Abstract
Direct in vivo evidence for the susceptibility of human neuronal cells to dengue virus has not been reported. In this study, we demonstrated that type 2 dengue (DEN-2) virus infection induced extensive apoptosis in the human neuroblastoma cell line SK-N-SH. Phospholipase A2 (PLA2) was activated by DEN-2 infection, which led to the generation of arachidonic acid (AA). Inhibition of PLA2 activity by the PLA2 inhibitors, AACOCF3 and ONO-RS-082, diminished DEN-2 virus-induced apoptosis. In contrast, the cyclooxygenase inhibitors aspirin and indomethacin, thought to increase AA accumulation by blocking AA catabolism, enhanced apoptosis. Exogenous AA induced apoptosis in a dose-dependent manner. Superoxide anion, which is thought to be generated through the AA-activated NADPH oxidase, was increased after infection. Pretreatment with superoxide dismutase (SOD) protected cells against DEN-2 virus-induced apoptosis. Furthermore, generation of superoxide anion was blocked by AACOCF3. In addition, the transcription factors, NF-κB and c-Jun, were found to be activated after DEN-2 virus infection. However, pretreatment of cells with oligodeoxynucleotides containing NF-κB, but not c-Jun, binding sites (transcription factor decoy) strongly prevented dengue virus-induced apoptosis. The finding that AACOCF3 and SOD significantly block activation of NF-κB suggests that this activation is derived from the AA-superoxide anion pathway. Our results indicate that DEN-2 virus infection of human neuroblastoma cells triggers an apoptotic pathway through PLA2 activation to superoxide anion generation and subsequently to NF-κB activation. This apoptotic effect can be either directly derived from the action of AA and superoxide anion on mitochondria or indirectly derived from the products of apoptosis-related genes activated by NF-κB.
Dengue virus, a mosquito-borne human pathogen, is a member of the Flaviviridae and is classified into four serotypes (Dengue virus type 1 through 4, designated here DEN-1, -2, -3, and -4 virus) (27, 74). Dengue disease, which is caused by dengue virus infection, is considered a major public health problem in Southeast Asia and Central America (25, 58). As a consequence of increasing travel to areas of endemicity, dengue infection has been imported to many parts of the world. Classic dengue fever generally presents in older children and adults with high fever, severe headache and retro-orbital pain, myalgia, arthralgia, nausea, and rash. The acute phase may last for up to a week, but prolonged recovery is common and is sometimes associated with fatigue and depression. In some cases, hemorrhagic manifestations (dengue hemorrhagic fever) and signs of circulatory failure occur, leading to sudden and often hypovolemic shock (dengue shock syndrome) (26, 88). An increasing number of cases have been reported with manifestations of encephalopathy and encephalitis, which cover a wide range of symptoms and signs from headache and clouded sensorium to convulsion, spasticity, and coma (34, 49, 73, 79). As a result, the etiology of the dengue encephalopathy and encephalitis has gained increased attention. However, encephalitis is still rare in dengue virus infections, and other flaviviruses, such as Japanese encephalitis virus, are major causes of encephalitis. Involvement of the central nervous system (CNS) has always been thought to be secondary to vasculitis with resultant fluid extravasation, cerebral edema, hypoperfusion, hyponatremia, and liver failure (28, 37). Direct involvement of the brain by the virus was originally thought to be unlikely (22, 60). However, patients with a diagnosis of dengue encephalitis based on the clinical characteristics of encephalitis and confirmed by cerebrospinal fluid (CSF) microscopy and electroencephalographic changes have been reported (49, 55, 79). In these studies, the onset of encephalitis occurred early in the course of illness (on the second or third day), coinciding with the viremic phase of the disease as identified by reverse transcription-PCR in both CSF and blood. Using immunohistochemical procedures, dengue virus antigens were identified in the CNS and numerous immunolabeled cells were found in brain sections. It has been postulated that dengue virus crosses the brain-blood barrier (BBB) and directly invades the brain, causing encephalitis (79). Immunohistochemical analysis of newborn Swiss mice following intracerebral administration of DEN-1 virus showed that neurons were the major target cells of the dengue virus, and neurolysis was apparent when mice presented severe encephalitis (13, 14). In addition, a study of C3H/HeN mice also showed that encephalitis could be induced when DEN-2 virus was administered intravenously, and these mice subsequently became paralyzed and died (H. K. Sytwu, unpublished data).
In the present study, we investigated how dengue virus infection causes encephalopathy and encephalitis in humans. Although the pathogenesis of dengue virus-induced encephalopathy and encephalitis remains poorly understood, virus-induced neuronal cell death may be a crucial pathogenic event. Apoptotic cell death has been implicated as a cytopathologic mechanism in response to dengue virus infection both in vitro and in vivo (13, 14, 52). Apoptosis is an active process of cell death which occurs in response to a variety of stimuli, including various virus infections (16, 39, 43, 59, 78, 83), and is characterized by a number of distinct morphological features and biochemical processes, such as cell shrinkage, plasma membrane blebbing, chromatin condensation, and intranucleosomal cleavage (15, 36, 76). During the last stage of apoptosis, the cells break up into apoptotic bodies, which are then eliminated by phagocytosis. It has been suggested that apoptosis is a defense mechanism which allows the organism to control virus infection by elimination of infected cells (13); however, several viruses have been shown to induce apoptosis, which is detrimental to the host (39, 43, 78). The induction of apoptosis involves the activation of intracellular signaling systems, and the included pathways are very intricate. In general, in the downstream apoptotic pathways, release of cytochrome c from damaged mitochondria and activation of caspase (the so-called death protease) cascade are commonly observed (48, 70, 85). However, the upstream reactions have not been conclusively determined and are considered to vary depending on the type of apoptotic stimuli. The mechanism of dengue virus-induced apoptosis has been partially demonstrated by Marianneau et al. in a human hepatoma cell line (52). They proposed that the transcription factor NF-κB was involved in the induction of apoptosis. In the present study, we sought to identify the mechanism responsible for apoptosis in human neuroblastoma cells and to determine the upstream reactions that occur before NF-κB activation in DEN-2 virus-infected cells.
Arachidonic acid (AA), a lipid second messenger, is generated by hydrolysis of membrane phospholipids via phospholipase A2 (PLA2) (2, 10, 11). Among the various types of PLA2, a cytosolic PLA2 (cPLA2) which preferentially cleaves sn-2-arachidonyl-containing phospholipids has been reported to be activated in response to a variety of stressors, such as tumor necrosis factor alpha (TNF-α), FasL, and irradiation, and is essential for the induction of apoptosis (31, 40, 47, 54, 82). Malewicz et al. reported that dengue virus was able to activate PLA2 in BHK-21 cells and generate AA (50); however, the effect of AA on these cells was not assessed. In addition, elevated levels of serum PLA2 were observed in dengue patients (62). While the exact role of AA in the apoptotic pathway has not been clearly determined, it has been suggested that AA indirectly activates membrane-associated NADPH oxidase to generate reactive oxygen species (ROS), such as superoxide anion and hydrogen peroxide (11, 19, 28, 71). These ROS may cause mitochondrial membrane damage by peroxidative reactions and finally lead to cell death (44, 65). Furthermore, NF-κB, a well-known transcription factor, has been widely proposed to be involved in either protecting or promoting cell death in response to different stimuli in various cell types (3, 21, 46, 52). NF-κB-activating stimuli generally seem to use oxidative stress as a common signal transduction pathway to elicit their response, and ROS have been implicated as messengers in the activation of NF-κB (4, 51, 68). Based on all this information, three correlated candidates, AA, ROS, and NF-κB, were used in the present study to explore the apoptotic pathway in DEN-2 virus-infected human neuroblastoma cells. Our results indicate that DEN-2 virus infection of human neuroblastoma cells triggers activation of PLA2 to generate AA, which subsequently induces apoptotic cell death by either directly damaging mitochondrial membrane or by stimulating generation of ROS, which cause peroxidative reactions on and alteration of the integrity of the mitochondrial membrane. Pretreatment of cells with the PLA2 inhibitors, ONO-RS-082 and AACOCF3 (41, 81), or the superoxide anion scavenger, superoxide dismutase (SOD), partially prevented DEN-2 virus-induced apoptosis. Furthermore, the transcriptional function of NF-κB may play a very important role in DEN-2 virus-induced apoptosis since double-stranded oligodeoxynucleotides containing the NF-κB binding sequence have been shown to have very efficient protective effects. These results suggest that a signaling pathway consisting sequentially of PLA2 activation, AA elevation, superoxide anion generation, and NF-κB activation is triggered in the DEN-2 virus-infected human neuroblastoma cells, which eventually leads to apoptosis.
MATERIALS AND METHODS
Virus and cell lines.
A local Taiwanese strain of DEN-2 PL046, isolated from patients with dengue fever, was generously provided by the National Institute of Preventive Medicine, Taiwan, Republic of China. Virus propagation was carried out in C6/36 mosquito cells utilizing RPMI 1640 medium containing 5% fetal bovine serum (FBS) (GIBCO BRL). SK-N-SH cells, a human neuroblastoma cell line purchased from the American Type Culture Collection, and BHK-21 cells were grown in RPMI 1640 medium containing 10% FBS and 1× nonessential amino acids.
Western immunoblot analysis.
SK-N-SH cell monolayers were rinsed with phosphate-buffered saline (PBS; pH 7.4), were lysed with radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10 μM EDTA, 0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 1% deoxycholate) (32) containing a cocktail of protease inhibitors, 20 μg of phenylmethylsulfonyl fluoride per ml, 2 μg of leupeptin per ml, and 2 μg of aprotinin per ml, and were stored in aliquots at −70°C. Expression of proteins was measured by Western blot analysis using specific antibodies. Briefly, cell lysates were thawed, mixed with an equal volume of 2× sampling buffer (0.16 M Tris-HCl [pH 6.8], 4% SDS, 0.143 M 2-mercaptoethanol, 33.3% glycerol, 1% bromophenol blue), separated by SDS–15% polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane (Hybond-C extra; Amersham). The nonspecific antibody-binding sites on the membrane were blocked with 5% skim milk in PBS, and membranes were then reacted with specific antibodies. The blots were treated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit immunoglobulins (Santa Cruz) and developed with an ECL kit system (Amersham).
