Abstract
Dengue fever is a mosquito-transmitted disease of great public health importance. Dengue lacks adequate vaccine protection and insecticide-based methods of mosquito control are proving increasingly ineffective. Here we review the emerging use of mosquitoes transinfected with the obligate intracellular bacterium Wolbachia pipientis for vector control. Wolbachia often induces cytoplasmic incompatibility in its mosquito hosts, resulting in infertile progeny between an infected male and an uninfected female. Wolbachia infection also suppresses the replication of pathogens in the mosquito, a process known as “pathogen blocking”. Two strategies have emerged. The first one releases Wolbachia-carriers (both male and female) to replace the wild mosquito population, a process driven by cytoplasmic incompatibility and that becomes irreversible once a threshold is reached. This suppresses disease transmission mainly by pathogen blocking and frequently requires a single intervention. The second strategy floods the field population with an exclusively male population of Wolbachia carrying mosquitoes to generate infertile hybrid progeny. In this case, transmission suppression depends largely on decreasing the population density of mosquitoes driven by infertility and requires continued mosquito release. The efficacy of both Wolbachia-based approaches has been conclusively demonstrated by randomized and non-randomized studies of deployments across the world. However, results conducted in one setting cannot be directly or easily extrapolated to other settings because dengue incidence is highly affected by the conditions into which the mosquitoes are released. Compared to traditional vector control methods, Wolbachia-based approaches are much more environmentally friendly and can be effective in the medium/long term. On the flip side, they are much more complex and cost-intensive operations, requiring a substantial investment, infrastructure, trained personnel, coordination between agencies, and community engagement. Finally, we discuss recent evidence suggesting that the release of Wolbachia-transinfected mosquitoes has a moderate risk of spreading potentially dangerous genes in the environment.
Keywords: Arboviruses, biocontrol, Wolbachia pipientis, genetically modified organism, symbiosis, genomics, environmental risk
Graphical Abstract
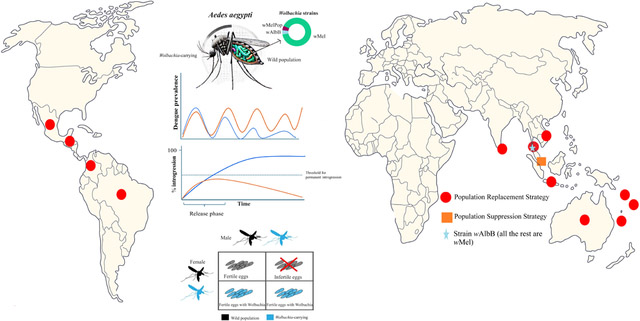
1. Introduction to Wolbachia
Wolbachia pipientis is an obligate intracellular Gram-negative bacterium that infects a wide range of invertebrate hosts, primarily arthropods and nematodes.
This bacterium can infect a variety of organs and tissues depending on the host and on the strain, but the reproductive system is the one organ system that is consistently infected (Werren et al., 2008; Pietri et al., 2016) .
1.1. Transmission and supergroup classification:
Wolbachia appears to have entered an endosymbiotic relationship with arthropods and nematodes around 100 My ago (Fenn et al., 2006), and spreads largely by vertical transmission through the cytoplasm of the egg from mothers to their offspring (Rodriguero, 2013). Currently, Wolbachia infects about 50% of terrestrial arthropod species (although with a variable prevalence) and also onchocercid and non-filarial plant nematodes.
Thirty-three strains (“supergroups”) of Wolbachia have been reported so far. These phylogenetic supergroups are arbitrarily named using capitalized letters of the Latin alphabet and reflect an early split between arthropod and nematode hosts. Supergroups A, B, E, H, I, and K correspond to arthropods and supergroups C, D, J, and L correspond to nematode species (Fenn et al., 2006; Lo et al., 2007; Wang et al., 2020; Kaur et al., 2021).
1.2. Nature of Wolbachia’s endosymbiotic relationship with its eukaryotic host:
Wolbachia’s endosymbiotic relationship with its eukaryotic hosts is assumed to be based (like most known examples of endosymbiosis) on metabolic complementation. Wolbachia is unable to synthesize many of the materials needed for membrane generation and it depletes the host of sphingolipids and ceramides (Hosokawa et al., 2010; Jiménez et al., 2019) while providing its host with cofactors necessary for iron metabolism, nucleotide synthesis precursors, FAD and glutathione (Bi and Wang, 2020; Manoj et al., 2021; Sanaei et al., 2021). Another documented benefit of Wolbachia infection for the host is a sizeable reduction in the burden of pathogens. This is particularly true in the case of insects, where a strong pathogen-blocking effect has been demonstrated for Wolbachia.
The virus-blocking effect of Wolbachia in mosquitoes has been studied in some detail (see review by Ant et al., 2023). This effect appears to be restricted to single-stranded, positive-sense RNA viruses belonging to the families Flaviviridae and Togaviridae, which include viruses of medical importance such as the causative agents of dengue fever, Chikungunya, Zika, Mayaro virus disease, yellow fever and West Nile fever.
At the molecular level, the observed virus-blocking effect appears to be primarily caused by Wolbachia -mediated disruption of vesicular traffic, which prevents the formation of the subcellular compartments that serve as a scaffold for viral replication. A second virus-blocking mechanism is the formation of lipid droplets, which restrict the availability of cholesterol for the formation of viral capsids (Geoghegan et al., 2017). Wolbachia’s virus-blocking effect may also be attributable to the priming of the mosquito innate immune system, leading to ROS production, an antagonistic modulation of autophagic flux, and the production of RNA binding proteins (Pan et al., 2012; Terradas and McGraw, 2017; Bhattacharya et al., 2020).
1.3. Endosymbiotic relationship in arthropods:
In terrestrial arthropods, the symbiotic association with the host is non-obligatory. Within mosquitoes, Wolbachia are found naturally in about 30% of species but are naturally absent from most Aedes aegypti, which is the main vector for arboviral disease transmission (Inácio da Silva et al., 2021). The non-obligatory symbiotic nature of Wolbachia’s relationship with its arthropod hosts is also reflected in its mixed symbiotic genomic profile, i.e. in a genomic sequence divergence that does not directly parallel that of the host due to frequent instances of host switching (Perreau and Moran, 2022).
In terrestrial arthropods, Wolbachia is frequently associated with sex interference, which skews the reproduction of the host toward females to facilitate its own transmission (Rodriguero, 2013). At least four mechanisms of sex interference have been described, namely cytoplasmic incompatibility (CI, which produces inviable offspring), male killing, the feminization of populations, and parthenogenesis (Yen and Barr, 1973; Werren et al., 1995; Rodriguero, 2013; Kaur et al., 2021; Manoj et al., 2021). All these sex interference mechanisms favor the reproduction of Wolbachia carriers by restricting transmission on the patrilineal line (Rodriguero, 2013). In the case of CI, female mosquitoes carrying Wolbachia produce viable Wolbachia-carrying offspring, regardless of whether the male carries Wolbachia, whereas fertility of Wolbachia-carrying male mosquitoes depends on whether the female is already infected with the same strain or not. This is illustrated in Figure 1 and is one of the key mechanisms upon which Wolbachia-based vector control strategies are based.
Fig 1. Reproductive viability of A. aegypti depending on the presence of Wolbachia.
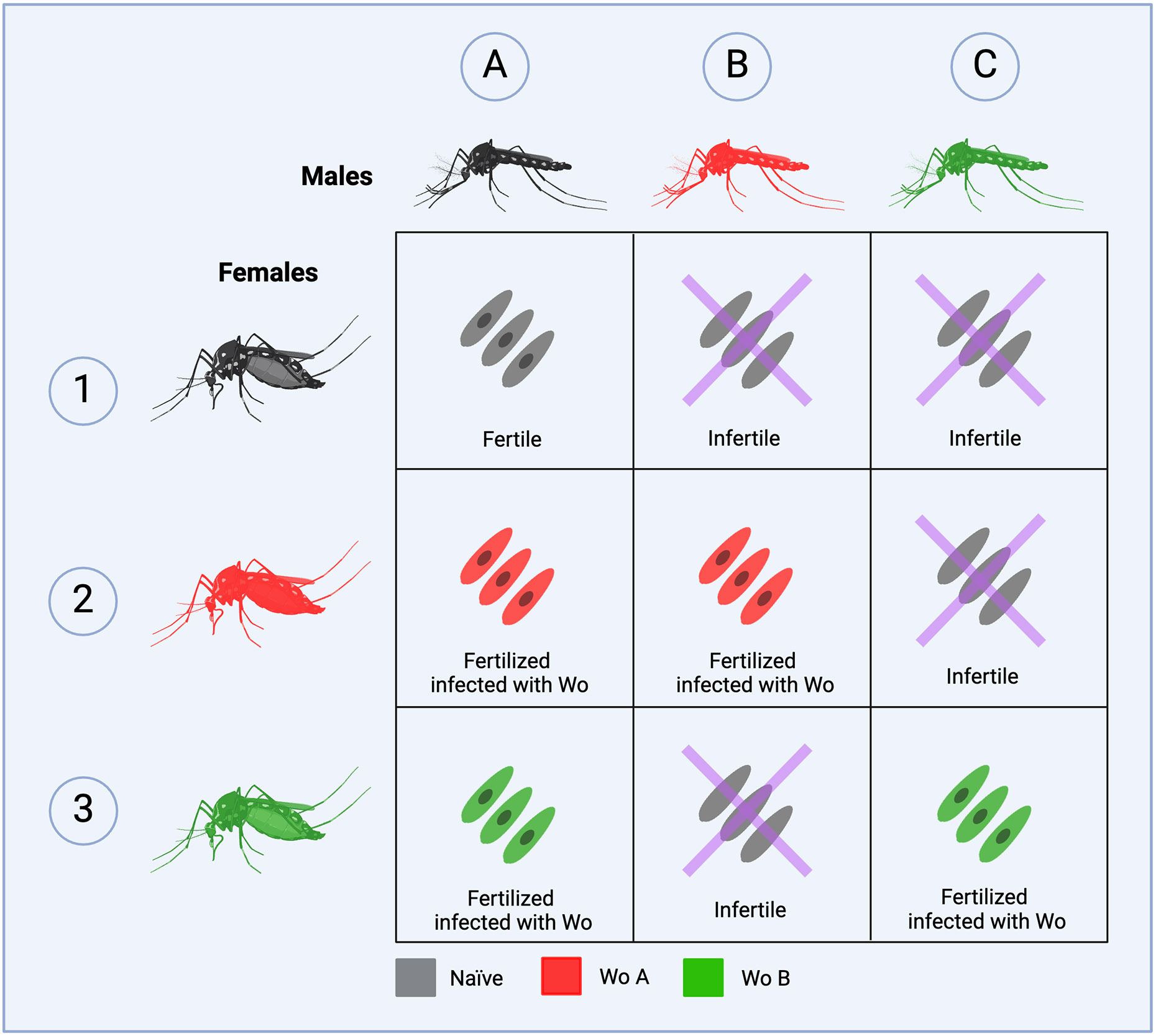
Female mosquitoes carrying Wolbachia produce viable offspring regardless of whether the male carries Wolbachia or not and the offspring is infected with Wolbachia. The outcome of a cross between a male mosquito carrying Wolbachia and a female mosquito depends on the status of the female (1-A, 2-A, 3-A). If the female is Wolbachia -free (1-B, 1-C) or if it carries a different strain of Wolbachia (2-C, 3-B), fertilization does not occur, resulting in infertile eggs. However, if the female harbors the same Wolbachia strain as the male (2-B, 3-C), offspring is produced, likely carrying Wolbachia. This figure has been adapted from (Wang et al., 2021).
In contrast to the non-obligatory nature of the association between Wolbachia and terrestrial arthropods, the association of Wolbachia with nematodes is very frequently obligate. It does not involve sex interference, as Wolbachia is generally already fixed in the population, making a mechanism favoring the introgression of infected hosts redundant. Consistent with the stable nature of its association with these hosts, in nematodes the Wolbachia genome is typically trimmed of non-essential genes and its evolutionary divergence tends to parallel that of the host (Werren et al., 2008; Perreau and Moran, 2022).
2. Epidemiology of vector-borne viral diseases.
Epidemic outbreaks of vector-transmitted viral diseases are hindering economic and social development worldwide due to direct healthcare costs as well as indirect costs related to reduced productivity (OMS, 2017).
2.1. Causative agents:
Vector-transmitted viruses are known as arboviruses. They include the causative agents of dengue (DENV), yellow fever (YFV), Chikungunya (CHIKV) and Zika (ZIKV). These are positive-sense RNA viruses belonging to the families Flaviviridae and Togaviridae that originated in either Africa or Southeast Asia from sylvatic cycles involving Aedes spp. mosquitoes with primate hosts. After being introduced in the Americas by humans, all four now circulate in human urban cycles in the Americas vectored by the anthropophilic mosquitoes A. aegypti and A. albopictus (Weaver et al., 2018). Mayaro virus is the only one of these viruses that is endogenous to the Americas (de Thoisy et al., 2003). The West Nile virus is another Togavirus transmitted by Aedes mosquitoes. It may have originated in the Middle East and it infects birds, which helped spread it across the world (Rappole, 2000).
2.2. Mosquito vectors:
A. aegypti is the primary urban vector for dengue, but this disease can also be transmitted by other Aedes species with unique ecologies such as A. albopictus, A. polynesiensis, and A. scutellaris. In a mosquito, to achieve transmission, an arbovirus present in a bloodmeal must invade the midgut epithelium, disseminate in haemolymph, and eventually establish an infection in the salivary glands (Wu et al., 2019).
A. aegypti proliferates in artificial containers placed in or near homes. Given that it is a day-biting mosquito, transmission is local (occurring primarily within households), focal and heterogeneous, with foci varying in space and time. These foci are connected at short distances by a combination of human and mosquito movement and at longer distances by human movement alone (Stoddard et al., 2013; Salje et al., 2017).
Before they can be transmitted to a new host, pathogens transmitted by mosquitoes need a significant period of development in the insect vector, known as the “extrinsic incubation period” (EIP). Given that EIP is long relative to the insect lifespan; the majority of pathogen transmission is done by old mosquitoes (Ye et al., 2016).
Understanding basic ecological and microevolutionary processes in mosquito populations is necessary for the efficient implementation of many vector control measures. For example, knowledge of the rates of ongoing gene flow, fine scale mosquito movement, and adaptive genomic changes are important for predicting the spread of Wolbachia infection (Barton and Turelli, 2011; Hoffmann et al., 2011) or insecticide resistance (Yan et al., 1998).
The genetic population structure of a mosquito population is reflective of a complex combination of historical and contemporary factors such as the dispersal ability and mating patterns of the species, its demographic history, and environmental barriers to dispersal (Balloux and Lugon-Moulin, 2002). Genetic tools for probing the genetic population structure of a given population include microsatellites, mitochondrial DNA (mtDNA), and single nucleotide polymorphisms (SNPs). Microsatellites are most useful for the study of subtle population structure because of their high polymorphism, co-dominant expression, and extensive genomic distribution (Brown et al., 2011; Rašić et al., 2014). By contrast, mtDNA markers can only provide information on long-term accumulated effects of female dispersal due to their moderate mutation rate and maternal mode of inheritance (Urdaneta-Marquez and Failloux, 2011). More recently, next generation sequencing offers an opportunity to generate SNP markers at a genome-wide level, allowing the detection of weak genetic structure caused by recent ecological and evolutionary processes while providing reliable inferences of demographic history. Genome-wide SNP profiling is thus emerging as the ideal method for determining patterns of dispersal, gene flow and genetic structure at all spatial scales for populations of A. aegypti (Rašić et al., 2014; Schmidt et al., 2018).
The overall levels of genetic differentiation for A. aegypti are relatively low (Hlaing et al., 2010), consistent with the recent spread of this species throughout the tropics from Africa (Kotsakiozi et al., 2018). In South-East Asia, the population structure over small and large distances shows a disjunctive structure that is indicative of human-mediated dispersal of mosquito eggs, larvae and adults along major human transportation routes coupled with low mosquito dispersal rates (Huber et al., 2004; Hlaing et al., 2010).
The observed restricted dispersal of A. aegypti favors the success of localized interventions such as conventional responses based on insecticides or removal of larval habitats following a dengue outbreak and the use of sterile insect approaches (see Box-1).
BOX-1. VECTOR CONTROL METHODS.
1. Source Reduction.
Source reduction consists of limiting mosquito access to potential breeding sites. Typically, most of the mosquito production is concentrated in certain breeding sites that are highly productive. Identifying these sites makes this approach very effective, although some breeding sites are cryptic (hidden) and/or unreachable (Baldacchino et al., 2015).
Limiting effective mosquito access is achieved by removing, turning over, or draining temporary water containers, covering permanent ones, or managing them with larvicidal treatments. There are a variety of larvicidal treatments. One of them is copepods, which are predators of early (first and second) mosquito instar larvae (Marten and Reid, 2007). This treatment is ideal for large, permanent water-filled containers and has successfully eradicated A. aegypti in several interventions (Marten and Reid, 2007; Kay & Vu 2005; Nam et al., 2012) but does not work for temporary breeding sites because copepods need water and food. Another larvicidal treatment is the use of specific bacteria, such as Bacillus thuringensis var. israeliensis (Bti) and Lisinobacillus sphericus (Lsph), whose secreted toxins are activated in the gut of larva, disrupting all membranes (Guidi et al., 2013). These are treatments that are typically used in the form of aerial spray in large areas, for example in floodplains and coastal wetlands. Spinosad is a product derived from the fermentation of a soil actinomycete with strong larvicidal properties, although its non-target effects on other aquatic species restrict its use to artificial breeding sites (Marina et al., 2014). Finally, Insect Growth Regulators (IGRs) pyriproxyfen, methoprene, and diflubenzuron are used as larvicides and they are also ovicides. They are most effective for the targeted treatment of most productive breeding sites and are relatively safe for non-target organisms (Marina et al., 2014; Mulla, 1995).
