Abstract
1. Isolates representing seven bacterial genera capable of growth on L-threonine medium, and possessing high L-threonine 3-dehydrogenase activity, were examined to elucidate the catabolic route. 2. The results of growth, manometric and enzymic experiments indicated the catabolism of L-threonine by cleavage to acetyl-CoA plus glycine, the glycine being further metabolized via L-serine to pyruvate, in all cases. No evidence was obtained of a role for aminoacetone in threonine catabolism or for the metabolism of glycine by the glycerate pathway. 3. The properties of a number of key enzymes in L-threonine catabolism were investigated. The inducibly formed L-threonine 3-dehydrogenase, purified from Corynebacterium sp. B6 to a specific activity of about 30-35 mumol of product formed/min per mg of protein, exhibited a sigmoid kinetic response to substrate concentration. The half-saturating concentration of substrate, [S]0.5, was 20mM and the Hill constant (h) was 1.50. The Km for NAD+ was 0.8mM. The properties of the enzyme were studied in cell-free extracts of other bacteria. 4. New assays for 2-amino-3-oxobutyrate-CoA ligase were devised. The Km for CoA was determined for the first time and found to be 0.14mM at pH8, for the enzyme from Corynebacterium sp. B6. Evidence was obtained for the efficient linkage of the dehydrogenase and ligase enzymes. Cell-free extracts all possessed high activities of the inducibly formed ligase. 5. L-Serine hydroxymethyltransferase was formed constitutively by all isolates, whereas formation of the 'glycine-cleavage system' was generally induced by growth on L-threonine or glycine. The coenzyme requirements of both enzymes were established, and their linked activity in the production of L-serine from glycine was demonstrated by using extracts of Corynebacterium sp. B6. 