DNA fragmentation assay.
Genomic DNA was extracted from apoptotic SK-N-SH cells according to a published method (45). Briefly, cell suspensions in PBS were incubated with 70% ethanol for 24 h at −20°C. The resulting cells were centrifuged at 500 × g for 5 min to remove ethanol, and the cell pellets were resuspended in 100 μl of PC buffer (192 mM Na2HPO4, 4 mM citric acid [pH 7.8] and incubated at room temperature (RT) for 30 min. After centrifugation at 1,000 × g for 5 min, the supernatants were collected and vacuum concentrated in new microcentrifuge tubes with a SpeedVac for 15 min. Three microliters of 0.25% Nonidet P-40 (NP-40) solution and 3 μl of RNase A solution (1 mg/ml) were added, followed by incubation at 37°C for 30 min. After incubation, 3 μl of proteinase K solution (1 mg/ml) was added, followed by incubation at 37°C for another 30 min. The resulting DNA-containing extracts were then analyzed by 2% agarose gel electrophoresis in 1× Tris-borate-EDTA (TBE) buffer with ethidium bromide.
PI staining and measurement of apoptotic cells.
The mock- and DEN-2-virus-infected SK-N-SH cells in culture plates were trypsinized and then fixed with 75% ethanol at 4°C for 1 h. The fixed cells were washed twice with PBS and treated with RNase A (0.5 mg/ml) and propidium iodide (PI) (50 μg/ml) for 15 min at RT. Chromatin condensation of the stained cells was visualized by fluorescence microscopy (Fluovert, FU; Leitz). Percentages of apoptotic cells were measured by quantifying the subdiploid cells by flow cytometry with Modfit software (Verity Software House, Inc.).
TUNEL assay.
Apoptosis-induced DNA strand breaks were end labeled with fluorescein isothiocyanate (FITC)-dUTP by use of terminal deoxynucleotidyltransferase (TdT) with a commercial kit (In Situ Cell Death Detection Kit; Boehringer Mannheim) according to the manufacturer's instructions. Briefly, 1 × 107 to 2 × 107 cells were washed twice with PBS and transferred into a V-bottom 96-well plate and fixed with paraformaldehyde solution (4% in PBS) for 30 min at RT. The fixed cells were centrifuged at 500 × g for 5 min to remove fixative and then were washed twice with PBS. Cells were resuspended in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 min on ice. After two washings with PBS, cells were resuspended in terminal TdT-mediated dUTP-biotin nick end labeling (TUNEL) reaction mixture and incubated for 60 min at 37°C in a humidified atmosphere in the dark. Labeled cells were then washed twice with PBS and visualized under a Leitz fluorescence microscope.
Assay for PLA2 activity.
PLA2 activity was determined by analysis of AA generation as described by Perez et al. (67). Briefly, SK-N-SH cells were prelabeled with 5 μCi of [5,6,8,9,11,12,14,15-3H]arachidonic acid (208.2 Ci/mmol; Amersham) per ml for 36 h. After labeling, the cells were washed gently three times with serum-free RPMI 1640 medium and infected with DEN-2 virus at a multiplicity of infection (MOI) of 5 in RPMI 1640 medium containing 1% FBS and 1× nonessential amino acids. At various time points after infection, cells in the culture plates were washed three times and cultured with fresh RPMI 1640 medium (with 1% FBS) for 1 h. The culture supernatants were then collected and centrifuged at 2,000 × g for 5 min. Radioactive AA released in the supernatants was measured by scintillation counting.
Superoxide anion detection.
Generation of superoxide anion was determined using a Lumimax Superoxide Anion Detection Kit (Stratagene) with some modifications. A total of 2 × 106 SK-N-SH cells in culture plates were trypsinized and washed twice with PBS. The cells were then resuspended in 200 μl of superoxide anion assay medium and added to 200 μl of superoxide anion reagent mixture (10 μl of 4.0 mM luminol solution plus 10 μl of 5.0 mM enhancer medium plus 180 μl of superoxide anion assay medium) and incubated at RT for 30 min. The relative amounts of superoxide anion were measured by collecting the reaction mixtures and detecting chemiluminescence with a luminometer (New Horizon).
Isolation of cytosol.
Cytosols for detection of cytochrome c were isolated by the method described by Kluck et al. (38). Briefly, 2 × 107 SK-N-SH cells were trypsinized and washed twice with PBS and then resuspended in 500 μl of extraction buffer [220 mM mannitol, 68 mM sucrose, 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)-KOH [pH 7.4], 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 10 μM cytochalasin B, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitors cocktail [Boehringer Mannheim]). After 30 min on ice, cells were broken with a glass Dounce homogenizer, using 40 strokes of the B pestle, and centrifuged at 14,000 × g for 15 min. Supernatant fluids were harvested, and protein concentration was determined by the Bradford assay (Bio-Rad) with bovine serum albumin as a standard.
Subcellular fractionation.
Mitochondrial fractions were prepared by the method described by Vander Heiden et al. (84). Briefly, 2 × 107 SK-N-SH cells were trypsinized and resuspended in 0.8 ml of ice-cold buffer A (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, and protease inhibitors cocktail). Suspended cells were then passed through an ice-cold glass Dounce homogenizer with 40 strokes of the B pestle. Unlysed cells, cell debris, and nuclei were pelleted by a 10-min, 1,000 × g centrifugation. The supernatant fluids were then centrifuged at 10,000 × g for 25 min, and pellets were resuspended in buffer A and represented the mitochondrial fractions.
Detection of NF-κB and c-Jun activation.
Mercury vectors containing either an NF-κB-like promoter region with four copies of NF-κB consensus sequence (CGGGAATTTC) or an AP1-like promoter region with four copies of c-Jun–c-Fos consensus sequence (TGAGTCAG), a downstream reporter gene, and secreted alkaline phosphatase (SEAP) were purchased from Clontech. SK-N-SH cells in 12-well culture plates were transiently transfected with each Mercury vector (5 μg/well) by use of a Lipofectamine reagent (GIBCO BRL) according to the manufacturer's instruction. Twenty-four hours after transfection, the cells were infected with DEN-2 virus at an MOI of 5. At different times after infection, culture supernatants were collected and the activities of NF-κB and c-Jun were determined by detecting the activity of SEAP using a 1-step PNPP ELISA (enzyme-linked immunosorbent assay) Kit (Pierce) with p-nitrophenyl phosphate disodium salt (PNPP) as a soluble substrate.
RESULTS
Apoptosis of SK-N-SH cells induced by DEN-2 virus infection.
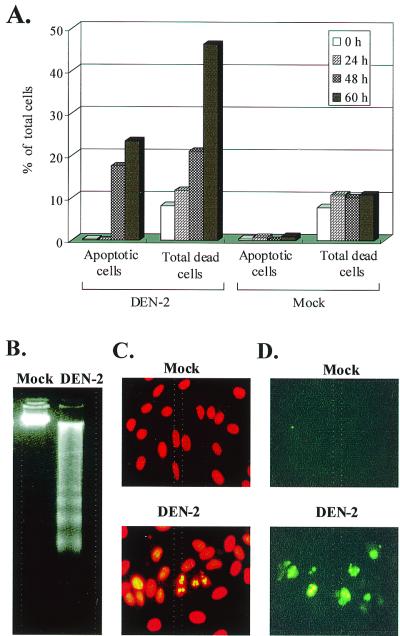
The cytopathologic effect of DEN-2 virus infection in SK-N-SH cells became apparent 36 to 48 h postinfection (p.i.), concurrent with the period in which the infected cells actively produced large quantities of virus. The cytopathologic effect was apparent in the pattern of cell death observed by trypan blue staining. To determine whether apoptosis contributed to DEN-2 virus-induced cell death, the SK-N-SH cells were fixed with 75% ethanol and analyzed by PI staining. Subdiploid cells were then quantified by flow cytometry with Modfit software. As shown in Fig. 1A, when apoptotic cells were observed at 48 h p.i., the number of apoptotic cells was fewer than, but positively proportional with, the number of dead cells determined by trypan blue staining. The numbers of apoptotic cells and dead cells were very similar when determined at 48 h p.i.; however, the higher proportion of dead cells at 60 h p.i. was due to the similar flow cytometric characteristics of these cells in late apoptosis and necrosis. One well-defined biochemical hallmark of apoptosis is the internucleosomal DNA fragmentation (ladder formation) generated during the apoptotic process, which can be visualized by agarose gel electrophoresis with ethidium bromide staining. In the present study, DNA laddering was observed at 48 h p.i. and became most obvious at 60 h p.i. (Fig. 1B). In addition, the morphological alteration of nuclei was directly observed by fluorescence microscopy. When the DEN-2 virus- and mock-infected cells were stained with PI at 48 h p.i., many of the DEN-2 virus-infected cells, but not the mock-infected cells, exhibited chromatin condensation, a characteristic of apoptosis (Fig. 1C). To determine whether chromosomal DNA breaks could be generated during DEN-2 virus infection, the DEN-2- and mock-infected cells were labeled and analyzed by TUNEL assay and microscopy for the presence of DNA fragmentation in the nuclei. After an appropriate incubation period of 60 h, at which DNA laddering was most significant, the cells were end labeled with FITC-dUTP by using TdT. Consistent with the results of PI staining, many of the DEN-2-infected cells, but not mock-infected cells, exhibited the characteristics of DNA breakage (Fig. 1D). In the late infection stage, defined as 72 h p.i., the typical apoptotic morphology transitioned to a morphology with the characteristic appearance of necrosis with diffused DNA in the PI staining and observations of DNA laddering (data not shown). The apoptotic response was delayed at a lower MOI (0.1 and 0.5) for about 12 to 18 h, which is equal to the time for one generation of dengue virus in SK-N-SH cells (data not shown).