Note that wild adult mosquitoes can be used as carriers of larvicide compounds, in an approach known as autodissemination (Devine et al., 2009).. Females are contaminated by larvicide using contamination devices such as treated nets or dissemination stations with modified ovitraps. In small-scale field experiments, these interventions led to increased mortality at the pulpal stage and effects on egg production and hatchability (Devine et al., 2009; Ohba et al., 2013). Males that are released as part of a Sterile Insect Technique intervention (see “Mating based methods” section below) can also be coated with pyriproxyfen for purposes of autodissemination.
2. Chemical adulticides:
The most frequently used ones are pyrethroids. Other adulticides used in vector control campaigns are organophosphates and carbamates.
These chemicals are sprayed to reduce the frequency of females in high-density mosquito populations or in rapidly-expanding populations, particularly during epidemics (Caputo et al., 2015). However, these insecticides are toxic for non-target insect species, aquatic vertebrates and fish by causing neurotoxic disruptions and impairing their predatory abilities (Faria et al., 2021; Herbert et al., 2021; Reiber et al., 2021). Its longer-term impact is limited due to a combination of resistance and insufficient follow-through that allows neighboring mosquito populations to reinfest the target area (Snetselaar et al., 2014; Marcombe et al., 2014).
Indoor-Residual Spraying (IRS) and Insecticide-Treated Materials (ITMs) leverage A. aegypti’s habit of resting in homes before and after blood-feeding, allowing the selective targeting of resting places with adulticides (Rizzo et al., 2012).
3. Entomopathogenic fungi:
Certain fungi such as Beauveria brassiana and Metarhizium anisopliae have larvicidal and adulticide activity. Their application through fungus-impregnated cloth or applied in screens around the home shows promise as mosquito control method.
4. Traps:
Aedes-targeting traps target gravid females (ovitraps or sticky/gravid traps) or females seeking blood meals (BG-sentinel traps). These traps are usually used for surveillance, and the addition of larvicide or autocide eliminates the risk of the trap becoming a source of adult mosquitoes (Mackay et al., 2013; Montenegro et al., 2020). Lethal ovitrap control programs have also been implemented as a means to reduce adult mosquito density (Ritchie et al., 2009) and can be enhanced through the use of attractants or oviposition stimulants.
5. Mating-based methods:
These methods seem ideal to control low-density populations because they can reach the target population more effectively (Iyaloo et al., 2014). There are two basic approaches, namely the Sterile Insect Technique (SIT) (Alphey et al., 2010; Lofgren et al., 1994) and a variety of molecular genetic methods..
Sterile Insect Technique:
this method relies on the release of large numbers of sterile males to suppress mosquito population density (Alphey et al., Lofgren et al., 1994). Sterilization techniques include exposure to radiation or chemicals, although these treatments generally have a negative impact on fitness, which in turn limits the effectiveness of an intervention that requires significant introgression into the wild mosquito population. RNAi is a promising approach, but at the moment the production of the necessary reagents is too expensive for large-scale treatment (Whyard et al., 2015).
Molecular Genetics Methods:
RIDL is based on the delivery of female-acting transgenes into the wild population by genetically modified males, interfering with development and/or inducing mortality of their progeny. This approach has been successfully used to suppress two wild target A. aegypti populations in Brazil (Alphey, 2014). RNAi-based strategies are being developed to enhance insect immune recognition and degradation of viral RNA (Franz et al., 2006). Finally, HEG is an experimental technique for vector control based on the spread of sequence-specific endonucleases, disrupting selected targets (competence, fertility, sex-determining) genes in the process (Traver et al., 2009).
2.3. Prevalence patterns and trends:
Dengue fever, like most vector-transmitted diseases, exhibits a much larger level of interannual variation in transmission relative to most directly transmitted infections (Johansson et al., 2009; Lambrechts et al., 2011; Stewart-Ibarra and Lowe, 2013; van Panhuis et al., 2015; Oidtman et al., 2019). Dengue’s high interannual variability results from the intersection of a complex tapestry of epidemiological variables, including climate (van Panhuis et al., 2015), the mosquito’s genetic make-up (Ford et al., 2019), immune-mediated serotype dynamics (Salje et al., 2018), and virus-specific phenotypic variation (Bennett, 2003). In particular, climate plays a major role in the temporal and spatial distribution of mosquito-transmitted infections by affecting variables such as humidity, precipitation, and ambient temperature, which directly or indirectly influence the population of vectors and viruses (Gould and Higgs, 2009; Campbell et al., 2013; Huang et al., 2019). In the case of dengue, the duration of the virus’s EIP and the density, longevity, and frequency of vector biting are all sensitive to changes in environmental conditions.
The prevalence of dengue is growing worldwide (Tian et al., 2022). This growth is linked to urbanization, which promotes higher mosquito density by increasing human density, and because of the coexistence of human blood sources and mosquito breeding sites in close proximity in human settings. The expansion of the vector’s geographic range driven by climate change also contributes to this growth (Bonizzoni et al., 2013; Mayer et al., 2017).
3. Control Strategies for vector-transmitted viral diseases
3.1. Vaccines:
Ideally, the control of vector-borne viral disease should be based on prophylactic measures. Vaccination would be the most effective approach because it has the potential to clear the infection and to therefore prevent symptoms and completely block transmission. However, despite substantial progress in the development of these vaccines, only yellow fever has viable, effective, and safe vaccines that are currently available. One dengue vaccine, the Sanofi-Pasteur vaccine, has been licensed so far. This is a recombinant chimeric live-attenuated vaccine that reduces the risk of symptomatic disease but has little effect on transmission (Ferguson et al., 2016; Flasche et al., 2016). Other vaccines are in advanced stages of development but, to date, none has achieved balanced efficacy and safety for all dengue serotypes (Orellano et al., 2023; Al-Osaimi et al., 2024).
3.2. Mosquito vector control:
In the absence of vaccines, mosquito vector control is the most effective approach to control the prevalence of vector-borne disease (WHO, 2017b). Vector control methods fall into two broad categories, namely acute interventions and interventions whose goal is a medium/long-term population reduction (Baldacchino et al., 2015).
One approach is to target potential mosquito breeding sites by removing mosquito access to breeding sites or by killing larvae through treatment with chemicals, microbes or natural predators. Another one is to target adults (through exposure to insecticides or through traps) or can target the fecundity or fitness of adults (Wolbachia, Sterile Insect Technique and molecular genetic approaches). Box-1 discusses the range of vector control methods (other than Wolbachia-based ones) currently available in more detail.
The timing and choice of treatment is determined by the population dynamics of the target species and needs to be integrated between decision-makers, public authorities, scientists and the general public (Becker et al., 2010; Lavery et al., 2010).
3.3. Overview of Wolbachia-based approaches:
The release of Wolbachia-carrying mosquito vectors has been proposed in recent years as a preventative measure for the long-term management of vector-borne diseases that would have a lower impact on the environment and that could be integrated with other strategies in comprehensive approaches for vector control (Ritchie and Johnson, 2017).
Two main Wolbachia-based strategies have emerged. Both strategies involve releasing mosquitoes into the environment but differ in the way they interfere with disease transmission. The population replacement strategy suppresses transmission mainly through pathogen blocking, while the population suppression strategy reduces transmission primarily by lowering the mosquito density.
Below we discuss these two strategies in detail, compare them to each other and to insecticide-based methods of vector control, and discuss their environmental safety. This topic has been previously covered in previous works (Ross, 2021; Ogunlade et al., 2021); the present work brings these reviews up to date and provides an overview of the initial implementation studies.
A. aegypti has a cosmopolitan range, living in cities across the tropics, and serves as primary vector for several vector-borne viruses that are important for public health such as YFV, DENV, CHIKV, and ZIKV (Weaver et al., 2018), making it an ideal candidate for Wolbachia interventions. In addition, A. aegypti is not usually naturally infected with Wolbachia (WMP, 2023).
Wolbachia can be introduced artificially through transinfection, which involves injecting cytoplasm or tissue homogenate from a Wolbachia-infected insect into mosquito embryos using a fine needle (Hughes and Rasgon, 2014). Stably infected lines are then generated by selecting for Wolbachia-infected females for multiple generations. The transfer of Wolbachia to a new mosquito host is typically detrimental to the host, particularly on fertility (Fraser et al., 2017; Ant et al., 2018). Moreover, the negative impacts of Wolbachia transinfection on fitness tend to be underestimated under laboratory conditions because they become more pronounced under stress conditions such as after a long egg quiescence or under poor nutritional conditions (Caragata et al., 2014; Allman et al., 2020).
3.4. Strain-dependent variation in Wolbachia impact on the host:
A. aegypti has been stably transinfected with Wolbachia, where it has a broad somatic distribution. The impact of Wolbachia on fitness, pathogen blocking, and CI are highly dependent on the specific strain involved (Ferguson et al., 2015; Ulrich et al., 2016; Fraser et al., 2017; Ant et al., 2018). These impacts also depend on genetic variation in the mosquito hosts, particularly the level of pathogen-blocking (Ford et al., 2019).
Fitness:
Wolbachia exhibits high strain-dependent variability in its impact on A. aegypti fitness (Ulrich et al., 2016; Ant et al., 2018). High Wolbachia densities are associated with negative impact on fitness, including fecundity, longevity and egg survival over extended periods of quiescence (McMeniman and O’Neill, 2010; Ant et al., 2018). Given the negative correlation between infection density and fitness, selection would be expected to favor the evolution of genetic factors restricting tropism to the tissues required for transmission to the progeny (Pietri et al., 2016). This selective pressure likely depends on the fitness costs of this symbiosis, with higher costs resulting in a stronger and more rapid selection for restricted tropism. Supporting this idea, restricted tropism is seen in some native associations such as A. albopictus (Ant and Sinkins, 2018), and Glossina morsitans (Cheng et al., 2000), but not in all of them (Pietri et al., 2016).
Virus-blocking activity:
the level of viral blocking also varies considerably between strains (Ferguson et al., 2015) and is also sensitive to environmental conditions (Chrostek et al., 2021). Similar to the negative impact on fitness, viral blocking positively correlates with Wolbachia intracellular density (Lu et al., 2012; Martinez et al., 2017). Given that the virus-blocking effect is cell-autonomous, i.e. requires Wolbachia -virus co-infection (Moreira et al., 2009; Nainu et al., 2019), the distribution of Wolbachia in host tissues is also important to determine its potential to block transmission. Key tissues are the gut and salivary glands, which are the two organs critical for transmission (Wu et al., 2019).
In addition to exhibiting strong strain variation, the level of pathogen interference is also a highly virus serotype-Wolbachia strain-host interdependent trait. In the case of wMel, in A. aegypti there are differences in the level of resistance to superinfection with DENV depending on the DENV serotypes (Ferguson et al., 2015). Some degree of resistance is observed for all of them (Carrington et al., 2018), but with variations.
Cytoplasmic incompatibility:
the strength of CI is strain- and host-dependent. Most characterized Wolbachia infections in mosquitoes cause strong or complete CI (Fraser et al., 2017), but some don’t cause any (Ant et al., 2018). CI is also sensitive to environmental conditions, with heat or hatching from quiescent eggs weakening it in the wMel strain (Ross et al., 2017; Lau et al., 2021).
3.5. Strains used for Wolbachia-based interventions:
Two main strains are being used for vector control, the wMel strain and the wAlbB strain, reviewed in (Hoffmann et al., 2015).
wMel is the strain of choice for the World Mosquito Program-WMP and is also widely used as a model for basic research. (Flores and O’Neill, 2018). This strain exhibits relatively low host fitness costs, at least in laboratory conditions (Ross et al., 2019) and induces significant pathogen blocking, although the block is incomplete and can be variable when tested under realistic conditions (Carrington et al., 2018). Hot environments have the potential to impact the efficacy of dengue blocking and its fitness, as wMel shows reduced density and cytoplasmic incompatibility when A. aegypti larvae are reared at high temperature (Ulrich et al., 2016; Ross et al., 2017; Ant et al., 2018). Note that wMel can still persist in high temperature locations sheltering in areas with no direct exposure to sunlight such as houses or patios, so this strain should not be necessarily ruled out for interventions in hot environments.
wAlbB is similar to wMel in many ways, producing similar levels of CI induction and of pathogen blocking (Bian et al., 2010; Joubert et al., 2016; Flores et al., 2020), but it is much less susceptible to the effects of similar high rearing temperatures, achieving higher densities at larval rearing temperatures well suited for population replacement in a hot environment. wAlbB also has a low impact on many aspects of host fitness (Axford et al., 2016; Ant et al., 2018). The accumulating evidence supporting the use of this strain in hot environments has been recently reviewed (Maciel-de-Freitas et al., 2024). However, parameters that are relevant for mosquito release interventions can differ under some environmental conditions. When wAlbB-infected A. aegypti hatch from eggs that are more than a few weeks old, a substantial proportion of females that hatch are infertile (Lau et al., 2020, 2021). wAlbB also causes a reduction in adult longevity in lab-reared A. aegypti (Axford et al., 2016). The significance of this observation is unclear, though, as the average lifespan of mosquitoes is expected to be much shorter in the field (Strickman, 2006). Overall, this means that population replacement with wAlbB can be more effective in hot environments so long as there is not a long dry season (Ross et al., 2023).
3.6. Role of the community:
Releasing mosquitoes into the environment affects local communities where the release sites are targeted, particularly when biting female mosquitoes (as opposed to male mosquitoes) are released in areas of human habitation.
Community engagement is not only necessary for obtaining consent for the releases but also for the long-term success of these interventions (Murray et al., 2016; O’Neill et al., 2019b; Costa et al., 2021; Liew et al., 2021). Community education is essential to ensure that mosquito management is maintained at the household level and to prevent insecticide use during the release phase of population replacement (Tapia-Conyer et al., 2012; Jeelani et al., 2015; Dhar-Chowdhury et al., 2016). The community can also get directly involved with the deployment of mosquito release interventions through hosting mosquito traps, participating in monitoring activities, or helping with the mosquito egg distribution. Therefore, community engagement can be important to ensure adequate monitoring and high coverage. Finally, continued community support is also necessary to avoid building mistrust and jeopardizing future releases (Nading, 2015).
Three major challenges in community engagement have been identified: poverty, lack of information, and resistance to interventions (Tapia-López et al., 2019). Therefore, successful community engagement involves addressing economic and informational barriers, ensuring transparent communication and fostering community ownership and involvement in the interventions process. These efforts need to start early, need to be sustained, and need to involve a broad range of stakeholders, including residents, the general public, and professional communities.
Information sessions, making literature available and accessible to the public, and ethical public participation and engagement sessions are important to inform and answer direct questions from members of the community. They help address concerns, shape public perception and combat misinformation (Popovici et al., 2010; McNaughton, 2012). The establishment of feedback mechanisms to gauge levels of public acceptance and understanding is also essential for the project's scalability and long-term success (McNaughton, 2012; Arellano et al., 2015; Dhar-Chowdhury et al., 2016).
A major component of quality engagement is community empowerment, which fosters a sense of ownership and responsibility. Responsive consultation processes are another key element in establishing a productive collaboration with community leaders. These consultation processes can take the form of the inclusion in technical and non-technical community experts in expect panels that captures the diversity of opinions relevant to different hazards.
3.7. Design Principles for vector control trials:
Since 2008, WHO has adopted the Grading of Recommendations Assessment Development and Evaluation (GRADET) methodology for evaluating evidence for policy and guideline recommendations regarding vector control (Guyatt et al., 2008). An initial rating is given based on the study design (see four level hierarchy of experimental designs below) and is modulated based on additional criteria. Large effect size and evidence of dose-response upgrade the rating, whereas inconsistency, risk of bias and imprecision down-grade it.
The general design principles for vector control trial design are outlined in the following two documents (Wilson et al., 2015) and (WHO, 2017a).
Well-defined, assessable endpoints that are critical for addressing the study question should be defined in advance and at least one of these primary outcomes has to be epidemiological. Entomological endpoints should not be used on their own but can be combined with epidemiological ones to evaluate an entomological effect.
In vector control studies, the experimental units are often groups of individuals (known as “clusters”). This reduces contamination between study arms and allows assessment of community-level effects of an intervention. In the case of dengue, the number of participants per cluster is typically between 2,000 and 3000, although it depends on background transmission rates, level of herd immunity, movement between clusters, and anticipated size effect of the intervention. Having sufficient replicates (multiple experimental units receiving the same intervention) is important to establish variability. Replicates are not to be confused with repeats, i.e. repeated measurements of the same item.
Generally, experimental designs (i.e. studies where investigators assign treatments to experimental units and observe the outcome) are considered superior to observational designs, where investigators report a relationship between intervention and outcome, as the latter are very prone to selection and reporting biases. Within experimental designs, a hierarchy of four levels has been established (Wilson et al., 2015) and (WHO, 2017a). Level 1 includes randomized controlled trials (RCTs), which minimize the risk of confounding bias by ensuring that characteristics of control and intervention groups are similar to one another. Level 2: includes other randomized controlled studies such as step-wedge cluster randomization (SWCR) or cross-over designs. A third level consists of comparative studies with non-randomized controls (“before-and-after”, cohort, case-control, time-series, and interrupted-time series). In these cases, the potential bias can be reduced by adjusting pre-intervention differences between the two groups using multivariate analysis (Hamel et al., 2011). Finally, the lowest level consists of vector control studies without a control group or using a non-contemporaneous control group. In these studies, longitudinal changes affecting the outcome but not linked to the intervention (such as rainfall or changes in diagnostic practices) are likely to impact epidemiological outcomes or mask an intervention effect.
In efficacy studies, morbidity data can be collected passively, i.e. when patients seek medical assistance, actively, i.e. reported by study staff visiting participants and screening them for disease, and during cross-sectional surveys. To calculate the incidence of disease (or infection) in each arm, rates of disease are computed as number of events divided by the sum of time at risk for the people studied. The protective efficacy of the intervention is then calculated as the rate of disease in the intervention group divided by the rate of disease in the control group and expressed as percentage.