6. L-Serine dehydratase, purified from Corynebacterium sp. B6 to a specific activity of about 4mumol of product formed/min per mg of protein, was found to exhibit sigmoid kinetics with an [S]0.5 of about 20mM and h identical to 1.4. Similar results were obtained with enzyme preparations from all isolates. The enzyme required Mg2+ for maximum activity, was different from the L-threonine dehydratase also detectable in extracts, and was induced by growth on L-threonine or glycine.
Full text
PDF


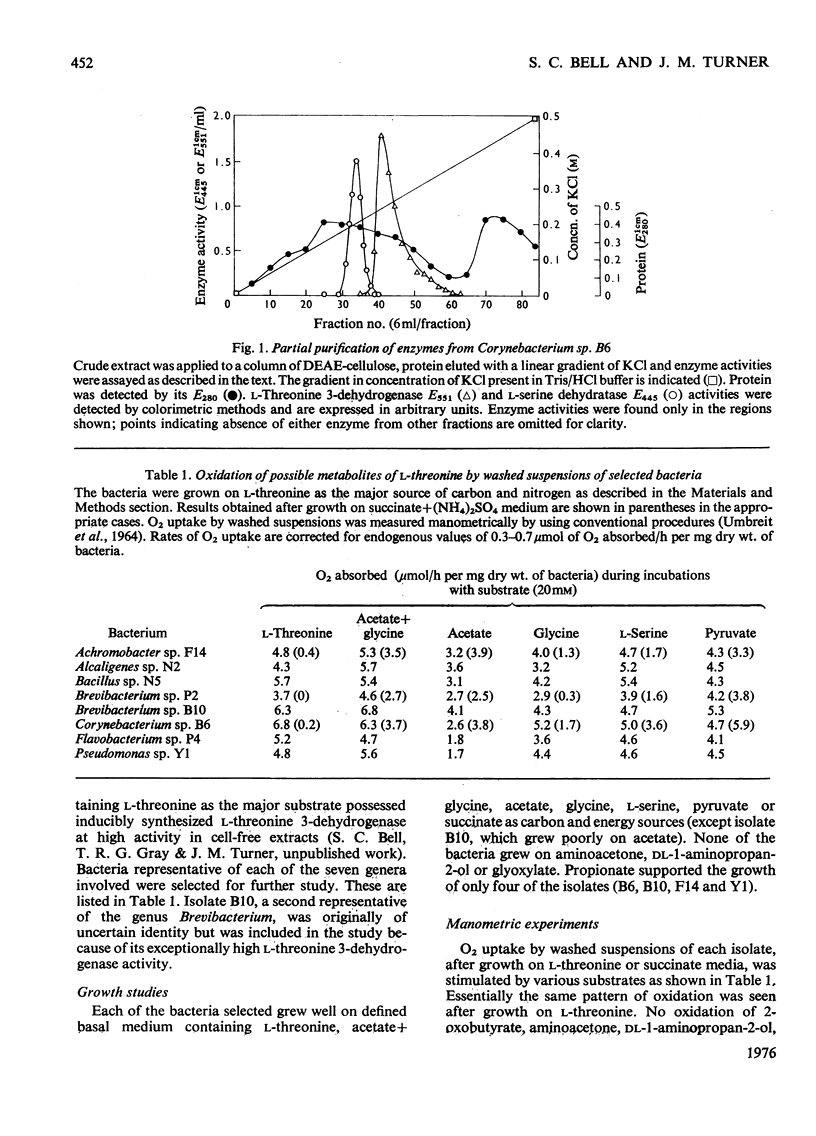
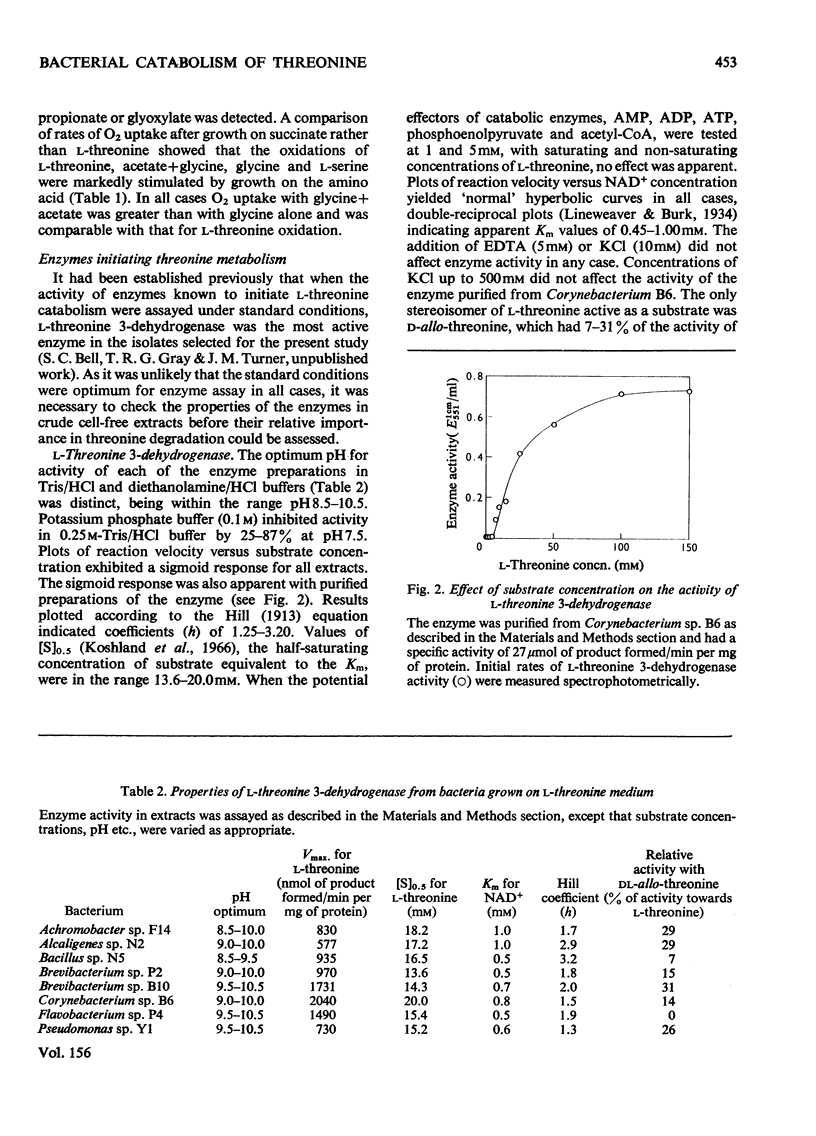

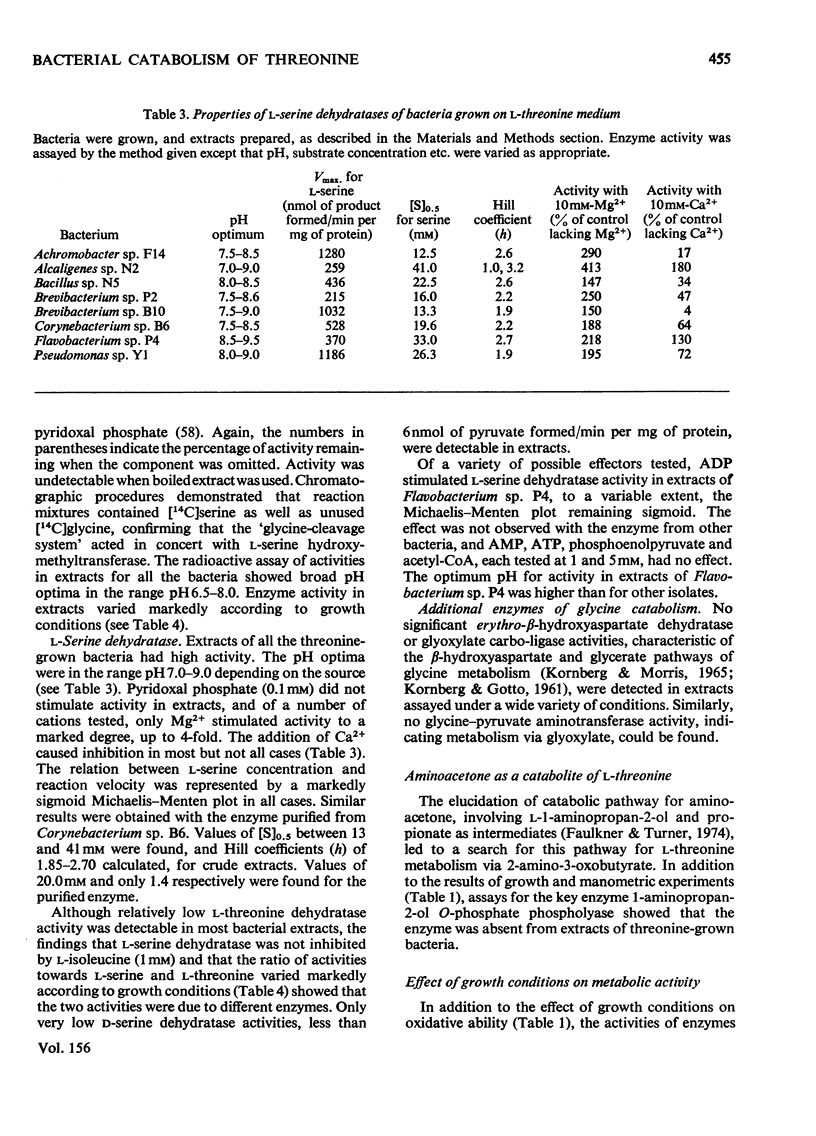
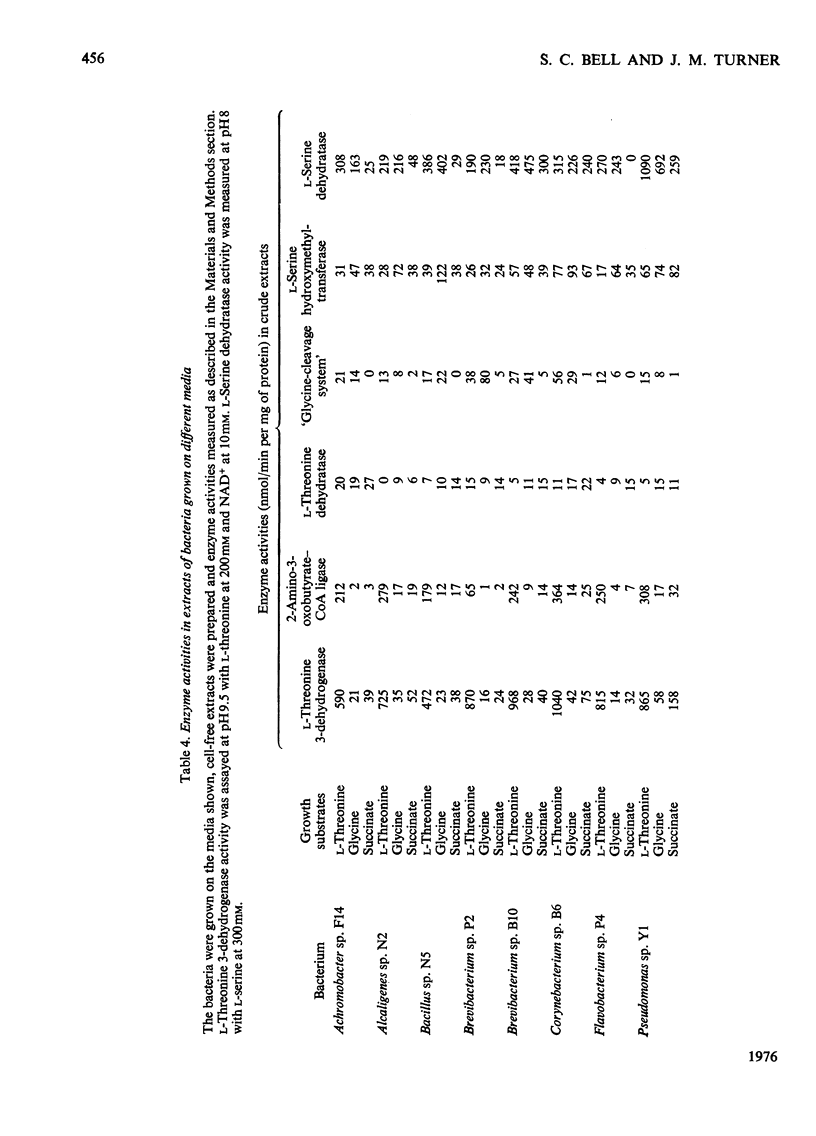


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. C., Turner J. M. L-Threonine catabolism via aminoacetone: a search for a pathway in bacteria. Biochem Soc Trans. 1976;4(3):497–500. doi: 10.1042/bst0040497. [DOI] [PubMed] [Google Scholar]
- Blackmore M. A., Turner J. M. Threonine metabolism via two-carbon compounds by Pseudomonas oxalaticus. J Gen Microbiol. 1971 Aug;67(2):243–246. doi: 10.1099/00221287-67-2-243. [DOI] [PubMed] [Google Scholar]
- Datta P. Purification and feedback control of threonine deaminase activity of Rhodopseudomonas spheroides. J Biol Chem. 1966 Dec 25;241(24):5836–5844. [PubMed] [Google Scholar]
- ELLIOTT W. H. A new threonine metabolite. Biochim Biophys Acta. 1958 Aug;29(2):446–447. doi: 10.1016/0006-3002(58)90215-4. [DOI] [PubMed] [Google Scholar]