FIG. 1.
Evidence for apoptosis in DEN-2 virus-infected human neuroblastoma cells. (A) Comparison of the percentages of apoptotic cells and dead cells. SK-N-SH cells were infected with DEN-2 PL046 virus at an MOI of 5 and were incubated for various time periods as indicated. Apoptotic cells were determined by PI staining and quantified as subdiploid cells by flow cytometry analysis as described in Materials and Methods. Dead cells were determined by trypan blue (0.5% in normal saline) staining and counted under a light microscope. The values represent the means of triplicate measurements from one of two similar experiments. Biochemical analyses (B to D) of DNA fragmentation were done as follows. (B) Genomic DNA laddering. DNA extracted from DEN-2 virus (MOI of 5)- and mock-infected cells at 60 h p.i. were subjected to a 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized on a UV light box. (C) Morphological alteration of nuclei of DEN-2 virus- and mock-infected cells was examined by chromatin condensation. (D) Morphological alteration of nuclei of DEN-2 virus- and mock-infected cells was examined by PI staining and DNA breakage. Breaks in cellular DNA were identified by TUNEL assay using FITC-dUTP labeling and were observed under a fluorescence microscope as described in Materials and Methods.
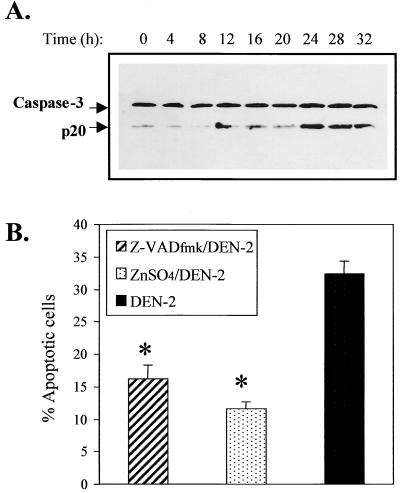
Activation and involvement of caspase-3 in apoptosis of DEN-2 virus-infected SK-N-SH cells.
Activation of the caspase cascade is an important series of events in the downstream apoptotic pathway and is essential for the induction of apoptosis (48, 61, 63, 70, 85). Caspase-3 is a member of the caspase family and appears to play an important role as an apoptotic effector in response to a variety of stimuli. Activation of caspase-3 is the result of cleavage of its 32-kDa precursor into a 20-kDa NH2-terminal fragment (p20 subunit) and 11-kDa COOH-terminal fragment (p11 subunit) (63). We assessed caspase-3 activation in the SK-N-SH cells in response to DEN-2 virus infection by Western blot analysis using monoclonal antibody specific to the p20 subunit. As shown in Fig. 2A, significant cleavage of the caspase-3 precursor was observed beginning at 24 h p.i. at an MOI of 5. Treatment with the pan-caspase inhibitor, Z-VADfmk (Z-Val-Ala-Asp[OME]-CH2F) (61), and ZnSO4, which is a potential inhibitor of endonucleases (5, 86), offered significant protection to SK-N-SH cells against DEN-2 virus-induced apoptosis (Fig. 2B). The protection afforded by Z-VADfmk was dose dependent, and the daily addition of fresh Z-VADfmk offered higher protection (data not shown). ZnSO4 at concentrations lower than 20 μM offered a dose-dependent protection; however, concentrations beyond 20 μM were cytotoxic (data not shown). These results indicate that certain Z-VADfmk-inhibitable cysteine proteases, including caspase-3 and probably caspase-6 or -7 and Zn2+-inhibitable endonucleases, are involved and necessary for DEN-2 virus-induced apoptosis of SK-N-SH cells.
FIG. 2.
Time course of caspase-3 activation and protection by inhibitors of caspase and endonuclease. (A) Caspase-3 activation. SK-N-SH cells were infected with DEN-2 PL046 virus at an MOI of 5. At the indicated times, cells were lysed and lysates were detected for cleavage of the caspase-3 by Western immunoblotting analysis using monoclonal antibody specific to caspase-3 p20 subunit (Santa Cruz). (B) Effects of a pan-caspase inhibitor, Z-VADfmk, and an endonuclease inhibitor, ZnSO4, on DEN-2 virus-induced apoptosis. SK-N-SH cells were pretreated or not pretreated with Z-VADfmk (100 μM) or ZnSO4 (20 mM) for 3 h and infected with DEN-2 virus (MOI of 5). Cells were then cultured in the presence of either inhibitor. After 60 h of infection, apoptotic cells were determined by PI staining and flow cytometry analysis as described in Materials and Methods. The values represent the means ± SD of triplicate determinations from one of two similar experiments, and the asterisks indicate a significant difference between the absence and presence of inhibitor (P < 0.01 by Student's t test).
AA, an apoptosis mediator, is generated in DEN-2 virus-infected cells through the activation of PLA2.
SK-N-SH cells were prelabeled with [3H]AA and infected with DEN-2 virus at an MOI of 5. PLA2 activity was measured by counting the radioactivity of released AA in the culture medium. As shown in Fig. 3A, an increase in AA was observed at 16 h after infection and continued to increase during the measurement period of 32 h. The DEN-2 virus E protein-specific monoclonal antibody (MAb) 56-3.1, which efficiently protected SK-N-SH cells from DEN-2 virus-induced apoptosis (data not shown), also blocked AA production. These results indicate that it is the DEN-2 virus, but not other mediators existing in the culture medium, that is responsible for PLA2 activation and AA production. Direct treatment of SK-N-SH cells with exogenous AA induced apoptosis in a dose-dependent manner (Fig. 3B). The exact intracellular AA concentrations within the exogenous AA-treated cells were difficult to determine; however, concentrations of exogenous AA lower than 25 μM were insufficient to induce apoptosis. DNA laddering in electrophoresis agarose gel (Fig. 3C) demonstrated exogenous AA-induced apoptosis, as shown by the extraction of cellular DNA at 48 h after AA treatment at a concentration of 100 μM.
FIG. 3.
Activation of PLA2 in response to DEN-2 virus infection and induction of apoptosis by AA. (A) Activation of PLA2 demonstrated by increased generation of AA. DEN-2 PL046 virus was incubated with MAb 8-1 specific to DEN-2 viral NS-1 protein or incubated with neutralizing MAb 56-3.1 specific to DEN-2 viral E protein for 1 h at 37°C. [3H]AA-prelabeled SK-N-SH cells were then infected with these virus-antibody mixtures. At the indicated times, the culture supernatants containing the mixtures were collected for radioactivity measurement. (B) Exogenous AA induced apoptosis of SK-N-SH cells in a dose-dependent manner. The percentages of apoptotic cells were determined at 48 h posttreatment. (C) DNA laddering induced by exogenous AA. SK-N-SH cells were mock infected or infected with DEN-2 PL046 virus at an MOI of 5. At 48 h p.i., cellular genomic DNA was extracted and subjected to a 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized on a UV light box. The values represent the means ± SD of triplicate measurements from one of three similar experiments.
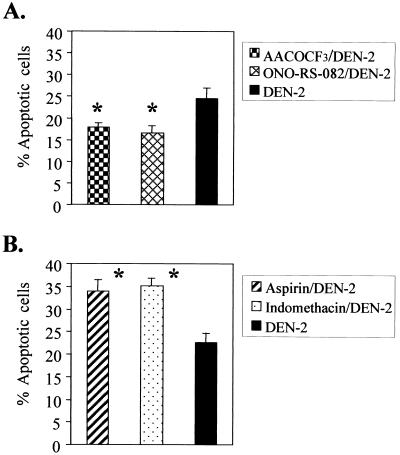
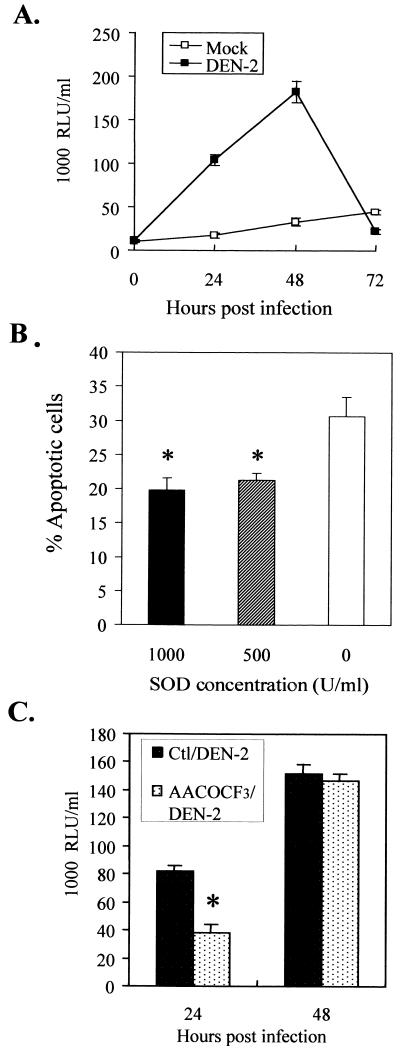
PLA2 inhibitors were protective against DEN-2 virus-induced apoptosis, while cyclooxygenase inhibitors enhanced apoptosis.