A reduction in the transmission of the virus between sites can also be measured based on virus sequencing data, a lower average dispersion distance travelled by the virus within the intervention area reduces the viral genetic diversity across serotypes. Viruses that continue to be imported into the area by human-mediated dispersal won’t have local ancestors, reducing the strong spatial clustering typically seen in dengue virus phylogenies (Lambrechts et al., 2015).
To avoid information and performance bias, study participants and outcome assessors should be blinded. Confirming the diagnosis with a molecular test is important because the symptomatology of vector-borne viral infection is generally not specific enough to allow unambiguous diagnosis (Indriani et al., 2020). The reporting of the results should follow the guidelines of CONSORT (Consolidated Standards of Reporting Trials) (Piaggio et al., 2012).
Optimal implementation is important, with attention to quality control (for example, through collaboration with national control programs), high coverage and participant compliance. These need to be measured because statistical power depends on the sensitivity of surveillance; if the sensitivity is decreased by false positives or by failure to detect repeat cases, the power of the study also decreases. Poor implementation can make negative results uninterpretable.
Compared to other efficacy studies, vector control studies have two unique challenges (WHO, 2017a). The first challenge is a highly variable spatiotemporal distribution of vector populations. Sampling sites should be selected randomly and repeated measurements should be taken to capture transmission over time. In this scenario, controlled time-series or controlled-interrupted time-series designs with an implementation over a large area are the most appropriate designs. The controlled-interrupted time-series design (with periodical monitoring for periods of 3-6 months for up to 5 years) has often been selected to measure dengue suppression in Wolbachia efficacy studies (O’Neill et al., 2019a; Utarini et al., 2021; Velez et al., 2023). The second challenge are contamination/spillover effects between different study arms. One example are movements of vector or humans between clusters, which dilute the intervention effects. Another example are community-level effects of an intervention, which can reduce transmission intensity in neighboring control studies. These types of contamination problems tend to be conservative so they don’t invalidate effects that are seen but may mask real effects. The experimental design can intentionally minimize contamination effects. For these modified designs, knowing the distance that the vector is likely to fly is important. Examples include (1) separation of clusters, i.e. including a buffer zone to eliminate any common boundary between intervention and control clusters; (2) increasing the cluster area; (3) monitoring outcomes in a sentinel population that is less mobile and (4) “fried egg” design in which intervention and control are administered throughout the cluster but outcome is only measured in the central part of the cluster (West et al., 2014).
3.8. Safety considerations:
The use of Wolbachia for disease vector control was deemed to be safe by an initial study whose goal was to produce a framework for authorization for the first deployment of mosquitoes in Cairns, Australia (Popovici et al., 2010).
This initial study by Popovici et al. included arguments addressing safety for humans based on our basic understanding of the biology of Wolbachia and also on experimental results. The most salient biological arguments included: (1) humans are already exposed to Wolbachia through bites from mosquitoes that are naturally infected with Wolbachia and through residues of Wolbachia-infected stored grain pests present in human food. (2) Wolbachia is an endosymbiont, and therefore it is not expected to persist in the environment outside mosquitoes carrying them. (3) Horizontal transmission does not occur easily or at high frequency. Wolbachia-infected and uninfected mosquitoes can inhabit the same habitat or predate on each other without acquiring the infection. Examples include A. aegypti (Wolbachia-free) and its close relative A. notoscriptus (naturally infected with Wolbachia) in Australia (Skelton et al., 2016) or A. aegypti and A. albopictus (also naturally infected with Wolbachia) in Asia (Gratz, 2004). In the latter case, the two species even inhabit the same larval containers and will ingest smaller units of the other species.
The critical experimental arguments were three. (1) Wolbachia was tested on chicken embryos as a potential human rickettsial pathogen and found to be non-pathogenic (Hertig, 1936); (2) Wolbachia was absent from the saliva of infected mosquitoes; and (3) human volunteers exposed to thousands of bites from wMelPop-infected mosquitoes over prolonged periods of time were found to be free of Wolbachia antibodies, suggesting that Wolbachia antigens are not injected into humans during the mosquito blood meal (Popovici et al., 2010).
The potential ecological of impact releasing Wolbachia-infected mosquitoes was also evaluated. This included an experimental evaluation of possible horizontal transfer of the wMelPop strain to two mosquito predators, two species of spiders that are not naturally infected, namely jumping spiders (Menemerus bivittatus, Salticidae), and daddy long legs spiders (Pholcus phalangioides, Pholcidae). A possible the horizontal transfer of this strain to non-predator species or to environments in the vicinity of mosquitoes was also tested. These experiments confirmed the absence of transmission of this strain of Wolbachia, at least at high frequency (Popovici et al., 2010).
However, the potential ecological impact of releasing Wolbachia-infected mosquitoes into the environment needs to be reassessed in light of new evidence regarding the genomic evolutionary profile of Wolbachia (Perreau and Moran, 2022).
The genome of Wolbachia strains isolated from nematodes conforms to the “closed” symbiosis category, as expected for obligate endosymbionts. These genomes co-evolve with those of the host, and only occasionally exchange genes within co-infected hosts (Perreau and Moran, 2022). By contrast, Wolbachia strains isolated from mosquitoes and other terrestrial arthropods have a genomic evolutionary profile that falls in the “mixed symbiosis” category. This means that, in addition to exchanging genes within a co-infected host, genetic material can be exchanged between different strains of Wolbachia. For example, there are multiple instances of interclade recombination between Wolbachia genomes belonging to supergroups A and B (Figure 2) and A and E (not shown). As a result, the phylogenetic clustering of Wolbachia genomes recovered from terrestrial arthropods does not necessarily parallel that of their hosts. The poor correspondence between 175 Wolbachia genomes belonging to supergroups A and B (the most frequent ones, arranged according to phylogenetic relatedness) and their host distribution is evident in Figure 2.
Figure 2. Distribution of Wolbachia genomes belonging to supergroups A and B by host.
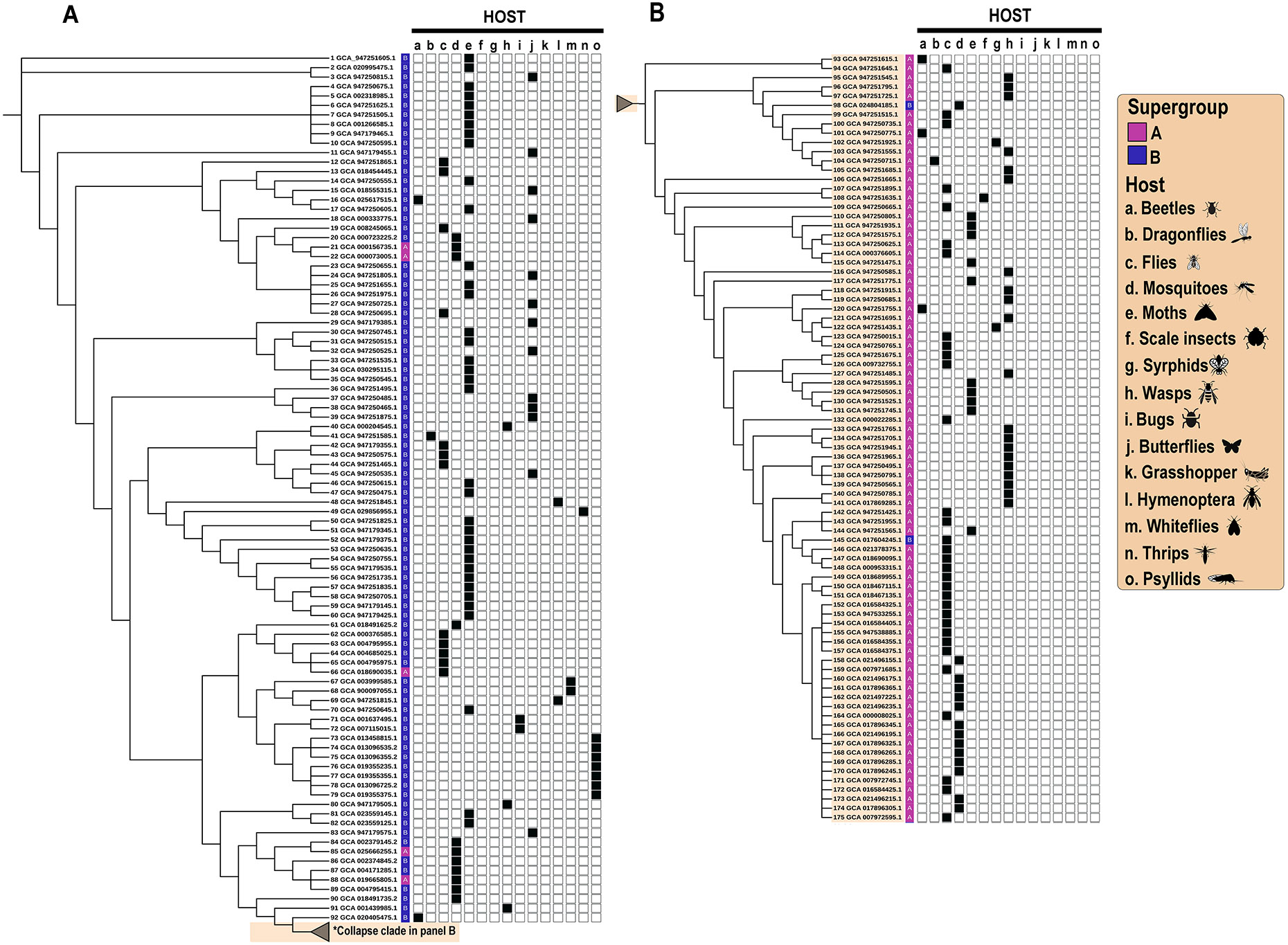
Panel Cladogram showing the phylogenetic relatedness of 175 Wolbachia genomes belonging to supergroups A and B. Panel A. Genomes in the majority supergroup B clade (with majority supergroup A collapsed). Panel B. Genomes belonging to the majority supergroup A clade. The supergroup classification for each individual sequence is shown in the first column of the grid, with supergroup A highlighted in pink and supergroup B highlighted in blue. Columns 2-16 in the grid show the host from which Wolbachia was isolated, denoted by letters of the alphabet. The correspondence of the letters to the common name and icon are shown in the legend to the right. The complete genome assemblies of Wolbachia were downloaded from NCBI on June 30, 2023. From this set of 212 genomes, we selected those belonging to the two predominant supergroups (supergroups A and B, n=176). For each of the samples included, the GenBank accession number, host and date processed are listed in Supplemental Table 1. The phylogenetic analysis was performed using Validated Bacterial Core Genomes VBCG with default settings (Tian et al., 2023). The cladogram was generated and annotated using Interactive Tree Of Life (ITOL) v6.9 (https://itol.embl.de/ accessed on March 2024 (Letunic and Bork, 2021). Supergroups were labeled based on the NCBI annotation, and if no records were found on NCBI, a literature search was performed using the NCBI GenBank accession number (Benson et. al. 2013; Schoch et al., 2020). The NCBI Taxonomy label was used to identify the Blast common name for each genome (Sayers et al., 2022).
The “mixed symbiosis” genomic profile of terrestrial arthropod Wolbachia strains implies the occasional co-infection of the same host (Wang et al., 2020). Superinfection with different strains of Wolbachia has been achieved in the laboratory (Joubert et al., 2016; Ant and Sinkins, 2018) and this scenario has also been confirmed in nature for the dwarf spider Oedothorax gibbosus (Halter et al., 2023). Co-infection implies transfer from another host or even from the environment, as seen in open symbioses (Le Clec’h et al., 2013; Thi Hue Kien et al., 2023). Natural A. aegypti infections have been occasionally detected (Balaji et al., 2019; Kulkarni et al., 2019; Zhang et al., 2022; Muharromah et al., 2023), although confirming detection in species previously thought to lack them is extremely challenging (Ross and Hoffmann, 2024). The fact that the Wolbachia strains apparently identified in these studies are not wMel or wAlbB suggest that this could be the result of a bacterial exchange with infected larvae from other mosquito species at shared breeding sites (Bennett et al., 2019; Zhu et al., 2023). Also supporting the ability of Wolbachia to infect new hosts and persist in the environment, predation and cannibalism have now been established as possible routes of Wolbachia transmission, at least within species (Le Clec’h et al., 2013; Thi Hue Kien et al., 2023) and it has now been established that Wolbachia can survive extracellularly for extensive periods of time (Porter and Sullivan, 2023). Taken together, this growing body of evidence implies a higher risk of Wolbachia to spread to non-target insects than originally anticipated.
In addition to exchanging material with other Wolbachia strains, Wolbachia can also potentially exchange genetic material with bacterial pathogens that are transmitted by arthropod vectors such as ticks, lice and flies. This this particularly true of other endosymbionts with mixed symbiotic profiles such as Rickettsia because they can co-infect the same cell as Wolbachia and could exchange material with it (Halter et al., 2023). Certain categories of genes under strong selection such as antibiotic resistance or virulence genes allowing bacterial host to colonize new niches or to colonize existing ones more effectively could drive this genetic exchange may thus have some environmental impact. This could be further facilitated by mobile genetic elements such as transposons and plasmids. Indeed, Wolbachia was found to harbor plasmids (Reveillaud et al., 2019), although these putative plasmids appear to be present in only a fraction of the isolates and their horizontal transfer between Wolbachia-infected cells has not been demonstrated (Martinez et al., 2022).
4. The population replacement strategy for biocontrol of vector-transmitted viral disease
4.1. Dynamics of introgression:
The goal of population replacement is to replace the field mosquito population by an introduced population of Wolbachia carriers. The spread of the released mosquitoes in the field is driven by CI, which provides an advantage to Wolbachia-carrying females mating with Wolbachia-free males, eliminating 50% of the hybrid offspring (Figure 1). Given that the fraction of hybrid progeny depends on the frequency of released mosquitoes present in the field at a given time, there is an unstable equilibrium point below which the frequency of released mosquitoes is expected to decline without further releases. This threshold in the order of 20-30%, but varies depending on host fitness parameters, level of CI, and maternal transmission rates (Barton and Turelli, 2011; Hancock et al., 2011; Hoffmann et al., 2011).
Given that the relative fitness of released vs. field mosquitoes is another major determinant of the threshold for stable introgression, it is important to match the fitness of released mosquitoes as closely as possible with that of the field mosquito population. The sensitivity to insecticides is a major factor driving mosquito fitness in the field. This complicates implementation efforts because the level of insecticide resistance in wild mosquito populations is highly variable, and it depends in part on the specific insecticide being used locally (Garcia et al., 2020). To even out the fitness of the release strain relative to that of field mosquito populations, field-derived A. aegypti males from the target area are reared and backcrossed with females from the wMel- (or wAlbB-) infected line for several (generally 6) generations (Hoffmann et al., 2011; Nazni et al., 2019; O’Reilly et al., 2019). Given that the degree of wMel-mediated pathogen interference in the carrier mosquitoes is affected by genetic variation (Ford et al., 2019), the degree of Wolbachia-mediated pathogen interference needs to be tested in the resulting Wolbachia-carrying release strain. An intrathoracic challenge with DENV1-4 is the most standardized and reproducible method at the moment (Simmons et al., 2024).
Past the threshold for stable introgression, the fraction of released mosquitoes is expected to continue to increase without further intervention. These introgression dynamics mean that reaching the stable introgression threshold requires a long and continuous release of Wolbachia-carrying mosquitoes (for 15-20 weeks) but that a single intervention can be sufficient to achieve long-term introgression. This has been demonstrated by longitudinal entomological monitoring in numerous studies showing a rapid establishment of wMel in the intervention areas during the first year-post release and persistence at very high level ever since without further intervention (Liang et al., 2022). This is shown in Table 1, which lists all randomized and non-randomized deployments across the world that (to our knowledge) have been reported.
Table 1.
