactosyltransferase in concanavalin A agglutination of erythrocytes. Biochem J. 1975 Jan;146(1):213–221. doi: 10.1042/bj1460213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Brew K. The preparation and characterization of two forms of bovine galactosyl transferase. Eur J Biochem. 1974 Oct 1;48(1):217–228. doi: 10.1111/j.1432-1033.1974.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Trayer I. P., Hill R. L. The purification and properties of the A protein of lactose synthetase. J Biol Chem. 1971 Nov;246(21):6666–6675. [PubMed] [Google Scholar]
- Wagner R. R., Cynkin M. A. Glycoprotein metabolism: a UDP-galactose-glycoprotein galactosyltransferase of rat serum. Biochem Biophys Res Commun. 1971 Oct 1;45(1):57–62. doi: 10.1016/0006-291x(71)90049-0. [DOI] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]