To further confirm the inductive role of AA in DEN-2 virus-induced apoptosis, SK-N-SH cells were pretreated with either of the two PLA2 inhibitors, ONO-RS-082 (41) and AACOCF3 (71, 81); the latter is a specific inhibitor for cPLA2. Both inhibitors have been reported to be able to block AA generation. In addition, cells were also pretreated with the cyclooxygenase inhibitors, aspirin and indomethacin (35, 66), that have been reported to cause AA accumulation by blocking the catabolism of AA. The treated cells were then infected with DEN-2 virus at an MOI of 5, and apoptotic cells were determined by PI staining and flow cytometry. The results show that both ONO-RS-082 and AACOCF3 were protective (percentage of cells reaching apoptosis, 16.53 and 17.88% treated versus 24.60% untreated; P < 0.01) (Fig. 4A), while aspirin and indomethacin enhanced apoptosis (percentage of cells reaching apoptosis, 33.99 and 35.10% treated versus 22.65% untreated; P < 0.01) (Fig. 4B). These results reveal the involvement and inductive role of AA in DEN-2 virus-induced SK-N-SH cell apoptosis. All of the four inhibitors were cytotoxic to SK-N-SH cells when used in higher concentrations; therefore, these inhibitors were used at their maximal tolerable concentrations.
FIG. 4.
Effects of inhibitors of PLA2 and cyclooxygenase on DEN-2 virus-induced apoptosis. SK-N-SH cells were pretreated with various inhibitors as indicated for 2 h and were infected with DEN-2 PL046 virus at an MOI of 5. Apoptotic cells were determined at 60 h p.i., and the inhibitors were maintained in the culture medium continuously for the duration of the experiment. (A) PLA2 inhibitors, AACOCF3 (10 μM) and ONO-RS-082 (10 μM), protected cells against DEN-2 virus-induced apoptosis. (B) Cyclooxygenase inhibitors, aspirin (5 mM) and indomethacin (10 μM), enhanced DEN-2 virus-induced apoptosis. The values represent the means ± SD of triplicate measurements from one of three similar experiments, and the asterisks indicate a significant difference between the absence and presence of inhibitor (P < 0.01 by Student's t test).
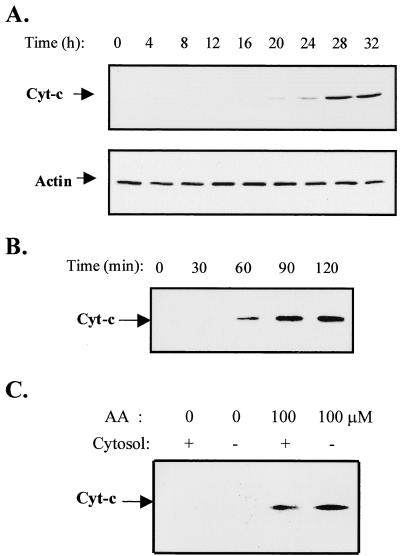
DEN-2 virus infection and AA treatment induced the mitochondrial release of cytochrome c.
Activation of caspase-3 is mediated by caspase-9, which is proteolytically activated by binding with Apaf-1 via their respective NH2-terminal CED-3 homologous domains in the presence of cytochrome c and ATP. Activated caspase-9 in turn cleaves and activates caspase-3 (85). Since the present study has demonstrated the activation of caspase-3 in DEN-2 virus-infected SK-N-SH cells, the release of cytochrome c, which also indicates mitochondrial damage, should be detectable. SK-N-SH cells were infected with DEN-2 virus at an MOI of 5, and cell cytosols were extracted at the indicated times and the presence of cytochrome c was detected by Western blot analysis using specific monoclonal antibody. The presence of actin in the cytosol fraction was measured as an internal control. As shown in Fig. 5A, the existence of cytochrome c in the cytosol fraction was first observed as a very weak band at 24 h p.i. and became obvious beginning at 28 h p.i. The effect of AA on cytochrome c release was also assessed. SK-N-SH cells were treated with exogenous AA at a concentration of 100 μM. Cell cytosols were collected and cytochrome c levels were measured as described above. As shown in Fig. 5B, exogenous AA treatment rapidly induced cytochrome c release by 1 h. Furthermore, direct treatment of isolated mitochondrial fraction with AA also caused cytochrome c release within 1 h, and additional cytosol did not enhance cytochrome c release (Fig. 5C). These results indicate that AA may directly exert a detrimental impact on the outer membrane of mitochondria.
FIG. 5.
Release of cytochrome c from mitochondria in response to DEN-2 virus infection and exogenous AA. SK-N-SH cells were infected with DEN-2 PL046 virus at an MOI of 5 (A) or treated with exogenous AA at a concentration of 100 μM (B). At the indicated times, cytosols were isolated and the presence of cytochrome c was detected by Western immunoblotting analysis using monoclonal antibody specific to cytochrome c. The levels of actin in the cytosols were demonstrated as an internal control. (C) Mitochondrial fractions were isolated from uninfected SK-N-SH cells and treated with AA (100 μM) in the presence or absence of cytosol for 30 min. Cytochrome c released into the buffer was detected as described above.
Superoxide anion generation was induced by DEN-2 virus infection in SK-N-SH cells and was involved in induction of apoptosis.
Generation of superoxide anion has been reported to be induced in response to a variety of stimuli and to cause apoptotic cell death (44, 65). The generation of superoxide anion was reported to be mediated by NADPH oxidase or 5-lipooxygenase (11, 21), both of which can be indirectly activated by AA. Because this study has demonstrated that AA is overgenerated in DEN-2 virus-infected SK-N-SH cells, the potential involvement of superoxide anion and the relationship between AA and superoxide anion in DEN-2 virus-induced apoptosis were assessed. Our results show that the level of intracellular superoxide anion was increased by 24 h p.i. and peaked at 48 h p.i. (Fig. 6A). The abrupt decrease in superoxide anion detected at 72 h p.i. was due to the few surviving cells and the short life of superoxide anion. The involvement of superoxide anion in DEN-2 virus-induced apoptosis was further assessed by treating cells with SOD prior to virus inoculation. The results showed that SOD protected SK-N-SH cells from DEN-2 virus-induced apoptosis in a dose-dependent manner (percentage of cells reaching apoptosis after treatment with SOD at 1,000 U/ml, 19.73% ± 1.85% [mean ± standard deviation {SD}]; 500 U/ml, 21.24% ± 1.07%; and 0 U/ml, 30.60% ± 2.89%; P < 0.01) (Fig. 6B). To determine whether AA is responsible for DEN-2 virus-induced superoxide anion generation, SK-N-SH cells were pretreated for 30 min with a cPLA2-specific inhibitor, AACOCF3, and intracellular levels of superoxide anion were measured after DEN-2 virus infection. The results indicate that treatment with AACOCF3 significantly delayed superoxide anion generation by about 24 h. As shown in Fig. 6C, at 24 h p.i., the level of superoxide anion in AACOCF3-treated cells was less than half of that in the nontreated cells (38 × 1,000 versus 82 × 1,000 RLU/ml); however, no difference in the levels of superoxide anion was found in cells from these groups at 48 h p.i.
FIG. 6.
Superoxide anion is generated downstream of PLA2 and is essential for apoptosis. (A) Generation of superoxide anion. SK-N-SH cells were infected with DEN-2 PL046 virus at an MOI of 5. At the indicated times, culture supernatants were collected and the amount of superoxide anion was detected using a Lumimax Superoxide Anion Detection Kit as described in Materials and Methods. (B) DEN-2 virus-induced apoptosis is blocked by SOD. SK-N-SH cells were pretreated with SOD at 1,000, 500, and 0 U/ml for 1 h and then were infected with DEN-2 PL046 virus at an MOI of 5. After 60 h of infection, apoptotic cells were determined by PI staining and flow cytometry analysis. (C) A cPLA2-specific inhibitor, AACOCF3, delayed DEN-2 virus-induced superoxide anion generation. SK-N-SH cells were pretreated with AACOCF3 (10 μM) for 1 h and then were infected with DEN-2 PL046 virus at an MOI of 5. At 24 and 48 h p.i., culture supernatants were collected and the amount of superoxide anion was measured. The values represent the means ± SD of triplicate measurements from one of two similar experiments. An asterisk indicates a significant difference between the absence and presence of SOD (B) and AACOCF3 (C) (P < 0.01 by Student's t test). Ctl, control.
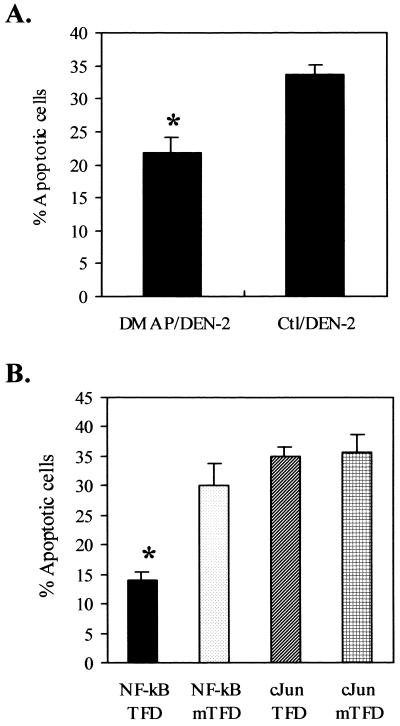
Transcription activity of NF-κB, but not c-Jun, was required for DEN-2 virus-induced apoptosis.