Wolbachia-based population replacement interventions for Dengue control.
| Location of the intervention |
Study period | Wo Strain |
Population covered |
Surface are of intervention (Km2) |
% introgression |
% decrease in Dengue prevalence |
Type of study | Dengue prevalence before intervention |
Dengue prevalence after intervention |
Sources | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Starting year |
End Year |
||||||||||
| Australia (the Cairns Region, the Cassowary Coast Region, the Douglas Shire and the Charters Towers Region) | 2011 | 2017 | wMel | 330 000 | 299 | >80 | 96 | Experimental: Non-Randomized and Controlled interrupted time series | Non-endemic country. Maximum of 915 cases in an epidemic year (2009) in Queensland (McBride, 2010) | Queensland is re-emerging. 23 autoctone cases in 2019 (Walker et al., 2021) |
https://www.worldmosquitoprogram.org/en/global-progress/australia (O’Neill et al., 2019a) |
| Vietnam (Tri Nguyen Island, Vinh Luong, Thu Dau Mot City, Binh Duong Province, and My Tho City) | 2013 | 2022 | wMelPop and wMel | 280 000 | 39 | 2,9 to 97,2 | Not available | No-experimental: Cohort study | The average incidence of 168 cases per 100,000 inhabitants during the years 2000 to 2015 (WMP, 2023) | As of October 15, 2023 (epidemiological week 41), Viet Nam has recorded a total of 113,962 dengue cases, with 31 fatalities. This marks a notable decrease of 56.8% compared to the corresponding period in 2022, where there were 264,078 cases and 128 deaths reported (WHO, 2023) |
https://www.worldmosquitoprogram.org/en/global-progress/vietnam (Ryan et al., 2020) |
| Brazil (Rio de Janeiro) | 2014 | 2021 | wMel | 960 000 | 95 | 32 | 38 | Quasi-experimental: Non-Randomized, Controlled interrupted time series | The State of Rio de Janeiro and its capital are hyperendemic. They have an average of 36,000 and 1,400 annual cases, respectively (Brazil, 2023) | State and its capital remain hyperendemic. On 2024, there have been more than 276,042 and 105,048 cases, respectively. There is also a report of 1,514 on 2023 and 3,040 on 2024 (EW 25) cases of Chikungunyaa |
https://www.worldmosquitoprogram.org/en/global-progress/brazil/rio-de-janeiro
a https://lookerstudio.google.com/u/0/reporting/6e4f2039-e55e-49a0-8732-874f69672241/page/p_yi8kb4cl3c |
| Brazil (Niterói) | 2015 | 2019 | wMel | 520 000 | 140 | 40-80 | 69 | In 2013, Niterói recorded 19 cases. 376 in 2014 839 in 2015 (Brazil, 2023) | In 2016, Niterói recorded 3,345 cases. 861 in 2017 1, 652 in 2018 353 in 2019 74 in 2020 31 in 2021 56 in 2022 (Brazil, 2023) 1,606 in 2024 (EW 25) a |
ahttps://lookerstudio.google.com/u/0/reporting/6e4f2039-e55e-49a0-8732-874f69672241/page/p_yi8kb4cl3c https://www.worldmosquitoprogram.org/en/global-progress/brazil/niteroi (Pinto et al., 2021) |
|
| Colombia (Bello, Itagüí, Medellín, Sabaneta) | 2015 | 2019 | wMel | 4 490 000 | 183 | >60 | 73 to 96 | Mixed Experimental: Non-Randomized, Controlled Interrupted Time Series And Non-experimental: Case-control study | On average, 3,433 cases were reported in Medellin, 430 in Bello, 698 in Itagüí, and 79 in Sabaneta between 2008 and 2014 (Colombia, 2023) | On average, 3,433 cases were reported in Medellin, 430 in Bello, 698 in Itagüí, and 29 in 2024 (EW 24)(Colombia, 2023) |
https://app.powerbi.com/view?r=eyJrIjoiOTIxMzE4MGItNjg4MC00ZmUyLWIwMzctODhlOWFjNzMyZmViIiwidCI6ImE2MmQ2YzdiLTlmNTktNDQ2OS05MzU5LTM1MzcxNDc1OTRiYiIsImMiOjR9 (Vélez-Bernal, 2023; Velez et al., 2023) |
| Malaysia (Selangor, Kuala Lumpur, Putrajaya and Penang Stateb) | Mar 2017 | 2019 | wAlbB | NA | NA | >90 (Kuala Lumpur) | 40 (5 to 65IC) in Kuala Lumpur | Experimental: Non-Randomized, Controlled Before-and-After | Malaysia consistently experiences a high prevalence of Dengue fever, with an annual report of nearly 100,000 cases, as outlined in the following statistics: 2014: Approx. 110,000 cases 2015: Approx. 120,000 2016: 101,000 cases 2017: 81,000 cases 2018: 80,000 cases 2019: 130,000 casesc |
Up until October 2023, there has been a substantial 100.5% surge in the number of dengue cases, reaching a cumulative total of 96,443 cases. This marks a significant increase from the 48,109 cases reported in 2022. In the same year, Malaysia registered approximately 64,078 cases of dengue fever nationwide, with 26,365 cases in 2021 and 90,304 cases in 2020. Regarding fatalities, by October 22, 2023, a total of 73 deaths, compared to 29 deaths during the corresponding period in 2022. In October 2023, the daily average of cases exceeded 300. Currently, there are 73 dengue hotspot locations nationwide, with Selangor state having the highest number of hotspots [51], while other states have single-digit countsd |
bhttps://wolbachia.nih.gov.my/wolbachia-operational-realization-by-phase/ chttps://www.ijidonline.com/article/S1201-9712(22)00505-7/fulltext d https://reliefweb.int/report/malaysia/malaysia-dengue-prevention-and-control-dref-operation-ndeg-mdrmy010#:~:text=In%202022%2C%20Malaysia%20documented%20roughly,the%20same%20period%20in%202022. (Ahmad et al., 2021) |
| Indonesia (Yogyakarta) | March 2017 | December 2017 | wMel | 311 700 (control and release area | 26 | >95 | NA | Experimental: Randomized Controlled Trial | 1,745 cases of Dengue in 2016 and 414 cases in 2017 (Arguni et al., 2022) | The protective efficacy of the intervention was 77.1% similar against the four dengue virus serotypes. The incidence of hospitalization for virologically confirmed dengue was lower among participants who lived in intervention clusters (0.4%) than among those who lived in control clusters (3.0%) 2021) | (Utarini et al., 2021) |
| Indonesia (Yogyakarta) | 2017 | 2021 | wMel | 1 760 000 | 539 | >60 | 77 | Mixed Experimental: Randomized Controlled Trial And Non-Randomized, Controlled Interrupted Time Series | A remarkable 83% drop in the occurrence of hemorrhagic Dengue was witnessed in the city of Yogyakarta, along with a substantial 78% decline in the number of Dengue cases (Indriani et al., 2023) |
https://www.worldmosquitoprogram.org/en/global-progress/indonesia/yogyakarta-city (Indriani et al., 2023) |
|
| Fiji (Lami-Suva-Nakasi, Nadi and Lautoka | 2018 | 2019 | wMel | 350 000 | 116 | >75. High established in all 12 reporting areas in Suva, 5 of 6 in Nadi, and 5 of 5 in Lautoka) | Ongoing monitoring | Experimental: Non-Randomized, Controlled Before-and-After And Non-Randomized, Controlled Interrupted Time Series | More than 4,000 cases in 2018 (WMP, 2023) | More than 8,000 cases compatible with Dengue as of EW 41, 2023 (WHO, 2023) |
https://www.worldmosquitoprogram.org/en/global-progress/fiji (Simmons et al., 2024) |
| Kiribati (South Tarawa and Betio) | June 2018 | June 2019 | wMel | 25 000 | 1,5 | >55. Intermediate to high established in Betio and western Bairiki) | Ongoing monitoring | Experimental: Non-Randomized, Controlled Before-and-After And Non-Randomized, Controlled Interrupted Time Series | In 2018, a total of 1,802 cases of dengue were reported nationwide (Yoon et al., 2019) | There were approximately 55 Dengue-compatible cases in 2021, 3 in 2022, and none in 2023 nationwide (WHO, 2023) |
https://www.worldmosquitoprogram.org/en/global-progress/kiribati (Simmons et al., 2024) |
| Sri Lanka (Colombo) | 2018 | June 2021 | wMel | 240 000 | 20 | Ongoing | Ongoing | In Colombo, there were approximately 13,000 annual cases reported between 2010 and 2017, accounting for roughly 24% of Sri Lanka's annual cases during that period (Sri Lanka, 2023) | In Colombo, there were 12,869 reported cases of dengue fever in 2022 and 14,747 in 2023, constituting an average of 19% of the total cases in the country (Sri Lanka, 2023) | https://www.worldmosquitoprogram.org/en/global-progress/sri-lanka | |
| Vanuatu (Port Vila) | July 2018 | March 2019 | wMel | 62 000 | 39 | >75. (High established in 10 of 12 reporting areas) | Ongoing | Experimental: Non-Randomized, Controlled Before-and-After And Non-Randomized, Controlled Interrupted Time Series | An environmental disaster declaration from December 30, 2016, to May 31, 2017, reported 2,820 suspected cases, of which 22.7% (641 cases) were confirmed nationwidef | Across the nation, there have been documented instances indicative of Dengue fever, with reported cases numbering 77 in 2021, 108 in 2022, and 77 as of EW 41 in 2023 (WHO, 2023) |
https://www.worldmosquitoprogram.org/en/global-progress/vanuatu/port-vila f https://reliefweb.int/report/vanuatu/vanuatu-dengue-fever-outbreak-dref-operation-n-mdrvu003-final-report (Simmons et al., 2024) |
| Mexico (La Paz) | January 2019 | 2021 | wMel | 250 000 | 50 | Ongoing | Ongoing | La Paz is located in Baja California Sur, and the entire state has reported the following cases: 166 (2016), 30 (2017), and 186 (2018)(México, 2023a) | Baja California Sur: 5 cases (2021), 91 (2022) and 761 (2023)(México, 2023b) | https://www.worldmosquitoprogram.org/en/global-progress/mexico | |
| New Caledonia (Mont-Dore and Dumbéa) | January 2022 | June 2022 | wMel | 150 000 | 144 | >71 | NA | The DENV virus has led to three notable outbreaks: the first occurred in 2008-2009, resulting in 9,589 reported cases; the second occurred in 2012-2013, with 11,240 cases reported; and the third took place in 2016-2018, accounting for 7,266 cases. In 2021, 117 cases were reported (Inizan et al., 2019) | 117 cases was reported at 2021. Three cases on 2022 (only one autochthonous) and Nine cases were confirmed on 2023, with 66,7% of them being importede |
https://www.worldmosquitoprogram.org/en/global-progress/new-caledonia
e https://dass.gouv.nc/votre-sante-maladies/la-dengue-le-chikungunya-et-le-zika |
|
| Honduras (Tegucigalpa) | Aug 2023 | No yet | wMel | 87 000 | 3,3 | Ongoing | Ongoing monitoring | 24,700 cases and more than 400 severe Dengue cases in 2022 (WMP, 2023) | Ongoing | https://www.worldmosquitoprogram.org/en/global-progress/honduras | |
| Laos (Vientian) | No yet | wMel | 56 000 | 12 | Not yet | Not yet | The last major epidemic in 2019 resulted in 39,091 cases of Dengue, with 10,813 cases in Vientiane alone (WMP, 2023) | Up until EW 41, 2023, a total of 28 041 Dengue cases have been documented at the country (WHO, 2023) | https://www.worldmosquitoprogram.org/en/global-progress/laos | ||
The mathematical models of dengue transmission and its suppression through vector control have been recently reviewed by Ogunlade et al., (2023). Wolbachia-based Interventions have imperfect efficacy, meaning that their impact will depend on local dengue transmission intensity as quantified by force of infection (FOI) and reproduction number (R0). Wolbachia reduces transmission by a fixed proportion. The release of mosquitoes carrying the wMel strain has been predicted on the basis of lab studies to achieve 70% reduction in R0 (O’Reilly et al., 2019). Mathematical models suggest that a reduction in infectiousness in wMel-infected A. aegypti in a similar range (80%) could be sufficient to reduce the basic reproductive number to below 1, potentially eliminating the disease over a decade. This is only true in settings in which dengue is endemic with low-to-moderate transmission, as the impact is predicted to be smaller in the highest transmission settings. Even a more moderate immediate efficacy of the intervention of 50% is predicted to decrease global incidence by at least 70% (Ferguson et al., 2015; Dorigatti et al., 2018).
4.2. Mechanisms of disease transmission suppression:
A high level of introgression of Wolbachia-carrying mosquitoes reduces the vectorial capacity of mosquitoes mainly through pathogen blocking, but Wolbachia infection also modulates mosquitoes’ efficiency as vectors in other ways. Wolbachia can also alter transmission in other ways such as by lowering the vector’s fitness, by lengthening its EIP (which reduces virus transmission through saliva (Ye et al., 2015)), by increasing mosquito locomotion and metabolism (Hoffmann et al., 2011), or by altering mosquito feeding habits, (Turley et al., 2009).
As mentioned above, the transinfection of Wolbachia into A. aegypti (a species in which it is largely non-native) typically reduces the host’s fitness, although with strong strain-dependent variation (Ulrich et al., 2016; Ant et al., 2018). Because most Wolbachia-transfected lines originate from few or just one female, Wolbachia invasions can cause a dramatic reduction of mitochondrial haplotype diversity within and among populations (Armbruster et al., 2003). There is a growing body of evidence suggesting that mitochondrial genetic variation is maintained by selective pressure (Rašić et al., 2014; Kurbalija Novičić et al., 2015) and might play an important role in allowing insects to modulate metabolic rate according to varying environmental conditions (Arnqvist et al., 2010). The loss of mitochondrial diversity following Wolbachia invasion is therefore likely to affect the performance of infected populations (Hoffmann et al., 2015).
Wolbachia-induced fitness loss is a double-edged sword. On the one hand poor mosquito fitness impairs disease transmission by lowering mosquito density and also by shortening mosquito average lifespan, which reduces transmission by decreasing the fraction of old mosquitoes in the population (Hoffmann et al., 2011). Poor fitness also helps to contain infections within the area of intervention (Barton and Turelli, 2011; Hancock and Godfray, 2012). On the other hand, impaired fitness can interfere with stable introgression of the released mosquitoes, as was shown in the case of the strain wMelPop (Nguyen et al., 2015).
4.3. Population replacement strategy in targets that are naturally infected with Wolbachia:
Alternate vectors being considered as targets for Wolbachia biocontrol can be naturally infected with Wolbachia. In this case, the potential impact of their resident Wolbachia strains must be considered. These naturally infected mosquitoes can be transinfected, generating a “superinfected” line with a resident and a transinfected strain. Transinfection has been successful in A. albopictus naturally carrying the wAlbB strain, artificially generating a triple Wolbachia infection that yielded a new pattern of cytoplasmic incompatibility (Fu et al., 2010). In cases where superinfection is not tolerated (which appears to be the case for Cx. quinquefasciatus) (Jeffries and Walker, 2016), the resident strain can be cured through the use of antibiotics prior to transinfection with the desired Wolbachia strain (Jeffries and Walker, 2016).
The mating of released transinfected mosquitoes with the wild population naturally infected with Wolbachia is expected to result in bidirectional CI (Sinkins et al., 2005). Assuming no difference in fitness between the released and wild mosquito populations, the population that reaches the highest local frequency would likely reach fixation given that females infected with this strain would be at a reproductive advantage (i.e. will have more males to mate with that are compatible)(Jeffries and Walker, 2016). However, fitness costs typically associated with a transinfected Wolbachia strain would decrease the probability of success of the invasion and replacement of the wild mosquito population (Jeffries and Walker, 2016).
The presence of other microorganisms within the mosquito population also needs to be considered as a factor that could affect the introduction of any transinfected Wolbachia strain into that population (Jeffries and Walker, 2016). For example, the bacteria Asaia, which is stably associated with many mosquito species, has been found to prevent or reduce Wolbachia establishment, possibly by competing with Wolbachia within a host (Favia et al., 2007).
4.4. Logistics of mosquito releases
Production:
The continuous release of Wolbachia-carrying mosquitoes requires the rearing of Wolbachia-inoculated mosquitoes on an industrial scale in “mosquito factories”. These specialized facilities require tightly controlled environments that reproduce optimal mosquito breeding and maintenance conditions and observe strict biosafety measures to prevent accidental release and to ensure the safety of personnel (WMP, 2023). They include egg collection systems, larval rearing setups, and insectaries for adult mosquitoes. For detailed logistics see :[https://cutt.ly/Tezsr6DJ].“Mosquito factories” can produce millions of mosquitoes per week. Wing measurements can be used to get a rough indication of the fitness competitiveness of the produced mosquitoes (Stephens and Juliano, 2012; Ross et al., 2014).
Design:
Pre-release entomological surveys are important to inform the density and the duration of the release, as a very high baseline abundance of A. aegypti in the target area appears to interfere with the introgression of released mosquitoes (Simmons et al., 2024; Ribeiro dos Santos et al., 2022). The genetic population structure of the target population also needs to be taken into consideration, including rates of ongoing gene flow, fine scale mosquito movement and adaptive genomic changes. A limited dispersal limits the ability to replace wild mosquito population in large geographical areas. In these situations, release of Wolbachia-infected mosquitoes should be done in multiple sites/villages in a given area rather than at a single site and expect a rapid spread throughout the area (Hlaing et al., 2010).
The target area has to be relatively small, ranging between 3 km2 and 100 km2, in quadrants of 250 m x 250 m (Shepard et al., 2022). Spatial and temporal continuity appear to be critical variables for the successful establishment of a Wolbachia-carrying mosquito population (Hu et al., 2015). Given these constraints, the population replacement strategy can only be realistically implemented in urban areas, which have good accessibility and a high mosquito population density.
Distribution:
The release of Wolbachia-carrying mosquitoes into the environment is meticulously coordinated to maximize coverage and is generally done weekly. Handling conditions are important because rearing, storage, transport, and release procedures can affect mosquito quality (Chung et al., 2018; Crawford et al., 2020)..
Adults are released at strategic points across designated areas (typically in the morning hours) on a pre-determined grid, either by staff driving through neighborhoods or using drone technology (Guo et al., 2022). It requires the prior transfer of larvae to release containers where they can pupate and eclose, which requires additional handling of the immature stages by laboratory personnel.
Alternatively, eggs produced by remote mass-rearing can be shipped to the release site. There, they can easily be cut into strips and transported in egg containers or “Mozzie boxes” to designated areas for local hatching and dispersal (WMP, 2023). Overall, egg-based distribution is far less laborious than adult releases, facilitating a wider roll-out, and it has been successfully used in in Townsville, Australia (wMel strain)(O’Neill et al., 2019b) and in Kuala Lumpur, Malaysia (wAlbB strain)(Nazni et al., 2019). However, because storage and transport can reduce Wolbachia-infected egg viability (Allman et al., 2020), this can lead to increased production requirements and produce lower quality mosquitoes so it may not be adequate for all programs.
Monitoring introgression:
measuring entomological endpoints is important to link any impact detected on pathogen incidence to the mosquito release intervention. Monitoring typically starts after 4 weeks of releases, capturing adult mosquitoes at predetermined locations through sentinel traps. The most frequent traps are BG-Sentinel ovitraps, which exploit the tendency of Aedes mosquitoes to lay their eggs in small containers (Li et al., 2016). This allows the estimation of Aedes species composition and population size over time. A Gravitrap Aegypti Index (GAI) (traps positive for A. aegypti divided by total functional gravitraps per site) can be used as an estimate of adult mosquito population size in an area (Barrera et al., 2013; Montenegro et al., 2020). Resulting eggs are returned to the lab, raised to adults and a subsample is used for PCR analysis (Simões et al., 2011).