- Faulkner A., Turner J. M. Microbial metabolism of amino alcohols. Aminoacetone metabolism via 1-aminopropan-2-ol in Pseudomonas sp. N.C.I.B. 8858. Biochem J. 1974 Feb;138(2):263–276. doi: 10.1042/bj1380263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. L., Elliott W. H. The enzymic formation of aminoacetone from threonine and its further metabolism. Biochem J. 1964 Sep;92(3):537–549. doi: 10.1042/bj0920537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. L. The activation of L-threonine dehydrogenase by potassium ions. Biochem J. 1964 Sep;92(3):550–555. doi: 10.1042/bj0920550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Pickard M. A., Turner J. M. Aminoacetone formation and utilization by pseudomonads grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):105–114. doi: 10.1099/00221287-54-1-105. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols. 1-Aminopropan-2-ol and ethanolamine metabolism via propionaldehyde and acetaldehyde in a species of Pseudomonas. Biochem J. 1973 May;134(1):167–182. doi: 10.1042/bj1340167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., GOTTO A. M. The metabolism of C2 compounds in micro-organisms. 6. Synthesis of cell constituents from glycollate by Pseudomonas sp. Biochem J. 1961 Jan;78:69–82. doi: 10.1042/bj0780069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., MORRIS J. G. THE UTILIZATION OF GLYCOLLATE BY MICROCOCCUS DENITRIFICANS: THE BETA-HYDROXYASPARTATE PATHWAY. Biochem J. 1965 Jun;95:577–586. doi: 10.1042/bj0950577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lessie T. G., Whiteley H. R. Properties of threonine deaminase from a bacterium able to use threonine as sole source of carbon. J Bacteriol. 