Apoptosis is achieved through various signal transductions, and AA has been implicated in some of the signal transduction pathways resulting in apoptosis. It has been reported that cPLA2 is activated by mitogen-activated protein kinase (45), the activity of which can be blocked by the protein kinase inhibitor 6-dimethylaminopurine (DMAP) (53). To assess the involvement of protein phosphorylation reactions in the DEN-2 virus-triggered apoptotic signaling pathways, SK-N-SH cells were pretreated with DMAP before DEN-2 virus infection and 60 h after infection, and the percentage of apoptotic cells was then determined. The results show that DMAP protected cells against apoptosis (percentage of cells reaching apoptosis, 21.85% ± 2.32% [mean ± SD] of DMAP pretreated versus 33.68% ± 1.54% of untreated; P < 0.01) (Fig. 7A). DMAP exerted no obvious effect on either the quantity or the time course of viral protein synthesis or on progeny virus production during the first 24 h of infection (data not shown). Since phosphorylation reactions were probably involved in DEN-2 virus-induced apoptosis, two transcription factors, NF-κB and c-Jun, which require phosphorylation for their activity and have been reported to be downstream mediators of AA (4, 11, 28), were assessed for their involvement in DEN-2 virus-induced apoptosis. Both of these transcription factors are known to be important mediators of apoptosis in response to a variety of stresses (11, 46, 71, 80). To investigate the involvement of these transcription factors, we pretreated SK-N-SH cells with synthetic oligonucleotides (transcription factor decoy [TFD]) consisting of the binding motifs for NF-κB and c-Jun and mutant oligonucleotides (mTFDs) with one nucleotide substitution in the binding motif prior to DEN-2 virus infection. This single nucleotide substitution diminished transcription factor binding. The percentage of apoptotic cells was measured at 60 h p.i., and the results indicate that NF-κB, but not c-Jun, plays a determinant role in the DEN-2 virus-induced apoptosis. The percentages of apoptotic cells were as follows: 13.91% ± 1.37% (mean ± SD) for NF-κB TFD, 30.04% ± 3.67% for NF-κB mTFD, 34.97% ± 1.69% for c-Jun TFD, and 35.79% ± 2.85% for c-Jun mTFD (Fig. 7B). The protection of NF-κB TFD was dose dependent; a concentration under 25 μM showed no significant protection. In contrast to the study of Marianneau et al. with HepG2 cells (52), which showed that 1 μM NF-κB TFD blocked apoptosis, our results show that much higher doses of the NF-κB TFD are required for SK-N-SH cells. The reason for the different requirement is not really understood; however, our explanation according to our work on HepG2 cells is that SK-N-SH cells are much more susceptible to dengue virus than HepG2 cells. First, the infection rate of SK-N-SH cells with dengue virus is almost 100%, but it is only 30% or less for HepG2 cells. Second, SK-N-SH cells produced a much higher titer (>107 PFU/ml) of dengue virus than HepG2 cells produced (105 to 106 PFU/ml). These results indicate that SK-N-SH cells suffer more stress than HepG2 cells during dengue virus infection.
FIG. 7.
Kinase reactions and activity of NF-κB, but not c-Jun, are required for DEN-2 virus-induced apoptosis. (A) A protein kinase inhibitor, DMAP, significantly decreased DEN-2 virus-induced apoptosis. SK-N-SH cells were pretreated with DMAP (5 mM) for 1 h and then were infected with DEN-2 PL046 virus at an MOI of 5. Apoptotic cells were counted by PI staining and flow cytometry at 60 h p.i. (B) NF-κB TFD, but not c-Jun TFD, blocked DEN-2 virus-induced apoptosis. SK-N-SH cells were pretreated for 6 h with 100 μM concentrations of each of the following oligonucleotides competing for transcription factor binding: NF-κB TFD (5′-AGTTGAGGGGACTTTCCCAGGC-3′), NF-κB mTFD (5′-AGTTGAGGCGACTTTCCCAGG-3′), c-Jun TFD (5′-CGCTTGATGACTCAGCCGGAA-3′), and c-Jun mTFD (5′-CGCTTGATGACTTGGCCGGAA-3′) (boldface characters represent the oligonucleotide substitution in the binding motif). The cells were then infected with DEN-2 PL046 virus at an MOI of 5. Apoptotic cells were measured at 60 h p.i. The values represent the means ± SD of triplicate measurements from one of three similar experiments. An asterisk indicates a significant difference between the absence and presence of DMAP (A) and NF-κB TFD (B) (P < 0.01 by Student's t test). Ctl, control.
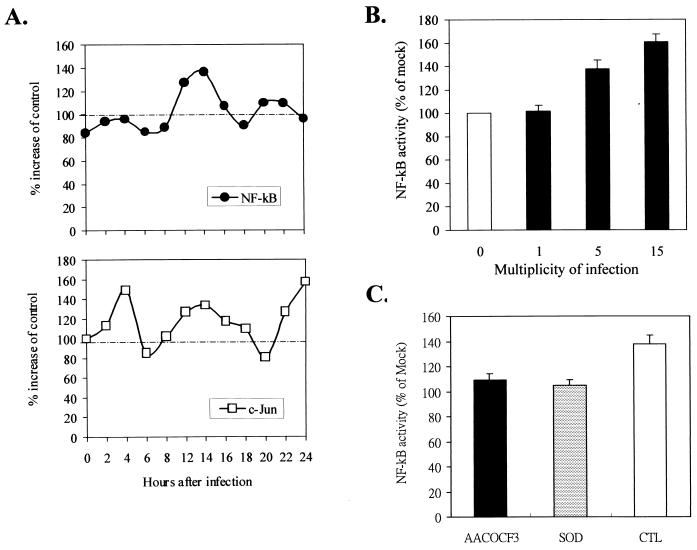
NF-κB activation in DEN-2 virus-infected cells through the AA-superoxide anion pathway.
SK-N-SH cells transiently transfected with Mercury vectors containing NF-κB and c-Jun promoter regions were infected with DEN-2 virus at an MOI of 5. The transfection process and transcription activity assay were done as described in Materials and Methods. Activation of NF-κB occurred at 12 to 16 h p.i., while activation of c-Jun occurred early at 4 h p.i. (Fig. 8A). The multiple peaks of c-Jun activity found after infection were probably due to different transcription factors, such as c-Fos, which shares the same promoter-binding motif as c-Jun. The activation of NF-κB by DEN-2 virus was dose dependent, with a higher MOI of inoculating virus inducing stronger NF-κB activity (Fig. 8B). Pretreatment of cells with AACOCF3 and SOD significantly blocked NF-κB activation. The values of NF-κB activity were as follows, as a percentage of mock infection: 109.2% ± 3.9% (mean ± SD) (AACOCF3 treated) and 105.6% ± 5.2% (SOD treated) versus 136.8% ± 8.4% (untreated), (P < 0.05) (Fig. 8C). These results indicate that NF-κB activation in DEN-2 virus-infected SK-N-SH cells is at least partially dependent on AA-dependent generation of superoxide anion.
FIG. 8.
NF-κB is activated downstream of PLA2 activation and superoxide anion generation. (A) Activation of NF-κB and c-Jun following DEN-2 virus infection. SK-N-SH cells were pretransfected with pNFκB-SEAP and pAP1-SEAP mercury vectors by Lipofectamine as described in Materials and Methods. At 24 h posttransfection, cells were mock infected (control [CTL]) or infected with DEN-2 PL046 virus at an MOI of 5 and maintained at 4°C for 1 h to allow virus binding. The virus inoculum was replaced by fresh culture medium before shifting to an incubator at 37°C. Activities of NF-κB and c-Jun were measured by detecting the activity of SEAP using the 1-Step PNPP ELISA kit as described in Materials and Methods. (B) DEN-2 virus activated NF-κB in a dose-dependent manner. pNFκB-SEAP-transfected SK-N-SH cells were infected with various MOIs of DEN-2 PL046 virus, and the NF-κB activity was measured at 14 h p.i. (C) AACOCF3 and SOD blocked DEN-2 virus-induced NF-κB activation. pNFκB-SEAP-transfected SK-N-SH cells were treated with AACOCF3 (10 μM) or SOD (1,000 U/ml) for 1 h and then were infected with DEN-2 PL046 virus at an MOI of 5. Activities of NF-κB were determined at 14 h p.i. The values represent the means ± SD of triplicate measurements from one of two similar experiments. An asterisk indicates a significant difference between the absence and presence of inhibitor (P < 0.05 by Student's t test).
DISCUSSION
Although dengue virus can infect a variety of cells in vitro, only very few cell types have been identified to be infected in vivo. The most recognized target cells for dengue virus in humans are the mononuclear phagocytes (24). In recent years, dengue viral antigens have been found by immunofluorescence staining and in situ hybridization in liver (23, 52, 72) and vascular tissues (W. J. Shieh, personal communication) of patients with fatal dengue infections. In addition, evidence of CNS infection has also been reported (13, 14, 49, 60). Isolation of DEN-3 virus and detection of DEN-2 virus by PCR in CSF provided strong evidence that dengue virus has neurovirulent properties and can cause encephalitis in both primary and secondary infections (49, 60). An increasing number of dengue patients with severe manifestations of encephalitis and encephalopathy has been reported (49, 73). Although the pathogenetic mechanism of the neural manifestations remains unclear, it has been strongly suggested that the neural pathology is induced by the detrimental effects of cytotoxic cytokines, such as TNF-α (30), interleukin-1 (9), and cytotoxic factors (77) released from virus-infected monocytes and activated T lymphocytes. However, the possibility that direct damage of neuronal cells occurs with dengue virus infection cannot be excluded, since only a few encephalitis cases have been reported. Vascular endothelial cells have demonstrated a similar susceptibility to dengue virus infection. Dengue virus infects endothelial cells in vitro and causes apoptosis (1); however, vascular leakage is common while evidence for infection in vivo in endothelial cells is rare. An explanation for the few cases of neural cell infection has been suggested as being due to the low titer of virus in blood, which does not allow virus to pass the BBB during the short viremia period. In addition, although there is no ideal animal model available for dengue studies, a mouse model has been widely employed and results in an encephalitis outcome after dengue virus inoculation via an intracerebral or intravenous route. Dengue virus can be isolated from the brains of infected mice, and neurons are the main infected cells. Since dengue virus can infect and directly cause damage to mouse neuronal cells, dengue virus may have a similar potential to infect human neuronal cells if it can pass through the BBB. Although the in vivo evidence for dengue virus infection in human neuronal cells is lacking, in vitro studies of the susceptibility of human and mouse neuroblastoma cells have been reported (13, 69). We have found that the DEN-2 virus is able to cause a productive infection in the human neuroblastoma cell line SK-N-SH. The infection was very efficient, since the SK-N-SH cells produced a titer of virus similar to that of BHK-21 cells (up to >2 × 107 PFU/ml), which are very susceptible to dengue virus infection and widely used for dengue virus titration. In addition, the well-known antibody-dependent enhancement (ADE) phenomenon was not observed in the SK-N-SH cells although they also bear the similar levels of Fc receptors (20), which play a role as an alternative port of entry for dengue virus in human monocytes/macrophages. A 65-kDa trypsin-sensible protein has been reported to be a putative receptor for dengue virus on the SK-N-SH cell surface (69). It is not known whether monocytes/macrophages also share the same protein for dengue virus binding (12).