4.5. Examples of implementation of the population replacement strategy across the world:
Since 2011, the World Mosquito Program (WMP) has launched pilot wMel population replacement programs in partnership with local organizations in 14 different countries, targeting areas with a combined population of 11M people. A separate initiative sponsored by Wellcome Trust, the National Health and Medical Research Council of Australia and the Ministry of Health of Malaysia using the wAlbB strain has also been implemented in Malaysia (Nazni et al., 2019; Ahmad et al., 2021). These programs primarily target dengue and their goal is to collect evidence of feasibility and determine the efficacy of the Wolbachia approach as a strategy for prevention of dengue.
The implementation sites for this initial phase are shown in red color in Figure 3 and the complete information for these studies including location, mosquito release period, surface of intervention and population covered, degree of introgression, impact on dengue prevalence, type of study, and source can be found in Table 1.
Fig 3. Implementation sites for population replacement interventions.
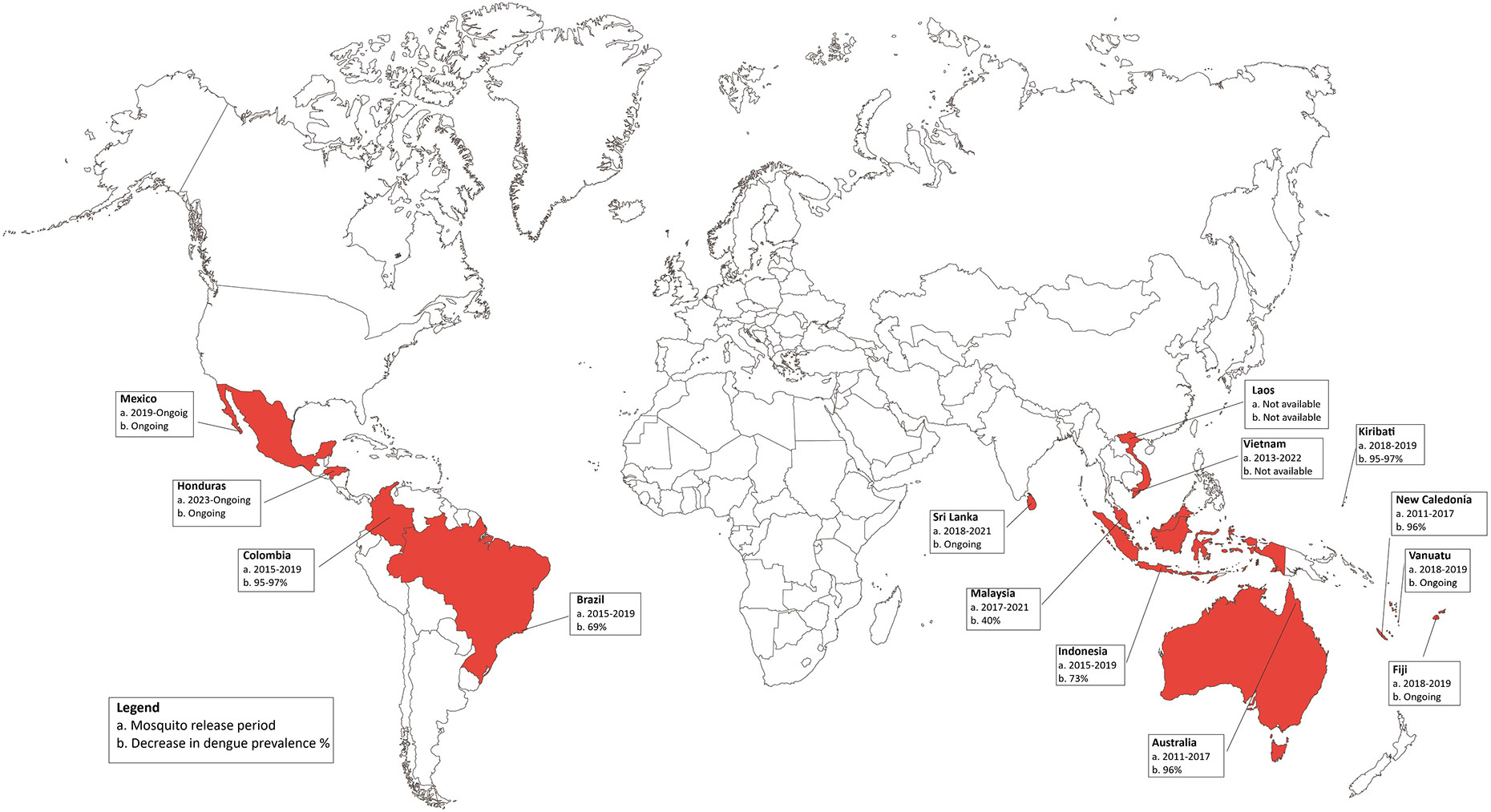
The map shows the countries where pilot studies using the replacement strategy of Wolbachia-transinfected mosquito release have been implemented, highlighted in red. The box lists the country, the mosquito release period and, in cases where Wolbachia’s prevalence was demonstrated to have reached the stable introgression threshold, the associated decrease in Dengue prevalence is listed (in percentage). Studies that are still in process are labelled as “ongoing”.
Mosquito releases led to the stable establishment of wMel in wild populations of A. aegypti in Cairns, Australia (O’Neill et al., 2019a); in two adjacent municipalities in Colombia (Bello, and Itaguí) (Velez et al., 2023); in Cali, Colombia (Velez et al., 2023); in Niteroi, Brazil (Pinto et al., 2021); in Yogyakarta, Indonesia (Utarini et al., 2021; Indriani et al., 2023), and in Fiji (Simmons et al., 2024).
A single study also reported high levels of introgression (>80%) following the release of wAlbB mosquitoes in six different urban areas of Kuala Lumpur, Malaysia but these high levels were only maintained in two of the areas (Nazni et al., 2019). Note that Kuala Lumpur has two relatively long dry seasons, which may have severely affected the fitness of the wAlbB strain of Wolbachia in the area. In Medellín, Colombia, wMel failed to stabilize in 7 of the 18 areas included in the study, but this result is likely attributable to accessibility problems, which effectively interfered with the continuous release of mosquitoes (Velez et al., 2023). In Rio de Janeiro, Brazil, wMel release only achieved incomplete introgression (Ribeiro dos Santos et al., 2022), probably due to a combination of inconsistent accessibility (it covered a very large area −87km2-) and an inability to reach the threshold stable introgression in areas of high mosquito density. Supporting this idea, locations and seasonal periods with the highest incidence of dengue and Chikungunya showed a lower average degree of introgression (Ribeiro dos Santos et al., 2022). In Vanuatu, wMel introgression only failed to reach >75% in 2 out of 12 areas of intervention, which could have been caused by concurrent use of insecticide, and in Kiribati one of the two study areas has only reached 50% introgression after 3.5 years of the last release, likely due to the very high baseline abundance of A. aegypti (Simmons et al., 2024).
In these controlled studies with a documented success in reaching the threshold for stable introgression, a substantial suppression in dengue cases is typically seen within the intervention sites within a year (Table 1). The Cairns study in Australia reported a decrease of 94% (O’Neill et al., 2019a); similarly, the Aburrá valley studies (Medellín, Bello and Itagüí) reported a 95-97% reduction in dengue prevalence (Velez et al., 2023); the Yogyakarta study a 78% reduction (Indriani et al., 2023), and the Niteroi study a 69% reduction (Pinto et al., 2021).
The impacts on dengue prevalence reported by these population replacement studies are well in line with lab studies that estimate a 70% reduction in R0 for dengue in wMel-carrying A. aegypti mosquitoes (O’Reilly et al., 2019), although (as mentioned above) the degree of blocking is affected by genetic variation in mosquitoes (Ford et al., 2019). Note that these reported impacts were likely underestimates because they only measure effects on the local transmission in the release zone and are unable to account fully for human and mosquito movement between treated and untreated clusters (Cavany et al., 2022) (see also discussion of contamination effects above). In addition, dengue’s extreme seasonal and interannual variation means that global transmission reduction intervention predictions are only approximations. Average R0 values overestimate the impact during transmission intensity peaks (as peak R0 values may be substantially higher); the converse is true during seasonal troughs. Therefore, results need to be corrected for seasonal variation (Ferguson, 2018).
Studies reporting more modest or unstable introgression also report more modest reductions in dengue prevalence, with the state of Rio de Janeiro reporting an intermediate level of dengue prevalence reduction (38%), and the Kuala Lumpur study reporting a 43% reduction over all intervention sites (Nazni et al., 2019). In the latter study, with the inclusion of additional release and control sites (20 and 76, respectively) and a more extended period of dengue monitoring, the impact on prevalence went up to 62% (Hoffmann et al., 2024). This study also reported that the level of dengue reduction increased with Wolbachia frequency, with a 75.8% reduction estimated at 100% Wolbachia frequency (Hoffmann et al., 2024). Taken together, these observations suggest a dose-response effect between the level of introgression and the level of dengue prevalence suppression, but the Yogyakarta study reported only cluster-level wMel frequencies higher than 80% as being protective (Utarini et al., 2021), (Table 1), so the question of a possible threshold effect below which introgression with Wolbachia-carrying mosquitoes fails to suppress dengue remains open.
The Yogyakarta (Indriani et al., 2023), Aburrá Valley (Velez et al., 2023) and Kuala Lumpur (Nazni et al., 2019; Hoffmann et al., 2024) studies are particularly powerful because they were cluster-randomized, minimizing the risk of confounding bias by ensuring that the characteristics of the control and intervention groups are similar to one another (Wilson et al., 2015; WHO, 2017a). The Yogyakarta study is also unique in reporting virologically-confirmed dengue (VCD) (Indriani et al., 2023).
Further, a secondary analysis of the trial data in the Yogyakarta study examining the spatial and temporal distribution of virologically-confirmed dengue cases over a 27-month study period demonstrated an even larger intervention effect than measured in the primary analysis, providing strong evidence suggesting that dengue cases reported in the intervention clusters may have acquired their infection outside their cluster of residence (Dufault et al., 2022).
However, the results reported by studies in one setting cannot be directly or easily extrapolated to other settings because dengue incidence is highly affected by the environment into which Wolbachia-carrying mosquitoes are released. These include environmental conditions, host genetic background, immune-mediated serotype dynamics, and virus-specific phenotypic variation. Note that, unlike mathematical model predictions (Ogunlade et al., 2023), these population replacement interventions very seldom led to its complete eradication. This is true even in areas where the degree of introgression was very high and where dengue is not considered endemic such as the Cairns study (Ryan et al., 2020). This is likely due to movement of people and mosquitoes from adjacent areas and to an incomplete coverage of the area.
5.0. The population suppression strategy for biocontrol of vector-transmitted viral disease
This approach, also known as incompatible insect technique (IIT), was first proposed by Beard and colleagues, and it is based on the release of males carrying Wolbachia strains that cause CI (sterility) when mated with females not infected with the same Wolbachia strain (Figure 1) (Beard et al., 1993). Above a certain threshold, the decreased fertility rate results in a reduction in the density of the vector mosquito population, which in turn lowers the risk of pathogen transmission (O’Neill, 2018; Tantowijoyo et al., 2020).
The goal is to achieve the low-risk threshold, which is an average number of females trapped (GAI index) <0.1.
This strategy is reminiscent of the classical sterile insect technique (SIT), which involves the release of males that have been sterilized by irradiation or exposure to chemicals so that mating with field females yields non-viable eggs, although IIT has less impact on the fitness of released males because it does not involve a toxic treatment (Lofgren et al., 1974; Alphey et al., 2010).
5.1. Implementation of the population suppression strategy:
The population suppression strategy comes with a unique set of challenges. The first challenge is a high effectiveness threshold that requires very large release volumes, with ratios of up to 50 Wolbachia-carrying male to 1 naïve male, which corresponds to 6-7 mosquitoes per resident per week (Crawford et al., 2020). An alternative approach for skewing the ratio of Wolbachia-carrying males to field females is to suppress the field population of mosquitoes by traditional vector control methods prior to the release of IIT males. Note that (assuming a near constant immigration from non-release areas) field mosquito density suppression does not increase linearly with the number of Wolbachia-carrying mosquitoes released. Eight is the released-to-field mosquito ratio above which increasing the size of release has a limited but additional impact on suppression levels (Ching et al., 2021; Zeng et al., 2022).
The need to flood the area with males limits the area that can be effectively covered (Xi et al., 2005; Axford et al., 2016; Pagendam et al., 2020). This area restriction makes the population suppression approach highly vulnerable to the impact of migration of mosquitoes from untreated surroundings, making complete mosquito eradication in release sites impossible. In addition, the impact of mosquito releases is short-lived because both hatch rates and the numbers of adult females quickly recover after releases stop. To counter the impact of migration of females from neighboring, non-intervention areas, and to prevent the recovery of non-IIT mosquito population, this approach requires continuous weekly releases.
The accidental release of residual numbers of Wolbachia-infected females can lead to the establishment of Wolbachia in the field population, which would restore the fertility of matings with Wolbachia-carrying males, rendering this strategy ineffective (Xi et al., 2005). However, the establishment of a Wolbachia-carrying population would still suppress transmission through pathogen blocking (Ross, 2021). While the threshold for stable introgression of Wolbachia in the field mosquito population is high, it can be achieved with a very small number of released females when the field mosquito population has been nearly eliminated (Pagendam et al., 2020).
No mosquito sex-sorting system has been approved by WHO’s vector control advisory group yet, but standard sex-sorting technology exhibits a 01.-0.3% female contamination rate. This rate can be further decreased using high-fidelity sex-sorting technology (Crawford et al., 2020). Alternatively, residual females can be sterilized using a low-dose irradiation (IIT-SIT) or Wolbachia introgression can be suppressed through the release of irradiated males or of a second bi-directionally incompatible strain that also exhibits CI and preserves high competitiveness (Ching et al., 2021).
Finally, for the population suppression approach to work, released male mosquitoes need to be able to physically meet field female mosquitoes. This means that to ensure comprehensive coverage the release of mosquitoes needs a high level of granularity. In high rises, for example, mosquitoes need to be released in several floors.
While all these challenges can be addressed, the population replacement strategy represents a highly resource-intensive approach that is only practicable in highly-urbanized, high-rise areas.
5.2. Results of studies of deployment of the population suppression strategy:
The implementation of the population suppression approach has been piloted by the National Environmental Agency (NEA) of Singapore (NEA, 2023). An initial trial was implemented in two high-rise public housing estates in Singapore over a period of 10 and 18 weeks, respectively. In Phase I of this study, the average number of A. aegypti females caught in Gravitraps only dropped by 51 to 65% in release sites and Wolbachia-carrying males were found to have a short life-span (~4.5 days). In addition, Wolbachia was detected in field populations, suggesting that imperfect sex-sorting can potentially lead to Wolbachia’s establishment in the population (Ching et al., 2021).
In Phase II (weeks 10-70 and 18-58, respectively), these problems were addressed by IIT-SIT (site 1), or using high-fidelity sex sorting (site 2), by increasing the frequency of releases to twice a week, by adding an upper floor release site, and by doubling release areas to include buffer areas against migration from adjacent non-release areas. As a result of these modifications to the implementation design, suppression of mosquito density to low-risk levels was achieved at week 9 (site 1) and 17 (site 2) and maintained throughout the duration of the trial, although a complete eradication was not achieved at either release site.
The observed suppression of mosquito density led to a suppression of dengue incidence of 71% (site 1) and 88% (site 2). Importantly, dengue suppression was also seen in buffer areas, with some spillover effect up to 1km away from the core, which is consistent with the flight range of individual mosquitoes (Ching et al., 2021).
This trial shows that mosquito density can be suppressed below the low-risk threshold through the continuous release of Wolbachia-carrying male mosquitoes so long as the immigration of wild-type females from neighboring areas can be addressed, an adequate distribution of males in high-rise buildings can be achieved, and the establishment of Wolbachia in the field mosquito population can be prevented. Once achieved, suppression can likely be maintained more efficiently through IIT-SIT and lower-volume releases.
6. Comparative analysis of Wolbachia-based vector control methods
6.1. Comparison between population replacement and population suppression:
The two main strategies of Wolbachia-based vector control differ in the desired features for the strain-mosquito combination; both approaches require strong CI, but population replacement is more dependent on pathogen blocking and on minimal fitness costs to the released strain. They also differ in the sex of the released mosquitoes (which is exclusively male in population suppression), in the target ratio of release vs. wild mosquitoes (much higher in population suppression), in the reversibility of the intervention (population replacement typically requires a single intervention, whereas population suppression requires continuous mosquito release), and in the area covered (smaller in population suppression).
Population replacement is much more cost-effective because it is expected to be permanent in the mid-term (requiring a single intervention). This is a crucial advantage over the population suppression approach and insecticide-based methods. Based on the monitoring of the earliest wMel Wolbachia release sites in N. Queensland, Wolbachia can be self-sustained at a high prevalence for at least 7-10 years post-release. Supporting the long-term efficacy of this approach, long-term follow-up of population replacement studies found that both the Wolbachia genomes used and their virus-blocking properties were found to be highly stable, suggesting that possible adaptations of the virus to Wolbachia-mediated pathogen interference do not interfere with the continued effectiveness of the intervention, at least in the mid-term (Huang et al., 2020; Ahmad et al., 2021; Dainty et al., 2021). The observed long-term stability of virus blocking is likely due to the multiplicity of antiviral mechanisms in place and their intimate connection to housekeeping cellular mechanisms, both of which drastically reduce the possible selection of escape mutants (Ant et al., 2023). The requirement for an alternate infection in mosquito and human hosts is also likely a major contributor to the slow emergence of adaptive resistant virus populations (Thi Hue Kien et al., 2023). The fitness advantage confererred by Wolbachia-mediated resistance to pathogenic insect-specific viruses may also act as a source of selection to maintain the virus inhibitor phenotype, although the magnitude of this selective pressure remains to be determined in wild mosquito populations (Ahmad et al., 2021) and there is also evidence that Wolbachia may increase the pathogenicity of some insect-specific pathogens (Teixeira et al., 2008; Parry and Asgari, 2018; Altinli et al., 2020). Other reasons that the population replacement approach is more cost-effective is because this approach involves a lower ratio of released vs. field mosquitoes, and because it does not require mosquito sex segregation (Brady et al., 2020). In addition, it can cover larger areas.