1969 Nov;100(2):878–889. doi: 10.1128/jb.100.2.878-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G. Utilization of L-threnonine by a pseudomonad: a catabolic role for L-threonine aldolase. Biochem J. 1969 Nov;115(3):603–605. doi: 10.1042/bj1150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUBERGER A., TAIT G. H. The enzymic conversion of threonine to aminoacetone. Biochim Biophys Acta. 1960 Jun 17;41:164–165. doi: 10.1016/0006-3002(60)90388-7. [DOI] [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Higgins I. J., Turner J. M. Purification and properties of l-1-aminopropan-2-ol. NAD oxidoreductase from a pseudomonad grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):115–126. doi: 10.1099/00221287-54-1-115. [DOI] [PubMed] [Google Scholar]
- Rowsell E. V., Snell K., Carnie J. A., Al-Tai A. H. Liver-L-alanine-glyoxylate and L-serine-pyruvate aminotransferase activities: an apparent association with gluconeogenesis. Biochem J. 1969 Dec;115(5):1071–1073. doi: 10.1042/bj1151071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuner J. M. Microbial metabolism of amino ketones. Aminoacetone formation from 1-aminopropan-2-ol by a dehydrgenase in Escerichia coli. Biochem J. 1966 May;99(2):427–433. doi: 10.1042/bj0990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M. Microbial metabolism of amino ketones. L-1-aminopropan-2-ol dehydrogenase and L-threonine dehydrogenase in Escherichia coli. Biochem J. 1967 Jul;104(1):112–121. doi: 10.1042/bj1040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts A. J. Metabolism of threonine by penicillia: growth on threonine as the sole carbon and nitrogen source. J Gen Microbiol. 1972 Nov;73(1):71–83. doi: 10.1099/00221287-73-1-71. [DOI] [PubMed] [Google Scholar]