Dengue virus has been demonstrated to induce apoptosis in a variety of cultured cell lines, including mouse neuroblastoma cell lines (13). However, the detrimental impact of dengue virus on human neuroblastoma cells has not yet been determined. The present study is the first to demonstrate the involvement of a signaling pathway consisting sequentially of activation of PLA2, generation of superoxide anion, and finally activation of NF-κB in DEN-2 virus-induced apoptosis of human neuroblastoma cells. PLA2 plays a key role in cellular signaling by generating a wide array of biologically active lipid mediators (31, 40, 82). PLA2-mediated hydrolysis of phospholipids results in the release of AA, which may either exert direct effects or serve as a substrate for the generation of other lipid messengers, such as prostaglandins and leukotrienes (2). At least two types of PLA2 have been identified: 85-kDa PLA2, which when present in the cytosol is named cPLA2, and 14-kDa PLA2, which is present in cellular granules and is secreted into the extracellular medium upon stimulation and is named secretory PLA2 (sPLA2) (54). In the present study, it is not known whether DEN-2 virus activated both of these types of PLA2; however, it is clear that at least cPLA2 was activated since a cPLA2-specific inhibitor, AACOCF3, was shown to be protective. AA generation as a result of PLA2 activation has been reported in signal transduction pathways resulting in apoptosis. The involvement of AA in dengue virus infection was first reported by Malewicz et al. (50). In their studies, rapid PLA2 activation was detected in BHK-21 cells during the initiation of dengue virus infection. More recently, Nevalainen et al. reported that dengue virus infection caused elevation of serum PLA2 (62). Nevertheless, the former study did not offer observations concerning fatal outcome or discussion of the pathogenetic mechanism of the infected cells. In the present study, we have demonstrated that DEN-2 virus can cause apoptosis of human neuroblastoma cells characterized by DNA fragmentation through the direct or indirect actions of AA generated by activated PLA2. The involvement of AA in apoptosis has been implicated in previous studies involving various apoptotic stimuli. Activation of cPLA2 has been indicated to be essential for TNF-mediated cytotoxicity in L929, MCF-7S, WEHI, and U937 cells (18, 31, 90); L929 mutants that had lost the expression of cPLA2 could be rendered TNF sensitive by exogenous expression of cPLA2. Moreover, inhibition of cPLA2 expression by antisense oligonucleotides has been shown to render melanoma cells resistant to TNF-mediated cytotoxicity. Our results demonstrate that AA was generated after DEN-2 virus infection and that pretreatment of human neuroblastoma cells with a cPLA2-specific inhibitor, AACOCF3, or a PLA2 inhibitor, ONO-RS-082, which prevented DEN-2 virus-induced apoptosis, supports a role of PLA2 in the mediation of DEN-2 virus-induced apoptosis. The mechanism by which AA mediates apoptosis is not fully understood. Wissing et al. indicated that activation of cPLA2 was inhibited by caspase inhibitors, revealing that AA functions downstream of caspase activity (87). In the present study, we demonstrated that treatment of SK-N-SH cells with exogenous AA induced rapid cytochrome c release within 1 h, and moreover, direct treatment of mitochondrial fractions with AA in the absence of cytosolic fractions was able to stimulate cytochrome c release. Cytochrome c has been recognized as an effector for caspase activation, and release of cytochrome c into the cytosol is an indicator of mitochondrial outer membrane damage. Although the detailed mechanism of this detrimental impact remains unclear, it has been reported that AA could alter cell membrane integrity and cause lipid peroxidation (8). In addition, it has been proposed that neuronal mitochondrial dysfunction may be a critical event in neuronal cell death, and AA was indicated to be able to mediate alteration of mitochondrial function (6). Our results reveal that part of the DEN-2 virus-mediated apoptotic effect is derived from the direct action of AA on mitochondria and suggest that AA is also active upstream as a caspase inducer by stimulating cytochrome c release.
AA has been reported to stimulate the generation of ROS, which are generated in all aerobic cells (4, 11). ROS are mainly produced during normal mitochondrial respiration and are used by specialized phagocytic cells to destroy invading pathogens. ROS can react rapidly with a range of biological molecules, making them highly destructive. Fortunately, aerobic cells are endowed with a complex network of antioxidants to scavenge ROS or repair the damage; however, the overwhelming majority of these defenses, termed oxidative stress, are implicated in numerous disease states. Infection of mice T lymphocytes with dengue virus both in vitro and in vivo produced a cytotoxic factor, which stimulates macrophages to produce cytotoxin (CF2) (56). It was observed that CF2 induced production of superoxide anion by the spleen cells and that treatment with SOD inhibited superoxide anion production and cytoxicity. The enzymes for superoxide anion production include NADPH oxidase and 5-lipoxygenase, depending on the various cell types (4, 11). Our results show that superoxide anion was produced in human neuroblastoma cells in response to DEN-2 virus infection. The protective effect of SOD treatment demonstrates that superoxide anion was involved in the induction of DEN-2 virus-induced apoptosis. The production of superoxide anion was probably due to the elevation of intracellular AA, since treatment with the PLA2 inhibitors AACOCF3 and ONO-RS-082 reduced superoxide anion production in DEN-2 virus-infected cells. AA has been reported to be able to activate NADPH oxidase (11), which suggests that NADPH oxidase is the enzyme that stimulated superoxide anion generation in the DEN-2 virus-infected cells. Although ROS have been widely suggested to be involved in apoptosis, it is still not clear how superoxide anion can induce apoptosis. Except for their role in mediating oxidative modification of critical cellular targets, including proteins or lipids, and formation of peroxynitrite with nitric oxide, evidence has suggested that ROS can be utilized in signal transduction events. Two transcription factors, c-Jun and NF-κB, have been reported to be activated downstream of ROS. c-Jun, a transcription factor activated by c-Jun N-terminal kinase, is stimulated by a variety of stresses and required for the induction of apoptosis (71). NF-κB, another important transcription factor, is known to mediate the inducible expression of a wide variety of genes that are involved in inflammatory and other cytotoxic reactions in numerous cell types (3, 21, 46). Several genes implicated in apoptosis, such as those for transforming growth factor β (42), c-myc (17), p53 (89), and interleukin-1β converting enzyme (ICE) (7), have consensus NF-κB sites in their promoters. Activation of NF-κB in response to dengue virus infection has been identified in human hepatoma cells and was implicated to induce apoptosis (52). The results of the present study show that NF-κB is also activated in DEN-2 virus-infected human neuroblastoma cells. Significant blocking of NF-κB activation by AACOCF3 and SOD indicate that this activation was induced through the AA-superoxide anion pathway. Our experiments using NF-κB and c-Jun decoys indicated that NF-κB, but not c-Jun, is involved in DEN-2 virus-induced apoptosis of human neuroblastoma cells and that it is the product of NF-κB-activated genes but not the subunits of NF-κB such as p65 or p50 (80), that is essential for the induction of apoptosis. An alternative strategy to confirm the involvement of NF-κB in induction of apoptosis was performed by overexpression of dominant-negative mutant IκBα. Our preliminary results in transiently transfected SK-N-SH cells also show protection (data not shown), which support the importance of NF-κB in dengue virus-induced apoptosis. One of the target genes for NF-κB is the p53 gene, a tumor suppressor gene. The best known activities of p53 protein are cell cycle arrest and induction of apoptosis. Both of these activities probably require activation of latent p53 protein by incoming signals, often coupled with a substantial increase in overall cellular p53 levels (64). In the present study, we have observed a dramatic increase of p53 protein at 14 h p.i. in DEN-2 virus-infected SK-N-SH cells, which is consistent with the NF-κB peak (data not shown).
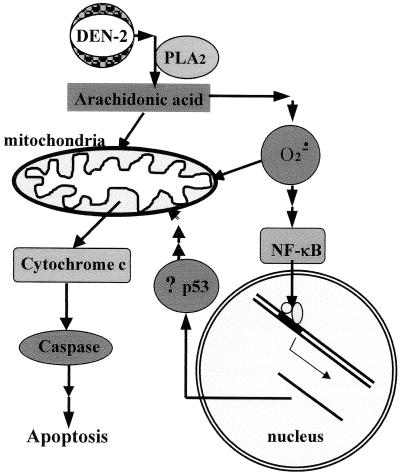
In conclusion, as illustrated in Fig. 9, DEN-2 virus-triggered apoptotic signaling includes the activation of PLA2s that sequentially generate AA, superoxide anion, and NF-κB activation, all of which are capable of inducing apoptotic damage but to different extents. The detrimental impacts may come from the direct action of AA and probably superoxide anion on the mitochondrial membrane and proteins that were generated by NF-κB which induce and/or enhance the apoptosis. It is not known how dengue virus could activate PLA2 to start the apoptotic signaling. In general, cPLA2 is believed to be activated by an upstream ligand-receptor interaction. Probable mediators within this pathway include phospholipase C (75), protein kinase C (57), ceramide-activated protein kinase (75, 91), Ca2+-calmodulin-dependent protein kinase (10), and mitogen-activated protein kinase (46). Our results show that the protein kinase inhibitor DMAP provides significant protection against apoptosis, indicating that a series of protein phosphorylation reactions occur during dengue virus infection and are essential for the induction of apoptosis. Although it has always been thought that intracellular synthesis of viral proteins seems to be essential to activate apoptotic pathways in the host cells, nevertheless, our previous Sindbis virus studies indicate that apoptotic signaling may first be triggered by the interaction of viral envelope protein with the endosomal membrane during the fusion process while newly synthesized viral proteins may enhance apoptosis (33). Lin et al. reported that a virus may trigger apoptotic signaling during the very early stage of infection before viral protein synthesis in their NF-κB studies (46). In addition, their results also indicate the involvement of NF-κB in mediating apoptosis. Further studies of the detailed interactions between dengue virus and susceptible cells are necessary to identify the key molecules on either cell or virus that are responsible for triggering apoptotic signaling.