On the negative side, this approach involves the release of biting (female) mosquitoes, with the possibility of increased frequency of biting in the initial phases. An additional potential risk of the population suppression approach is the possibility of Wolbachia spreading to non-target insects (see discussion on safety above). The possibility of enhancement (rather than suppression) of virus transmission also needs to be considered. There is only one study out of more than 50 involving A. aegypti and A. albopictus that reports evidence of enhancement of viruses known to cause pathology in humans However, there is strong evidence that Wolbachia can enhance insect viruses with DNA or negative-sense RNA genomes. This includes an A. albopictus densovirus (Teixeira et al., 2008), a Culex pipens densovirus (Teixeira et al., 2008), and the negative-sense RNA ISV Aedes anphevirus of A. aegypti (Parry and Asgari, 2018). In addition, there is evidence that Wolbachia increases the susceptibility of Culex pipiens mosquitoes to Plasmodium relictum, suggesting that Wolbachia may not be a safe approach for suppression of malaria (Zélé et al., 2014).
The population suppression approach is more labor-intensive but it has several advantages. The release of males (which are non-biting) facilitates regulatory approval and the fact that the released mosquitoes are infertile eases concerns about their ecological impact. Another advantage of this approach is that it is suitable for a broader range of mosquito species as it is not dependent on having a pathogen-blocking effect (Ross, 2021). However, while the released mosquitoes are not intended to persist in the population, their loss can increase the prevalence of other mosquito species coming to occupy the empty niche. This, in turn, can have unexpected consequences for pathogen transmission (Ritchie et al., 2013). A possible decreased effectiveness over time due to wild mosquitoes developing a mating preference against the released mosquitoes is a potential specific risk for this approach (Cator et al., 2021).
6.2. Comparison to insecticide-based approaches:
Compared to traditional insecticide-based approaches, Wolbachia-based strategies face significant barriers to adoption because they require infrastructure, trained personnel, political support and tight coordination between multiple agencies and the community (Damayanti et al., 2017). Wolbachia-based strategies are considerably more expensive than traditional approaches; even population replacement (which is the more cost-effective of the two Wolbachia-based approaches) is about 7 times more expensive than traditional approaches (Shepard et al., 2022).
On the positive side, Wolbachia-based strategies are more environmentally friendly, with no adverse effects on other species or ecosystems reported so far (Hu et al., 2021). This is unlike insecticide-based approaches, which are frequently toxic for non-target aquatic species (see Bti, Lsph and spinosad larvicides in the “source reduction” section and “chemical adulticides” section in Box-1).
Also, population replacement is often irreversible, necessitating a single intervention for at least a 7-10 years (Dainty et al., 2021; Ross et al., 2021). This is unlike insecticide-based interventions, which are becoming less effective for medium/long term vector control because of the challenges of sustaining these activities at scale and over the long term (Achee et al., 2015), allowing reinfestation with mosquitoes from neighboring areas. Another reason is the development of resistance to insecticides by genetic and behavioral mechanisms (Sparks and Nauen, 2015; Guedes et al., 2020; Zulfa et al., 2022).
Note that controlling mosquito population density is only one of the four key factors contributing to the spread of vector-borne disease; the other three are a deterioration of public health systems, poor sanitary conditions and environmental factors (Tapia-López et al., 2019). This adds an extra layer of complexity to assessments of cost-effectiveness of vector control strategies relative to interventions in other areas.
7. Final conclusions
Overall, the effectiveness of the population replacement approach has been supported by multiple non-randomized studies across the world and conclusively demonstrated by randomized studies. These studies show that stable introgression of Wolbachia-carrying mosquitoes results in decreased dengue prevalence within a year. The stability of the Wolbachia-induced antiviral properties and of strong CI expression in the transinfected line support a longer-term success for these interventions.
The effectiveness of the population suppression approach has been validated by an intervention in Singapore, although this study highlighted the importance of preventing the immigration of wild-type females from neighboring areas and of an adequate distribution of males in high-rise buildings.
Based on the initial results of all these studies, the WHO declared in March 2021 the release of Wolbachia-carrying mosquitoes an “intervention of interest for public health”. Further, wAlbB deployment has become operational and is being rolled out across Malaysia by the Malaysian Ministry of Health (Hoffmann et al., 2024).
Wolbachia-based vector control strategies should be used in conjunction with existing vector control measures and they are not necessarily intended to replace them(Ritchie and Johnson, 2017). When applied synchronously, traditional vector control and population suppression strategies (whose goal is to eliminate the vectors) are incompatible with population replacement strategies (whose goal is to replace the field vector population with transinfected vectors). However, applied sequentially, an initial population suppression intervention can facilitate subsequent introgression. Local knowledge of the ecology of mosquito populations should be used to coordinate release strategies with other vector control measures; for instance, seasonal breeding containers may need to be treated to remove sources of uninfected mosquitoes (Hoffmann et al., 2015).
Current releases focus on a few mosquito species but there is great potential for Wolbachia to control other vectors, pests and diseases (Gong et al., 2020; Mateos et al., 2020) . As mentioned in section 4.3, as the number of hosts expands, natural Wolbachia infections will need to be taken into consideration because they would be expected to interfere with replacement or suppression if the resident Wolbachia strain is compatible with the release strain (Gong et al., 2020).
For the purposes of this review, we have focused on the impact of Wolbachia-based approaches on dengue, but these two approaches should be multivalent. As mentioned above, A. aegypti is also the primary vector for transmission of YFV, CHIKV, ZIKV and Mayaro viruses, so decreasing mosquito population density should suppress the transmission of all these other viruses. Laboratory studies also suggest that the wMel strain of Wolbachia inhibits replication of Chikungunya, Plasmodium, (Moreira et al., 2009) (Shaw and Catteruccia, 2019), and Zika (Aliota et al., 2016), suggesting a polyvalent component to the population replacement strategy. This is further supported by the Niteroi field study, which (with a 69% introgression) reported a 56% reduction in Chikungunya and a 37% reduction in Zika (Pinto et al., 2021).
Wolbachia-based approaches hold great promise for the long-term disease vector control in urban environments. However, the potential of Wolbachia-carrying released mosquitoes to facilitate the evolution and the spread of potentially harmful genes in the environment and the potential to of Wolbachia to increase the transmission of some viruses have not been fully investigated. Also, synergistic combinations of Wolbachia-based and traditional approaches have not been fully exploited.
Supplementary Material
Highlights.
Discusses the elements that need to be considered in the deployment of a Wolbachia-based intervention and their rationale.
Presents the rationale for two main Wolbachia-based strategies for the biocontrol of vector-transmitted disease.
Lists the different deployments reported around the world and discusses their outcomes.
Compares Wolbachia-based biocontrol methods to other types of vector control strategies.
Discusses the environmental risks associated with the release of Wolbachia-infected mosquitoes in light of new genomic, epidemiological and experimental data suggesting that genetic exchange of Wolbachia across hosts is more frequent than anticipated.
Acknowledgements
The authors thank Ministerio de Ciencia Tecnología e Innovación de Colombia (MinCiencias Colombia).
Funding
This work was supported by MinCiencias Colombia postdoctoral fellowship 934-2023 to DML.
Abbreviations:
- CI
Cytoplasmic Incompatibility
- WMP
World Mosquito Program
- EIP
Extrinsic Incubation Period
- ARG
Antibiotic Resistance Gene
- DENV
Dengue Virus
- CHIKV
Chikungunya Virus
- ZIKV
Zika Virus
- WHO
World Health Organization
- DENV
dengue virus
- IRS
Indoor Residual Spraying
- ITMs
Insecticide-Treated Materials
- IIT
Incompatible Insect Technique
- SIT
Sterile Insect Technique
- GAI
Gravity Aegypti Index
- NEA
National Environmental Agency
- FOI
Force of Infection
References
- Achee NL, Gould F, Perkins TA, Reiner RC, Morrison AC, Ritchie SA, et al. (2015). A Critical Assessment of Vector Control for Dengue Prevention. PLoS Negl Trop Dis 9, e0003655. doi: 10.1371/journal.pntd.0003655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad NA, Mancini MV, Ant TH, Martinez J, Kamarul GMR, Nazni WA, et al. (2021). Wolbachia strain wAlbB maintains high density and dengue inhibition following introduction into a field population of Aedes aegypti: Wolbachia wAlbB in wild Aedes aegypti. Philosophical Transactions of the Royal Society B: Biological Sciences 376. doi: 10.1098/rstb.2019.0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Peinado SA, Velez ID, and Osorio JE (2016). The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep 6, 28792. doi: 10.1038/srep28792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, Fraser JE, Ritchie SA, Joubert DA, Simmons CP, and Flores HA (2020). Wolbachia’s Deleterious Impact on Aedes aegypti Egg Development: The Potential Role of Nutritional Parasitism. Insects 11, 735. doi: 10.3390/insects11110735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Osaimi HM, Kanan M, Marghlani L, Al-Rowaili B, Albalawi R, Saad A, et al. (2024). A systematic review on malaria and dengue vaccines for the effective management of these mosquito borne diseases: Improving public health. Hum Vaccin Immunother 20. doi: 10.1080/21645515.2024.2337985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L. (2014). Genetic Control of Mosquitoes. Annu Rev Entomol 59, 205–224. doi: 10.1146/annurev-ento-011613-162002 [DOI] [PubMed] [Google Scholar]
- Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, et al. (2010a). Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector-Borne and Zoonotic Diseases 10, 295–311. doi: 10.1089/vbz.2009.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, et al. (2010b). Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector-Borne and Zoonotic Diseases 10, 295–311. doi: 10.1089/vbz.2009.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinli M, Lequime S, Atyame C, Justy F, Weill M, and Sicard M (2020). Wolbachia modulates prevalence and viral load of Culex pipiens densoviruses in natural populations. Mol Ecol 29, 4000–4013. doi: 10.1111/mec.15609 [DOI] [PubMed] [Google Scholar]
- Ant TH, Herd CS, Geoghegan V, Hoffmann AA, and Sinkins SP (2018). The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog 14, e1006815. doi: 10.1371/journal.ppat.1006815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ant TH, Mancini MV, McNamara CJ, Rainey SM, and Sinkins SP (2023). Wolbachia-Virus interactions and arbovirus control through population replacement in mosquitoes. Pathog Glob Health 117, 245–258. doi: 10.1080/20477724.2022.2117939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ant TH, and Sinkins SP (2018). A Wolbachia triple-strain infection generates self-incompatibility in Aedes albopictus and transmission instability in Aedes aegypti. Parasit Vectors 11, 295. doi: 10.1186/s13071-018-2870-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano C, Castro L, DÃ-az-Caravantes RE, Ernst KC, Hayden M, and Reyes-Castro P (2015). Knowledge and Beliefs about Dengue Transmission and Their Relationship with Prevention Practices in Hermosillo, Sonora. Front Public Health 3. doi: 10.3389/fpubh.2015.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguni E, Indriani C, Rahayu A, Supriyati E, Yohan B, Hayati RF, et al. (2022). Dengue virus population genetics in Yogyakarta, Indonesia prior to city-wide Wolbachia deployment. Infection, Genetics and Evolution 102, 105308. doi: 10.1016/j.meegid.2022.105308 [DOI] [PubMed] [Google Scholar]
- Armbruster P, Damsky WE, Giordano R, Birungi J, Munstermann LE, and Conn JE (2003). Infection of New- and Old-World Aedes albopictus (Diptera: Culicidae) by the Intracellular Parasite Wolbachia: Implications for Host Mitochondrial DNA Evolution. J Med Entomol 40, 356–360. doi: 10.1603/0022-2585-40.3.356 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Dowling DK, Eady P, Gay L, Tregenza T, Tuda M, et al. (2010). Genetic Architecture of Metabolic Rate: Environment Specific Epistasis Between Mitochondrial And Nuclear Genes In An Insect. Evolution (N Y) 64, 3354–3363. doi: 10.1111/j.1558-5646.2010.01135.x [DOI] [PubMed] [Google Scholar]
- Axford JK, Ross PA, Yeap HL, Callahan AG, and Hoffmann AA (2016). Fitness of wAlbB Wolbachia Infection in Aedes aegypti: Parameter estimates in an outcrossed background and potential for population invasion. American Journal of Tropical Medicine and Hygiene 94, 507–516. doi: 10.4269/AJTMH.15-0608/-/DC2/SD2.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, Jayachandran S, and Prabagaran SR (2019). Evidence for the natural occurrence of Wolbachia in Aedes aegypti mosquitoes. FEMS Microbiol Lett 366. doi: 10.1093/femsle/fnz055 [DOI] [PubMed] [Google Scholar]
- Baldacchino F, Caputo B, Chandre F, Drago A, della Torre A, Montarsi F, et al. (2015). Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Manag Sci 71, 1471–1485. doi: 10.1002/ps.4044 [DOI] [PubMed] [Google Scholar]
- Balloux F, and Lugon-Moulin N (2002). The estimation of population differentiation with microsatellite markers. Mol Ecol 11, 155–165. doi: 10.1046/j.0962-1083.2001.01436.x [DOI] [PubMed] [Google Scholar]
- Barrera R, Mackay AJ, and Amador M (2013). A novel autocidal ovitrap for the surveillance and control of Aedes aegypti. J Am Mosq Control Assoc 29, 293–6. doi: 10.2987/13-6345R.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NH, and Turelli M (2011). Spatial Waves of Advance with Bistable Dynamics: Cytoplasmic and Genetic Analogues of Allee Effects. Am Nat 178, E48–E75. doi: 10.1086/661246 [DOI] [PubMed] [Google Scholar]
- Beard CB, O’Neill SL, Tesh RB, Richards FF, and Aksoy S (1993). Modification of arthropod vector competence via symbiotic bacteria. Parasitology Today 9, 179–183. doi: 10.1016/0169-4758(93)90142-3 [DOI] [PubMed] [Google Scholar]
- Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, et al. (2010). Mosquitoes and Their Control. Berlin, Heidelberg: Springer Berlin Heidelberg. doi: 10.1007/978-3-540-92874-4 [DOI] [Google Scholar]
- Bennett KL, Gómez-Martínez C, Chin Y, Saltonstall K, McMillan WO, Rovira JR, et al. (2019). Dynamics and diversity of bacteria associated with the disease vectors Aedes aegypti and Aedes albopictus. Sci Rep 9, 12160. doi: 10.1038/s41598-019-48414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SN (2003). Selection-Driven Evolution of Emergent Dengue Virus. Mol Biol Evol 20, 1650–1658. doi: 10.1093/molbev/msg182 [DOI] [PubMed] [Google Scholar]
- Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, & Sayers EW (2013). GenBank. Nucleic acids research, 41(Database issue), D36–D42. 10.1093/nar/gks1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya T, Newton ILG, and Hardy RW (2020). Viral RNA is a target for Wolbachia-mediated pathogen blocking. PLoS Pathog 16, e1008513. doi: 10.1371/journal.ppat.1008513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, and Wang Y (2020). The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci 27, 846–858. doi: 10.1111/1744-7917.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G, Xu Y, Lu P, Xie Y, and Xi Z (2010). The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6, e1000833. doi: 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X, and James AA (2013). The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29, 460. doi: 10.1016/J.PT.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady OJ, Kharisma DD, Wilastonegoro NN, O’Reilly KM, Hendrickx E, Bastos LS, et al. (2020). The cost-effectiveness of controlling dengue in Indonesia using wMel Wolbachia released at scale: a modelling study. BMC Med 18, 186. doi: 10.1186/s12916-020-01638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil: (2023). Sistema de Informação de Agravos de Notificação-SINAN. Brasil . Available at: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sinannet/cnv/denguebbr.def (Accessed August 12, 2023). [Google Scholar]
- Brown JE, McBride CS, Johnson P, Ritchie S, Paupy C, Bossin H, et al. (2011). Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti , a major vector of human diseases. Proceedings of the Royal Society B: Biological Sciences 278, 2446–2454. doi: 10.1098/rspb.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KM, Lin CD, Iamsirithaworn S, and Scott TW (2013). The Complex Relationship between Weather and Dengue Virus Transmission in Thailand. Am J Trop Med Hyg 89, 1066–1080. doi: 10.4269/ajtmh.13-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo B, Ienco A, Manica M, Petrarca V, Rosà R, and della Torre A (2015). New adhesive traps to monitor urban mosquitoes with a case study to assess the efficacy of insecticide control strategies in temperate areas. Parasit Vectors 8, 134. doi: 10.1186/s13071-015-0734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata EP, Rancès E, O’Neill SL, and McGraw EA (2014). Competition for Amino Acids Between Wolbachia and the Mosquito Host, Aedes aegypti. Microb Ecol 67, 205–218. doi: 10.1007/s00248-013-0339-4 [DOI] [PubMed] [Google Scholar]