FIG. 9.
Proposed model of the DEN-2 virus-induced apoptotic signaling pathways.
ACKNOWLEDGMENTS
We greatly appreciate Diane E. Griffin (Johns Hopkins University) and Ching-Len Liao (National Defense Medical Center) for stimulating discussions which contributed to this work.
This work was supported by grant NHRI-CN-CL8902P from the National Health Research Institute, Republic of China (ROC), and grant NSC 89-2320-B-016-45 from the National Science Council, ROC.
REFERENCES
- 1.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 2.Axelroid J, Burch R M, Jelsema C L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988;11:117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κ B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville M P, Bours V. Reactive oxygen intermediate-dependent NF-κB activation by interleukin-1-β requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booker J K, Reap E A, Cohen P L. Expression and function of Fas on cells damaged by gamma-irradiation in B6 and B6/lpr mice. J Immunol. 1998;161:4536–4541. [PubMed] [Google Scholar]
- 6.Camins A, Sureda F X, Gabriel C, Pallas M, Escubedo E, Camarasa J. Modulation of neuronal mitochondrial membrane potential by the NMDA receptor: role of arachidonic acid. Brain Res. 1997;777:69–74. doi: 10.1016/s0006-8993(97)00947-5. [DOI] [PubMed] [Google Scholar]
- 7.Casano F J, Rolando A M, Mudgett J S, Molineaux S M. The structure and complete nucleotide sequence of the murine gene encoding interleukin-1 beta converting enzyme (ICE) Genomics. 1994;20:474–481. doi: 10.1006/geno.1994.1203. [DOI] [PubMed] [Google Scholar]
- 8.Chan P H, Fishman R A. Alterations of membrane integrity and cellular constituents by arachidonic acid in neuroblastoma and glioma cells. Brain Res. 1982;248:151–157. doi: 10.1016/0006-8993(82)91156-8. [DOI] [PubMed] [Google Scholar]
- 9.Chang D M, Shaio M F. Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocytes exposed to dengue virus. J Infect Dis. 1994;170:811–817. doi: 10.1093/infdis/170.4.811. [DOI] [PubMed] [Google Scholar]
- 10.Clark J D, Lin L L, Kriz R W, Ramesha C S, Sultzman L A, Lin A Y, Milona N, Knopf J L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 11.Cui X L, Douglas J G. Arachidonic acid activates c-Jun N-terminal kinase through NADPH oxidase in rabbit proximal tubular epithelial cells. Proc Natl Acad Sci USA. 1997;94:3771–3776. doi: 10.1073/pnas.94.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daughaday C C, Brandt W E, McCown J M, Russell P K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981;32:469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despres P, Falmand M, Ceccaldi P E, Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despres P, Frenkiel M P, Ceccaldi P E, Santos C D D, Deubel V. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J Virol. 1998;72:823–829. doi: 10.1128/jvi.72.1.823-829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dive C, Gregory C, Phipps D, Evans D J, Milner D L, Wyllie A H. Analysis and discrimination of necrosis and apoptosis (programmed cell death) by multiparameter flow cytometry. Biochim Biophys Acta. 1992;1133:275–285. doi: 10.1016/0167-4889(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 16.Esolen L M, Park S W, Hardwick J M, Griffin D E. Apoptosis as a cause of death in measles virus-infected cells. J Virol. 1995;69:3955–3958. doi: 10.1128/jvi.69.6.3955-3958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by myc-protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 18.Fulda S, Sieverts H, Friesen C, Herr I, Debatin K M. The CD95 (APO-1/Fas) system mediates drug-induced apoptosis in neuroblastoma cells. Cancer Res. 1997;57:3823–3829. [PubMed] [Google Scholar]
- 19.Giardina C, Boulares H, Inan M S. NSAIDs and butyrate sensitize a human colorectal cancer cell line to TNF-alpha and Fas ligation: the role of reactive oxygen species. Biochim Biophys Acta. 1999;1448:425–438. doi: 10.1016/s0167-4889(98)00156-6. [DOI] [PubMed] [Google Scholar]
- 20.Gorini G, Ciotti M T, Starace G, Vigneti E, Raschella G. Fc gamma receptors are expressed on human neuroblastoma cell lines: lack of correlation with N-myc oncogene activity. Int J Neurosci. 1992;62:287–297. doi: 10.3109/00207459108999781. [DOI] [PubMed] [Google Scholar]
- 21.Grimm S, Bauer M K, Bauerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-κB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubler D J, Kuno G, Waterman S H. Neurological disorders associated with dengue infection. In: Pan T, Pathmanathan R, editors. Proceedings of the International Conference on Dengue/Dengue Haemorrhagic Fever. Kuala Lumpur: University of Malaysia Press; 1983. pp. 290–306. [Google Scholar]
- 23.Hall W C, Crowell T P, Watts D M, Barros V L R, Kruger H, Pinheiro F, Peters C J. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistological analysis. Am J Trop Med Hyg. 1991;45:408–417. doi: 10.4269/ajtmh.1991.45.408. [DOI] [PubMed] [Google Scholar]
- 24.Halstead S B, O'Rourke E J, Allison A C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977;146:218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halstead S B. Dengue hemorrhagic fever—a public health problem and a field for research. Bull W H O. 1981;58:1–21. [PMC free article] [PubMed] [Google Scholar]
- 26.Halstead S B. Pathogenesis of dengue: challenge to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 27.Henchal E A, Putnak R J. The dengue viruses. Clin Microbiol Rev. 1990;3:376–396. doi: 10.1128/cmr.3.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendarto S K, Hadinegoro S R. Dengue encephalopathy. Acta Paediatr Jpn. 1992;34:350–357. doi: 10.1111/j.1442-200x.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 29.Henderson L M, Chappell J B, Jones O T. Superoxide generation is inhibited by phospholipase A2 inhibitors. Role for phospholipase A2 in the activation of the NADPH oxidase. Biochem J. 1989;264:249–255. doi: 10.1042/bj2640249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hober D, Nguyen T L, Shen L, Ha D Q, Huong V T Q, Benyoucef S, Nguyen T H, Bui T M P, Loan H K, Le B L, Bouzidi A, Groote D D, Drouet M T, Deubel V, Wattre P. Tumor necrosis factor alpha levels in plasma and whole blood culture in dengue-infected patients: relationship between virus detection and pre-existing specific antibodies. J Med Virol. 1998;54:210–218. doi: 10.1002/(sici)1096-9071(199803)54:3<210::aid-jmv12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Jaattela M, Benedict M, Tewari M, Shayman J A, Dixit V W. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 32.Jan J T, Byrnes A P, Griffin D E. Characterization of a Chinese hamster ovary cell line developed by retroviral insertional mutagenesis that is resistant to Sindbis virus infection. J Virol. 1999;73:4919–4924. doi: 10.1128/jvi.73.6.4919-4924.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jan J T, Griffin D E. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J Virol. 1999;73:10296–10302. doi: 10.1128/jvi.73.12.10296-10302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen H L A, Bienfait H P, Jansen C L, van Duinen S G, Vriesendorp R, Schimsheimer R J, Groen J, Osterhaus A D M E. Fatal cerebral oedema associated with primary dengue infection. J Infect. 1998;36:344–346. doi: 10.1016/s0163-4453(98)94783-1. [DOI] [PubMed] [Google Scholar]
- 35.Kalgutkar A S, Crews B C, Rowlinson S W, Garner C, Seibert K, Marnett L J. Aspirin-like molecules that covalently inactivate cyclooxygenase-21211. Science. 1998;280:1268–1270. doi: 10.1126/science.280.5367.1268. [DOI] [PubMed] [Google Scholar]
- 36.Kerr J F, Wyllie A H, Currie A R. Apoptosis: a basic biological phenomenon with wide range implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kho L M, Sumarmo S P S, Wulur H, Jahja E, Gubler D J. Dengue hemorrhagic fever accompanied by encephalopathy in Jakarta. Southeast Asian J Trop Med Public Health. 1981;12:83–86. [PubMed] [Google Scholar]
- 38.Kluck R M, Bossy-Wetzel E, Green D R, Newmayer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 39.Koga Y, Tanaka K, Lu Y Y, Oh-Tsu M, Sasaki M, Kimura G, Nomoto K. Priming of immature thymocytes to CD3-mediated apoptosis by infection with murine cytomegalovirus. J Virol. 1994;68:4322–4328. doi: 10.1128/jvi.68.7.4322-4328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korystov Y N, Dobrovinskaya O R, Shaposshnikova V V, Eidus L K. Role of arachidonic acid metabolism in thymocyte apoptosis after irradiation. FEBS Lett. 1996;388:238–241. doi: 10.1016/0014-5793(96)00538-8. [DOI] [PubMed] [Google Scholar]
- 41.Kurosawa M, Hisada T, Ishizuka T. Effect of phospholipase A2 inhibitor ONO-RS-082 on substance P-induced histamine release from rat peritoneal mast cells. Int Arch Allergy Immunol. 1992;97:226–228. doi: 10.1159/000236123. [DOI] [PubMed] [Google Scholar]
- 42.Kyprianou N, Isaacs J T. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989;3:1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- 43.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1825–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Cathcart M K. Selective inhibition of cytosolic phospholipase A2 in activated human monocytes. Regulation of superoxide anion production and low-density lipoprotein oxidation. J Biol Chem. 1997;272:2404–2411. doi: 10.1074/jbc.272.4.2404. [DOI] [PubMed] [Google Scholar]