- Carrington LB, Tran BCN, Le NTH, Luong TTH, Nguyen TT, Nguyen PT, et al. (2018). Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 115, 361–366. doi: 10.1073/pnas.1715788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator LJ, Wyer CAS, and Harrington LC (2021). Mosquito Sexual Selection and Reproductive Control Programs. Trends Parasitol 37, 330–339. doi: 10.1016/j.pt.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavany SM, Huber JH, Wieler A, Tran QM, Alkuzweny M, Elliott M, et al. (2022). Ignoring transmission dynamics leads to underestimation of the impact of a novel intervention against mosquito-borne disease. medRxiv, 2021.11.19.21266602. doi: 10.1101/2021.11.19.21266602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Ruel TD, Zhou W, Moloo SK, Majiwa P, O’neill SL, et al. (2000). Tissue distribution and prevalence of Wolbachia infections in tsetse flies, Glossina spp. Med Vet Entomol 14, 44–50. doi: 10.1046/j.1365-2915.2000.00202.x [DOI] [PubMed] [Google Scholar]
- Ching NL, Huat TC, Seng CC, Youming N, Shuzhen S, and Lu D (2021). Wolbachia-mediated sterility suppresses Aedes aegypti populations in the urban tropics. medRxiv . doi: doi.org/ 10.1101/2021.06.16.21257922 [DOI] [Google Scholar]
- Chrostek E, Martins N, Marialva MS, and Teixeira L (2021). Wolbachia -Conferred Antiviral Protection Is Determined by Developmental Temperature. mBio 12. doi: 10.1128/mBio.02923-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H-N, Rodriguez SD, Gonzales KK, Vulcan J, Cordova JJ, Mitra S, et al. (2018). Toward Implementation of Mosquito Sterile Insect Technique: The Effect of Storage Conditions on Survival of Male Aedes aegypti Mosquitoes (Diptera: Culicidae) During Transport. Journal of Insect Science 18. doi: 10.1093/jisesa/iey103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombia: (2023). Portal SIVIGILA: Estadísticas de Vigilancia Rutinaria. Instituto Nacional de Salud. Available at: https://portalsivigila.ins.gov.co/Paginas/Vigilancia-Rutinaria.aspx (Accessed August 16, 2023). [Google Scholar]
- Costa GB, Smithyman R, O’Neill SL, and Moreira LA (2021). How to engage communities on a large scale? Lessons from World Mosquito Program in Rio de Janeiro, Brazil. Gates Open Res 4, 109. doi: 10.12688/gatesopenres.13153.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JE, Clarke DW, Criswell V, Desnoyer M, Cornel D, Deegan B, et al. (2020). Efficient production of male Wolbachia-infected Aedes aegypti mosquitoes enables large-scale suppression of wild populations. Nature Biotechnology 2020 38:4 38, 482–492. doi: 10.1038/s41587-020-0471-x [DOI] [PubMed] [Google Scholar]
- Dainty KR, Hawkey J, Judd LM, Pacidônio EC, Duyvestyn JM, Gonçalves DS, et al. (2021). wMel Wolbachia genome remains stable after 7 years in Australian Aedes aegypti field populations. Microb Genom 7, 641. doi: 10.1099/MGEN.0.000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damayanti B, Aryati S, Upik K, and Hari K (2017). Risk assessment on the release of Wolbachia infected Aedes aegypti. Jakarta. Available at: http://www.eliminatedengue.com/library/publication/document/yogyakarta/risk_assessment_on_the_release_of_wolbachia-infected_aedes_aegypti.pdf (Accessed August 8, 2023). [Google Scholar]
- de Thoisy B, Gardon J, Salas RA, Morvan J, and Kazanji M (2003). Mayaro Virus in Wild Mammals, French Guiana. Emerg Infect Dis 9, 1326–1329. doi: 10.3201/eid0910.030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine GJ, Perea EZ, Killeen GF, Stancil JD, Clark SJ, and Morrison AC (2009). Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proceedings of the National Academy of Sciences 106, 11530–11534. doi: 10.1073/pnas.0901369106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar-Chowdhury P, Haque CE, and Driedger SM (2016). Dengue Disease Risk Mental Models in the City of Dhaka, Bangladesh: Juxtapositions and Gaps Between the Public and Experts. Risk Analysis 36, 874–891. doi: 10.1111/risa.12501 [DOI] [PubMed] [Google Scholar]
- Dorigatti I, McCormack C, Nedjati-Gilani G, and Ferguson NM (2018). Using Wolbachia for Dengue Control: Insights from Modelling. Trends Parasitol 34, 102–113. doi: 10.1016/j.pt.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria M, Prats E, Rosas Ramírez JR, Bellot M, Bedrossiantz J, Pagano M, et al. (2021). Androgenic activation, impairment of the monoaminergic system and altered behavior in zebrafish larvae exposed to environmental concentrations of fenitrothion. Science of The Total Environment 775, 145671. doi: 10.1016/j.scitotenv.2021.145671 [DOI] [PubMed] [Google Scholar]
- Favia G, Ricci I, Damiani C, Raddadi N, Crotti E, Marzorati M, et al. (2007). Bacteria of the genus Asaia stably associate with Anopheles stephensi , an Asian malarial mosquito vector. Proceedings of the National Academy of Sciences 104, 9047–9051. doi: 10.1073/pnas.0610451104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn K, Conlon C, Jones M, Quail MA, Holroyd NE, Parkhill J, et al. (2006). Phylogenetic Relationships of the Wolbachia of Nematodes and Arthropods. PLoS Pathog 2, e94. doi: 10.1371/JOURNAL.PPAT.0020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson NM (2018). Challenges and opportunities in controlling mosquito-borne infections. Nature 559, 490–497. doi: 10.1038/s41586-018-0318-5 [DOI] [PubMed] [Google Scholar]
- Ferguson NM, Kien DTH, Clapham H, Aguas R, Trung VT, Chau TNB, et al. (2015). Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 7, 279ra37. doi: 10.1126/scitranslmed.3010370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson NM, Rodríguez-Barraquer I, Dorigatti I, Mier-y-Teran-Romero L, Laydon DJ, and Cummings DAT (2016). Benefits and risks of the Sanofi-Pasteur dengue vaccine: Modeling optimal deployment. Science (1979) 353, 1033–1036. doi: 10.1126/science.aaf9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flasche S, Jit M, Rodríguez-Barraquer I, Coudeville L, Recker M, Koelle K, et al. (2016). The Long-Term Safety, Public Health Impact, and Cost-Effectiveness of Routine Vaccination with a Recombinant, Live-Attenuated Dengue Vaccine (Dengvaxia): A Model Comparison Study. PLoS Med 13, e1002181. doi: 10.1371/journal.pmed.1002181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores HA, and O’Neill SL (2018). Controlling vector-borne disease by releasing modified mosquitoes. Nat Rev Microbiol 16, 508. doi: 10.1038/S41579-018-0025-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores HA, Taneja de Bruyne J, O’Donnell TB, Tuyet Nhu V, Thi Giang N, Thi Xuan Trang H, et al. (2020). Multiple Wolbachia strains provide comparative levels of protection against dengue virus infection in Aedes aegypti. PLoS Pathog 16, e1008433. doi: 10.1371/journal.ppat.1008433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford SA, Allen SL, Ohm JR, Sigle LT, Sebastian A, Albert I, et al. (2019). Selection on Aedes aegypti alters Wolbachia-mediated dengue virus blocking and fitness. Nat Microbiol 4, 1832–1839. doi: 10.1038/s41564-019-0533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AWE, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, et al. (2006). Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proceedings of the National Academy of Sciences 103, 4198–4203. doi: 10.1073/pnas.0600479103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, Flores HA, et al. (2017). Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog 13, e1006751. doi: 10.1371/journal.ppat.1006751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Gavotte L, Mercer DR, and Dobson SL (2010). Artificial Triple Wolbachia Infection in Aedes albopictus Yields a New Pattern of Unidirectional Cytoplasmic Incompatibility. Appl Environ Microbiol 76, 5887–5891. doi: 10.1128/AEM.00218-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GA, Hoffmann AA, Maciel-de-Freitas R, and Villela DAM (2020). Aedes aegypti insecticide resistance underlies the success (and failure) of Wolbachia population replacement. Sci Rep 10, 63. doi: 10.1038/s41598-019-56766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan V, Stainton K, Rainey SM, Ant TH, Dowle AA, Larson T, et al. (2017). Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat Commun 8, 526. doi: 10.1038/s41467-017-00610-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J-T, Li Y, Li T-P, Liang Y, Hu L, Zhang D, et al. (2020). Stable Introduction of Plant-Virus-Inhibiting Wolbachia into Planthoppers for Rice Protection. Current Biology 30, 4837–4845.e5. doi: 10.1016/j.cub.2020.09.033 [DOI] [PubMed] [Google Scholar]
- Gould EA, and Higgs S (2009). Impact of climate change and other factors on emerging arbovirus diseases. Trans R Soc Trop Med Hyg 103, 109–121. doi: 10.1016/j.trstmh.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz NG,. (2004) Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 18, 215–27. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Guedes RNC, Beins K, Navarro Costa D, Coelho GE, and da Bezerra HSS (2020). Patterns of insecticide resistance in Aedes aegypti: meta-analyses of surveys in Latin America and the Caribbean. Pest Manag Sci. doi: 10.1002/ps.5752 [DOI] [PubMed] [Google Scholar]
- Guidi V, Lehner A, Lüthy P, and Tonolla M (2013). Dynamics of Bacillus thuringiensis var. israelensis and Lysinibacillus sphaericus Spores in Urban Catch Basins after Simultaneous Application against Mosquito Larvae. PLoS One 8, e55658. doi: 10.1371/journal.pone.0055658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zheng X, Zhang D, and Wu Y (2022). Current Status of Mosquito Handling, Transporting and Releasing in Frame of the Sterile Insect Technique. Insects 13, 532. doi: 10.3390/insects13060532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336, 924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter T, Köstlbacher S, Rattei T, Hendrickx F, Manzano-Marín A, and Horn M (2023). One to host them all: genomics of the diverse bacterial endosymbionts of the spider Oedothorax gibbosus. Microb Genom 9. doi: 10.1099/mgen.0.000943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel MJ, Otieno P, Bayoh N, Kariuki S, Were V, Marwanga D, et al. (2011). The Combination of Indoor Residual Spraying and Insecticide-Treated Nets Provides Added Protection against Malaria Compared with Insecticide-Treated Nets Alone. The American Society of Tropical Medicine and Hygiene 85, 1080–1086. doi: 10.4269/ajtmh.2011.10-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PA, and Godfray HCJ (2012). Modelling the spread of Wolbachia in spatially heterogeneous environments. J R Soc Interface 9, 3045–3054. doi: 10.1098/rsif.2012.0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PA, Sinkins SP, and Godfray HCJ (2011). Population Dynamic Models of the Spread of Wolbachia. Am Nat 177, 323–333. doi: 10.1086/658121 [DOI] [PubMed] [Google Scholar]
- Herbert LT, Cossi PF, Painefilú JC, Mengoni Goñalons C, Luquet CM, and Kristoff G (2021). Acute neurotoxicity evaluation of two anticholinesterasic insecticides, independently and in mixtures, and a neonicotinoid on a freshwater gastropod. Chemosphere 265, 129107. doi: 10.1016/j.chemosphere.2020.129107 [DOI] [PubMed] [Google Scholar]
- Hertig M. (1936). The Rickettsia, Wolbachia pipientis (Gen. Et Sp. N.) and Associated Inclusions of the Mosquito, Culex pipiens. Parasitology 28, 453–486. doi: 10.1017/S0031182000022666 [DOI] [Google Scholar]
- Hlaing T, Tun-Lin W, Somboon P, Socheat D, Setha T, Min S, et al. (2010). Spatial genetic structure of Aedes aegypti mosquitoes in mainland Southeast Asia. Evol Appl 3, 319–339. doi: 10.1111/j.1752-4571.2009.00113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Ahmad NW, Keong WM, Ling CY, Ahmad NA, Golding N, et al. (2024). Introduction of Aedes aegypti mosquitoes carrying wAlbB Wolbachia sharply decreases dengue incidence in disease hotspots. iScience 27, 108942. doi: 10.1016/j.isci.2024.108942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. (2011). Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–459. doi: 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Ross PA, and Rašić G (2015). Wolbachia strains for disease control: ecological and evolutionary considerations. Evol Appl 8, 751–768. doi: 10.1111/eva.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Koga R, Kikuchi Y, Meng XY, and Fukatsu T (2010). Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A 107, 769–774. doi: 10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Huang M, Tang M, Yu J, and Zheng B (2015). Wolbachia spread dynamics in stochastic environments. Theor Popul Biol 106, 32–44. doi: 10.1016/j.tpb.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Hu L, Yang C, Hui Y, and Yu J (2021). Mosquito Control Based on Pesticides and Endosymbiotic Bacterium Wolbachia. Bull Math Biol 83, 58. doi: 10.1007/s11538-021-00881-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Yang Q, Hoffmann AA, Ritchie SA, van den Hurk AF, and Warrilow D (2020). Wolbachia Genome Stability and mtDNA Variants in Aedes aegypti Field Populations Eight Years after Release. iScience 23, 101572. doi: 10.1016/J.ISCI.2020.101572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-JS, Higgs S, and Vanlandingham DL (2019). Arbovirus-Mosquito Vector-Host Interactions and the Impact on Transmission and Disease Pathogenesis of Arboviruses. Front Microbiol 10. doi: 10.3389/fmicb.2019.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K, Le Loan L, Chantha N, and Failloux A-B (2004). Human transportation influences Aedes aegypti gene flow in Southeast Asia. Acta Trop 90, 23–29. doi: 10.1016/j.actatropica.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Hughes GL, and Rasgon JL (2014). Transinfection: a method to investigate Wolbachia –host interactions and control arthropod-borne disease. Insect Mol Biol 23, 141–151. doi: 10.1111/imb.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inácio da Silva LM, Dezordi FZ, Paiva MHS, and Wallau GL (2021). Systematic Review of Wolbachia Symbiont Detection in Mosquitoes: An Entangled Topic about Methodological Power and True Symbiosis. Pathogens 10, 39. doi: 10.3390/pathogens10010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriani C, Tanamas SK, Khasanah U, Ansari MR, Rubangi, Tantowijoyo W, et al. (2023). Impact of randomised wMel Wolbachia deployments on notified dengue cases and insecticide fogging for dengue control in Yogyakarta City. Glob Health Action 16. doi: 10.1080/16549716.2023.2166650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriani C, Tantowijoyo W, Rancès E, Andari B, Prabowo E, Yusdi D, et al. (2020). Reduced dengue incidence following deployments of Wolbachia-infected Aedes aegypti in Yogyakarta, Indonesia: a quasi-experimental trial using controlled interrupted time series analysis. Gates Open Res 4, 50. doi: 10.12688/gatesopenres.13122.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inizan C, Tarantola A, O’Connor O, Mangeas M, Pocquet N, Forfait C, et al. (2019). Dengue in New Caledonia: Knowledge and Gaps. Trop Med Infect Dis 4, 95. doi: 10.3390/tropicalmed4020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyaloo DP, Elahee KB, Bheecarry A, and Lees RS (2014). Guidelines to site selection for population surveillance and mosquito control trials: A case study from Mauritius. Acta Trop 132, S140–S149. doi: 10.1016/j.actatropica.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Jeelani S, Sabesan S, and Subramanian S (2015). Community knowledge, awareness and preventive practices regarding dengue fever in Puducherry – South India. Public Health 129, 790–796. doi: 10.1016/j.puhe.2015.02.026 [DOI] [PubMed] [Google Scholar]
- Jeffries CL, and Walker T (2016). Wolbachia Biocontrol Strategies for Arboviral Diseases and the Potential Influence of Resident Wolbachia Strains in Mosquitoes. Curr Trop Med Rep 3, 20–25. doi: 10.1007/s40475-016-0066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez NE, Gerdtzen ZP, Olivera-Nappa Á, Salgado JC, and Conca C (2019). A systems biology approach for studying Wolbachia metabolism reveals points of interaction with its host in the context of arboviral infection. PLoS Negl Trop Dis 13, e0007678. doi: 10.1371/JOURNAL.PNTD.0007678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MA, Cummings DAT, and Glass GE (2009). Multiyear Climate Variability and Dengue—El Niño Southern Oscillation, Weather, and Dengue Incidence in Puerto Rico, Mexico, and Thailand: A Longitudinal Data Analysis. PLoS Med 6, e1000168. doi: 10.1371/journal.pmed.1000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DHT, Hoang NLT, et al. (2016). Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog 12, e1005434. doi: 10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Shropshire JD, Cross KL, Leigh B, Mansueto AJ, Stewart V, et al. (2021). Living in the endosymbiotic world of Wolbachia: A centennial review. Cell Host Microbe 29, 879–893. doi: 10.1016/j.chom.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B, & Vu SN (2005). New strategy against Aedes aegypti in Vietnam. Lancet (London, England), 365(9459), 613–617. 10.1016/S0140-6736(05)17913-6 [DOI] [PubMed] [Google Scholar]