- 45.Liao C L, Lin Y L, Wang J J, Huang Y L, Yeh C T, Ma S H, Chen L K. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin K I, Lee S H, Narayanan R, Hardwick J M. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L L, Wartmann M, Lin A Y, Knoff J L, Seth A, Davis R J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Kim C N, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 49.Lum L C S, Lam S K, Choy Y S, George R, Harun F. Dengue encephalitis: a true entity. Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 50.Malewicz B, Parthsarathy S, Jenkin H M, Baumann W J. Rapid phospholipase A2 stimulation and diacylglycerol cholinephosphotransferase inhibition in baby hamster kidney cells during initiation of dengue virus infection. Biochem Biophys Res Commun. 1981;101:404–410. doi: 10.1016/0006-291x(81)91274-2. [DOI] [PubMed] [Google Scholar]
- 51.Manna S K, Aggarwal B B. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-kappa B activation and reactive oxygen intermediates. J Immunol. 1999;162:1510–1518. [PubMed] [Google Scholar]
- 52.Marianneau P, Cardona A, Edelman L, Deubel V, Despres P. Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marino M W, Dunbar J D, Wu L W, Zgaiza J R, Han H M, Guo D, Matsushita M, Nairn A C, Zhang Y, Kolesnick R, Jaffe E A, Donner D B. Inhibition of TNF signal transduction in endothelial cells by dimethylaminopurine. J Biol Chem. 1996;271:28624–28629. doi: 10.1074/jbc.271.45.28624. [DOI] [PubMed] [Google Scholar]
- 54.Mayer R J, Marshall L A. New insights on mammalian phospholipase A2(s); comparison of arachidonyl-selective and -nonselective enzymes. FASEB J. 1993;7:339–348. doi: 10.1096/fasebj.7.2.8440410. [DOI] [PubMed] [Google Scholar]
- 55.Miagostovich M P, Ramos R G, Nicol A F, Nogueira R M R, Cuzzi-Maya T, Oliveira A V, Marchevsky R S, Mesquita R P, Schatzmayr H G. Retrospective study on dengue fatal cases. Clin Neuropathol. 1997;16:204–208. [PubMed] [Google Scholar]
- 56.Misra A, Mukerjee R, Chaturvedi U C. Release of reactive oxygen intermediates by dengue virus-induced macrophage cytotoxin. Int J Exp Pathol. 1996;77:237–242. doi: 10.1046/j.1365-2613.1996.9900327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mohan N, Meltz M L. Induction of nuclear factor kappa B after low-dose ionizing radiation involves a reactive oxygen intermediate signaling pathway. Radiat Res. 1994;140:97–104. [PubMed] [Google Scholar]
- 58.Monath T P. Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–2400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morey A L, Ferguson D J, Fleming K A. Ultrastructural features of fetal erythroid precursors infected with parvovirus B19 in vitro: evidence of cell death by apoptosis. J Pathol. 1993;169:213–220. doi: 10.1002/path.1711690207. [DOI] [PubMed] [Google Scholar]
- 60.Nathanson N, Cole G A. Immunosuppression and experimental virus infection of the nervous system. Adv Virus Res. 1970;16:397–428. doi: 10.1016/S0065-3527(08)60028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nevalainen T J, Losacker W. Serum phospholipase A2 in dengue. J Infect. 1997;35:251–252. doi: 10.1016/s0163-4453(97)92966-2. [DOI] [PubMed] [Google Scholar]
- 63.Nicholson W D, Ali A, Thornberry N A, Vailancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raji S M, Smulson M E, Yamin T T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 64.Oren M. Lonely no more: p53 finds its kin in a tumor suppressor haven. Cell. 1997;90:829–832. doi: 10.1016/s0092-8674(00)80347-5. [DOI] [PubMed] [Google Scholar]
- 65.Orrenius S. Mechanisms of oxidative cell damage: an overview. In: Paoletti R, editor. Oxidative processes and antioxidants. New York, N.Y: Raven Press; 1994. pp. 53–71. [Google Scholar]
- 66.Pennisi E. Building a better aspirin. Science. 1998;280:1191–1192. doi: 10.1126/science.280.5367.1191. [DOI] [PubMed] [Google Scholar]
- 67.Perez L, Irurzun A, Carrasco L. Activation of phospholipase activity during Semliki Forest virus infection. Virology. 1993;194:28–36. doi: 10.1006/viro.1993.1231. [DOI] [PubMed] [Google Scholar]
- 68.Rahman A, Kefer J, Bando M, Niles W D, Malik A B. E-selectin expression in human endothelial cells by TNF-alpha-induced oxidant generation and NF-κB activation. Am J Physiol. 1998;275:L533–L544. doi: 10.1152/ajplung.1998.275.3.L533. [DOI] [PubMed] [Google Scholar]
- 69.Ramos-Castaneda J, Imbert J L, Barron B L, Ramos C. A 65-kDa trypsin-sensible membrane cell protein as a possible receptor for dengue virus in cultured neuroblastoma cells. J Neurovirol. 1997;3:435–440. doi: 10.3109/13550289709031189. [DOI] [PubMed] [Google Scholar]
- 70.Reed J C. Cytochrome c: can't live with it—can't live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- 71.Roberts M L, Cowsert L M. Interleukin-1 beta and reactive oxygen species mediate activation of c-Jun NH2-terminal kinases, in human epithelial cells, by two independent pathways. Biochem Biophys Res Commun. 1998;251:166–172. doi: 10.1006/bbrc.1998.9434. [DOI] [PubMed] [Google Scholar]
- 72.Rosen L, Khin M M, Tin U. Recovery of virus from the liver of children with fatal dengue: reflections on the pathogenesis of the disease and its possible analogy with that of yellow fever. Res Virol. 1989;140:351–360. doi: 10.1016/s0923-2516(89)80115-3. [DOI] [PubMed] [Google Scholar]
- 73.Row D, Weinstein P, Smith S M. Dengue fever with encephalopathy in Australia. Am J Trop Med Hyg. 1996;54:253–255. doi: 10.4269/ajtmh.1996.54.253. [DOI] [PubMed] [Google Scholar]
- 74.Russel P K, Nisalak A. Dengue virus identification by the plaque reduction neutralization test. J Immunol. 1967;99:291–296. [PubMed] [Google Scholar]
- 75.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-κB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 76.Searle J, Kerr J F, Bishop C J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;2:229–259. [PubMed] [Google Scholar]
- 77.Shaio M F, Cheng S N, Yuh Y S, Yang K D. Cytotoxic factors released by dengue virus-infected human blood monocytes. J Med Virol. 1995;46:216–223. doi: 10.1002/jmv.1890460309. [DOI] [PubMed] [Google Scholar]
- 78.Shen Y, Shenk T E. Viruses and apoptosis. Curr Opin Genet Dev. 1995;5:105–111. doi: 10.1016/s0959-437x(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 79.Thakare J, Walhekar B, Banerjee K. Hemorrhagic manifestations and encephalopathy in cases of dengue in India. Southeast Asian J Trop Med Public Health. 1996;27:471–475. [PubMed] [Google Scholar]
- 80.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 81.Thommesen L, Sjursen W, Gasvik K, Hanssen W, Brekke O L, Skattebol L, Holmeide A K, Espevik T, Johansen B, Laegreid A. Selective inhibitors of cytosolic or secretory phospholipase A2 block TNF-induced activation of transcription factor nuclear factor-kappa B and expression of ICAM-1. J Immunol. 1998;161:3421–3430. [PubMed] [Google Scholar]
- 82.Thorne T E, Johnson C V, Casey W M, Parks L W, Laster S M. The activity of cytosolic phospholipase A2 is required for the lysis of adenovirus-infected cells by tumor necrosis factor. J Virol. 1996;70:8502–8507. doi: 10.1128/jvi.70.12.8502-8507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tolskaya E A, Romanova L I, Kolesnikova M S, Smirnova E A, Raikhlin N T, Agol V I. Apoptosis-inducing and apoptosis-preventing functions of poliovirus. J Virol. 1995;69:1181–1189. doi: 10.1128/jvi.69.2.1181-1189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vander Heiden M G, Chandel N S, Williamson E K, Schumacker P T, Thompson C B. Bcl-XL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- 85.Vaux D L. CED-4—the third horseman of apoptosis. Cell. 1997;90:389–390. doi: 10.1016/s0092-8674(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 86.Villalba M, Ferrari D, Bozza A, Del Sennol L, Di Virgilio F. Ionic regulation of endonuclease activity in PC12 cells. Biochem J. 1995;311:1033–1038. doi: 10.1042/bj3111033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wissing D, Mouritzen H, Egeblad M, Poirier G G, Jaattela M. Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:5073–5077. doi: 10.1073/pnas.94.10.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Health Organization. Dengue hemorrhagic fever: diagnosis, treatment and control. Geneva, Switzerland: World Health Organization; 1986. [Google Scholar]
- 89.Wu H, Lozano G. NF-κB activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem. 1994;269:20067–20074. [PubMed] [Google Scholar]
- 90.Wu Y L, Jiang X R, Lillington D M, Allen P D, Newland A C, Kelsey S M. 1,25-dihydroxyvitamin D3 protects human leukemic cells from tumor necrosis factor-induced apoptosis via inactivation of cytosolic phospholipase A2. Cancer Res. 1998;58:633–640. [PubMed] [Google Scholar]
- 91.Yao B, Zhang Y, Delikat S, Basu S, Kolesnick R N. Ceramide-activated protein kinase is a Raf kinase. Nature (London) 1995;378:307–310. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]