- Kotsakiozi P, Evans BR, Gloria-Soria A, Kamgang B, Mayanja M, Lutwama J, et al. (2018). Population structure of a vector of human diseases: Aedes aegypti in its ancestral range, Africa. Ecol Evol 8, 7835–7848. doi: 10.1002/ece3.4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A, Yu W, Jiang J, Sanchez C, Karna AK, Martinez KJL, et al. (2019). Wolbachia pipientis occurs in Aedes aegypti populations in New Mexico and Florida, USA. Ecol Evol 9, 6148–6156. doi: 10.1002/ece3.5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurbalija Novičić Z, Immonen E, Jelić M, AnÐelković M, Stamenković-Radak M, and Arnqvist G (2015). Within-population genetic effects of mtDNA on metabolic rate in Drosophila subobscura . J Evol Biol 28, 338–346. doi: 10.1111/jeb.12565 [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Ferguson NM, Harris E, Holmes EC, McGraw EA, O’Neill SL, et al. (2015). Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect Dis 15, 862–866. doi: 10.1016/S1473-3099(15)00091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, et al. (2011). Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proceedings of the National Academy of Sciences 108, 7460–7465. doi: 10.1073/pnas.1101377108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M-J, Ross PA, Endersby-Harshman NM, and Hoffmann AA (2020). Impacts of Low Temperatures on Wolbachia (Rickettsiales: Rickettsiaceae)-Infected Aedes aegypti (Diptera: Culicidae). J Med Entomol 57, 1567–1574. doi: 10.1093/jme/tjaa074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M-J, Ross PA, and Hoffmann AA (2021). Infertility and fecundity loss of Wolbachia-infected Aedes aegypti hatched from quiescent eggs is expected to alter invasion dynamics. PLoS Negl Trop Dis 15, e0009179. doi: 10.1371/journal.pntd.0009179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery JV, Tinadana PO, Scott TW, Harrington LC, Ramsey JM, Ytuarte-Nuñez C, et al. (2010). Towards a framework for community engagement in global health research. Trends Parasitol 26, 279–283. doi: 10.1016/j.pt.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Le Clec’h W, Chevalier FD, Genty L, Bertaux J, Bouchon D, and Sicard M (2013). Cannibalism and Predation as Paths for Horizontal Passage of Wolbachia between Terrestrial Isopods. PLoS One 8, e60232. doi: 10.1371/journal.pone.0060232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, & Bork P (2021). Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic acids research, 49(W1), W293–W296. 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Su X, Zhou G, Zhang H, Puthiyakunnon S, Shuai S, et al. (2016). Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit Vectors 9. doi: 10.1186/S13071-016-1724-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Tan CH, Sun Q, Zhang M, Wong PSJ, Li MI, et al. (2022). Wolbachia wAlbB remains stable in Aedes aegypti over 15 years but exhibits genetic background-dependent variation in virus blocking. PNAS nexus 1, pgac203. doi: 10.1093/pnasnexus/pgac203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew C, Soh LT, Chen I, Li X, Sim S, and Ng LC (2021). “Community Engagement for Wolbachia-Based Aedes aegypti Population Suppression for Dengue Control: The Singapore Experience,” in Area-Wide Integrated Pest Management, (CRC Press; ), 747–761. doi: 10.1201/9781003169239-42 [DOI] [Google Scholar]
- Lo N, Paraskevopoulos C, Bourtzis K, O’Neill SL, Werren JH, Bordenstein SR, et al. (2007). Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int J Syst Evol Microbiol 57, 654–657. doi: 10.1099/IJS.0.64515-0/CITE/REFWORKS [DOI] [PubMed] [Google Scholar]
- Lofgren CS, Dame DA, Breeland SG, Weidhaas DE, Jeffery G, Kaiser R, et al. (1974). Release of chemosterilized males for the control of Anopheles albimanus in El Salvador. 3. Field methods and population control. Am J Trop Med Hyg 23, 288–97. doi: 10.4269/ajtmh.1974.23.288 [DOI] [PubMed] [Google Scholar]
- Lu P, Bian G, Pan X, and Xi Z (2012). Wolbachia Induces Density-Dependent Inhibition to Dengue Virus in Mosquito Cells. PLoS Negl Trop Dis 6, e1754. doi: 10.1371/journal.pntd.0001754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Sauer FG, Kliemke K, Garcia GA, Pavan MG, David MR, et al. (2024). Wolbachia strains wMel and wAlbB differentially affect Aedes aegypti traits related to fecundity. Microbiol Spectr 12. doi: 10.1128/spectrum.00128-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AJ, Amador M, and Barrera R (2013). An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasit Vectors 6, 225. doi: 10.1186/1756-3305-6-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoj RRS, Latrofa MS, Epis S, and Otranto D (2021). Wolbachia: endosymbiont of onchocercid nematodes and their vectors. Parasit Vectors 14, 245. doi: 10.1186/s13071-021-04742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcombe S, Farajollahi A, Healy SP, Clark GG, & Fonseca DM (2014). Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PloS one, 9(7), e101992. 10.1371/journal.pone.0101992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina CF, Bond JG, Muñoz J, Valle J, Novelo-Gutiérrez R, and Williams T (2014). Efficacy and non-target impact of spinosad, Bti and temephos larvicides for control of Anopheles spp. in an endemic malaria region of southern Mexico. Parasit Vectors 7, 55. doi: 10.1186/1756-3305-7-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten GG, and Reid JW (2007). Cyclopoid copepods. J Am Mosq Control Assoc 23, 65–92. doi: 10.2987/8756-971X(2007)23[65:CC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Martinez J, Ant TH, Murdochy SM, Tong L, Da Silva Filipe A, and Sinkins SP (2022). Genome sequencing and comparative analysis of Wolbachia strain wAlbA reveals Wolbachia-associated plasmids are common. PLoS Genet 18. doi: 10.1371/journal.pgen.1010406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Tolosana I, Ok S, Smith S, Snoeck K, Day JP, et al. (2017). Symbiont strain is the main determinant of variation in Wolbachia -mediated protection against viruses across Drosophila species. Mol Ecol 26, 4072–4084. doi: 10.1111/mec.14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos M, Martinez Montoya H, Lanzavecchia SB, Conte C, Guillén K, Morán-Aceves BM, et al. (2020). wolbachia pipientis Associated With Tephritid Fruit Fly Pests: From Basic Research to Applications. Front Microbiol 11. doi: 10.3389/fmicb.2020.01080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer SV, Tesh RB, and Vasilakis N (2017). The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop 166, 155. doi: 10.1016/J.ACTATROPICA.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJH (2010). Dengue fever: is it endemic in Australia? Intern Med J 40, 247–249. doi: 10.1111/J.1445-5994.2010.02196.X [DOI] [PubMed] [Google Scholar]
- McGraw EA, and O’Neill SL (2013). Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol 11, 181–193. doi: 10.1038/nrmicro2968 [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, and O’Neill SL (2010). A Virulent Wolbachia Infection Decreases the Viability of the Dengue Vector Aedes aegypti during Periods of Embryonic Quiescence. PLoS Negl Trop Dis 4, e748. doi: 10.1371/journal.pntd.0000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton D. (2012). The Importance of Long-Term Social Research in Enabling Participation and Developing Engagement Strategies for New Dengue Control Technologies. PLoS Negl Trop Dis 6, e1785. doi: 10.1371/journal.pntd.0001785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexico: (2023a). Histórico Boletín Epidemiológico: 2016-2022. Secretaria de salud. Available at: https://www.gob.mx/salud/acciones-y-programas/historico-boletin-epidemiologico (Accessed August 11, 2023). [Google Scholar]
- Mexico: (2023b). Panorama epidemiológico de dengue. Secretaria de Salud. Available at: https://www.gob.mx/cms/uploads/attachment/file/848245/Pano_dengue_31_2023.pdf (Accessed August 11, 2023). [Google Scholar]
- Montenegro D, Martinez L, Tay K, Hernandez T, Noriega D, Barbosa L, et al. (2020). Usefulness of autocidal gravid ovitraps for the surveillance and control of Aedes (Stegomyia) aegypti (Diptera: Culicidae) in eastern Colombia. Med Vet Entomol 34, 379–384. doi: 10.1111/mve.12443 [DOI] [PubMed] [Google Scholar]
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139, 1268–1278. doi: 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- Muharromah AF, Reyes JIL, Kagia N, and Watanabe K (2023). Genome-wide detection of Wolbachia in natural Aedes aegypti populations using ddRAD-Seq. Front Cell Infect Microbiol 13. doi: 10.3389/fcimb.2023.1252656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JV, Jansen CC, and De Barro P (2016). Risk Associated with the Release of Wolbachia-Infected Aedes aegypti Mosquitoes into the Environment in an Effort to Control Dengue. Front Public Health 4. doi: 10.3389/fpubh.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nading AM (2015). The lively ethics of global health GMOs: The case of the Oxitec mosquito. Biosocieties 10, 24–47. doi: 10.1057/biosoc.2014.16 [DOI] [Google Scholar]
- Nainu F, Trenerry A, and Johnson KN (2019). Wolbachia-mediated antiviral protection is cell-autonomous. J Gen Virol 100, 1587–1592. doi: 10.1099/JGV.0.001342/CITE/REFWORKS [DOI] [PubMed] [Google Scholar]
- Nam VS, Yen NT, Duc HM, Tu TC, Thang VT, Le NH, San LH, Loan LL, Huong VTQ, Khanh LHK, Trang HTT, Lam LZY, Kutcher SC, Aaskov JG, Jeffery JAL, Ryan PA, & Kay BH (2012). Community-based control of Aedes aegypti by using Mesocyclops in southern Vietnam. The American journal of tropical medicine and hygiene, 86(5), 850–859. 10.4269/ajtmh.2012.11-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazni WA, Hoffmann AA, NoorAfizah A, Cheong YL, Mancini MV, Golding N, et al. (2019). Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes aegypti for Dengue Control. Current Biology 29, 4241–4248.e5. doi: 10.1016/j.cub.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEA (2023). Wolbachia-Aedes Mosquito Suppression. National Environment Agency. Available at: https://www.nea.gov.sg/corporate-functions/resources/research/wolbachia-aedes-mosquito-suppression-strategy/phase-2-field-study (Accessed September 1, 2023). [Google Scholar]
- Nguyen TH, Le Nguyen H, Nguyen TY, Vu SN, Tran ND, Le TN, et al. (2015). Field evaluation of the establishment potential of wMelpop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors 8. doi: 10.1186/S13071-015-1174-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunlade ST, Meehan MT, Adekunle AI, and McBryde ES (2023). A Systematic Review of Mathematical Models of Dengue Transmission and Vector Control: 2010–2020. Viruses 15, 254. doi: 10.3390/v15010254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunlade ST, Meehan MT, Adekunle AI, Rojas DP, Adegboye OA, and McBryde ES (2021). A Review: Aedes-Borne Arboviral Infections, Controls and Wolbachia-Based Strategies. Vaccines (Basel) 9, 32. doi: 10.3390/vaccines9010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba S, Ohashi K, Pujiyati E, Higa Y, Kawada H, Mito N, et al. (2013). The effect of pyriproxyfen as a “population growth regulator” against Aedes albopictus under semi-field conditions. PLoS One 8, e67045. doi: 10.1371/journal.pone.0067045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oidtman RJ, Lai S, Huang Z, Yang J, Siraj AS, Reiner RC, et al. (2019). Inter-annual variation in seasonal dengue epidemics driven by multiple interacting factors in Guangzhou, China. Nat Commun 10. doi: 10.1038/S41467-019-09035-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMS (2017). La Asamblea Mundial de la Salud llega a una serie de acuerdos sobre el control de vectores , las enfermedades no transmisibles y los ODS.
- O’Neill SL (2018). “The use of Wolbachia by the world mosquito program to interrupt transmission of Aedes Aegypti transmitted viruses,” in Advances in Experimental Medicine and Biology, (Springer; New York LLC: ), 355–360. doi: 10.1007/978-981-10-8727-1_24 [DOI] [PubMed] [Google Scholar]
- O’Neill SL, Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, et al. (2019a). Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland. Aust. Gates Open Res. 3, 1547. doi: 10.12688/gatesopenres.13061.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, et al. (2019b). Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res 2, 36. doi: 10.12688/gatesopenres.12844.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly KM, Hendrickx E, Kharisma DD, Wilastonegoro NN, Carrington LB, Elyazar IRF, et al. (2019). Estimating the burden of dengue and the impact of release of wMel Wolbachia-infected mosquitoes in Indonesia: a modelling study. BMC Med 17, 172. doi: 10.1186/s12916-019-1396-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellano P, Reynoso J, Salomón OD, and Vezzani D (2023). Dengue vaccine acceptance and willingness to pay: a systematic review and meta-analysis. Public Health 224, 74–81. doi: 10.1016/j.puhe.2023.08.022 [DOI] [PubMed] [Google Scholar]
- Pagendam DE, Trewin BJ, Snoad N, Ritchie SA, Hoffmann AA, Staunton KM, et al. (2020). Modelling the Wolbachia incompatible insect technique: strategies for effective mosquito population elimination. BMC Biol 18, 161. doi: 10.1186/s12915-020-00887-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, et al. (2012). Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proceedings of the National Academy of Sciences 109. doi: 10.1073/pnas.1116932108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R, and Asgari S (2018). Aedes Anphevirus: an Insect-Specific Virus Distributed Worldwide in Aedes aegypti Mosquitoes That Has Complex Interplays with Wolbachia and Dengue Virus Infection in Cells. J Virol 92. doi: 10.1128/JVI.00224-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau J, and Moran NA (2022). Genetic innovations in animal-microbe symbioses. Nat Rev Genet 23, 23–39. doi: 10.1038/s41576-021-00395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG, and CONSORT Group, for the (2012). Reporting of Noninferiority and Equivalence Randomized Trials. JAMA 308, 2594. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- Pietri JE, DeBruhl H, and Sullivan W (2016). The rich somatic life of Wolbachia. Microbiologyopen 5, 923–936. doi: 10.1002/mbo3.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SB, Riback TIS, Sylvestre G, Costa G, Peixoto J, Dias FBS, et al. (2021). Effectiveness of Wolbachia-infected mosquito deployments in reducing the incidence of dengue and other Aedes-borne diseases in Niterói, Brazil: A quasi-experimental study. PLoS Negl Trop Dis 15, e0009556. doi: 10.1371/journal.pntd.0009556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovici J, Moreira LA, Poinsignon A, Iturbe-Ormaetxe I, McNaughton D, and O’Neill SL (2010). Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem Inst Oswaldo Cruz 105, 957–964. doi: 10.1590/S0074-02762010000800002 [DOI] [PubMed] [Google Scholar]
- Porter J, and Sullivan W (2023). The cellular lives of Wolbachia. Nat Rev Microbiol 21, 750–766. doi: 10.1038/s41579-023-00918-x [DOI] [PubMed] [Google Scholar]
- Rappole J. (2000). Migratory Birds and Spread of West Nile Virus in the Western Hemisphere. Emerg Infect Dis 6, 319–328. doi: 10.3201/eid0604.000401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rašić G, Filipović I, Weeks AR, and Hoffmann AA (2014). Genome-wide SNPs lead to strong signals of geographic structure and relatedness patterns in the major arbovirus vector, Aedes aegypti. BMC Genomics 15, 275. doi: 10.1186/1471-2164-15-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber L, Knillmann S, Kaske O, Atencio LC, Bittner L, Albrecht JE, et al. (2021). Long-term effects of a catastrophic insecticide spill on stream invertebrates. Science of The Total Environment 768, 144456. doi: 10.1016/j.scitotenv.2020.144456 [DOI] [PubMed] [Google Scholar]
- Reveillaud J, Bordenstein SR, Cruaud C, Shaiber A, Esen ÖC, Weill M, et al. (2019). The Wolbachia mobilome in Culex pipiens includes a putative plasmid. Nat Commun 10, 1051. doi: 10.1038/s41467-019-08973-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro dos Santos G, Durovni B, Saraceni V, Souza Riback TI, Pinto SB, Anders KL, et al. (2022). Estimating the effect of the wMel release programme on the incidence of dengue and chikungunya in Rio de Janeiro, Brazil: a spatiotemporal modelling study. Lancet Infect Dis 22, 1587. doi: 10.1016/S1473-3099(22)00436-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, and Johnson BJ (2017). Advances in Vector Control Science: Rear-and-Release Strategies Show Promise… but Don’t Forget the Basics. J Infect Dis 215, S103–S108. doi: 10.1093/infdis/jiw575 [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Montgomery BL, and Hoffmann AA (2013). Novel Estimates of Aedes aegypti (Diptera: Culicidae) Population Size and Adult Survival Based on Wolbachia Releases. J Med Entomol 50, 624–631. doi: 10.1603/ME12201 [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Rapley LP, Williams C, Johnson PH, Larkman M, Silcock RM, et al. (2009). A lethal ovitrap-based mass trapping scheme for dengue control in Australia: I. Public acceptability and performance of lethal ovitraps. Med Vet Entomol 23, 295–302. doi: 10.1111/j.1365-2915.2009.00833.x [DOI] [PubMed] [Google Scholar]
- Rizzo N, Gramajo R, Escobar MC, Arana B, Kroeger A, Manrique-Saide P, & Petzold M (2012). Dengue vector management using insecticide treated materials and targeted interventions on productive breeding-sites in Guatemala. BMC public health, 12, 931. 10.1186/1471-2458-12-931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguero M. (2013). Wolbachia, una pandemia con posibilidades Wolbachia, a pandemic with potential. Rev Soc Entomol Argent 72, 117–137. Available at: http://www.scielo.org.ar/pdf/rsea/v72n3-4/v72n3-4a01.pdf (Accessed August 7, 2023). [Google Scholar]
- Ross PA (2021). Designing effective Wolbachia release programs for mosquito and arbovirus control. Acta Trop 222, 106045. doi: 10.1016/j.actatropica.2021.106045 [DOI] [PubMed] [Google Scholar]
- Ross PA, Elfekih S, Collier S, Klein MJ, Lee SS, Dunn M, et al. (2023). Developing Wolbachia-based disease interventions for an extreme environment. PLoS Pathog 19, e1011117. doi: 10.1371/journal.ppat.1011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PA, Endersby NM, Yeap HL, and Hoffmann AA (2014). Larval Competition Extends Developmental Time and Decreases Adult Size of wMelPop Wolbachia-Infected Aedes aegypti. The American Society of Tropical Medicine and Hygiene 91, 198–205. doi: 10.4269/ajtmh.13-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PA, and Hoffmann AA (2024). Revisiting Wolbachia detections: Old and new issues in Aedes aegypti mosquitoes and other insects. Ecol Evol 14, e11670. doi: 10.1002/ECE3.11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PA, Ritchie SA, Axford JK, and Hoffmann AA (2019). Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 13, e0007357. doi: 10.1371/journal.pntd.0007357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, and Hoffmann AA (2017). Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog 13, e1006006. doi: 10.1371/journal.ppat.1006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. (2020). Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res 3, 1547. doi: 10.12688/gatesopenres.13061.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PA, Hien NT, Anh DD, Le NH, Yen NT, Phong TV, et al. (2021). Environmental factors influence the local establishment of Wolbachia in Aedes aegypti mosquitoes in two small communities in central Vietnam. Gates Open Res 5. doi: 10.12688/GATESOPENRES.13347.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H, Cummings DAT, Rodriguez-Barraquer I, Katzelnick LC, Lessler J, Klungthong C, et al. (2018). Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 557, 719–723. doi: 10.1038/s41586-018-0157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje H, Lessler J, Maljkovic Berry I, Melendrez MC, Endy T, Kalayanarooj S, et al. (2017). Dengue diversity across spatial and temporal scales: Local structure and the effect of host population size. Science (1979) 355, 1302–1306. doi: 10.1126/science.aaj9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei E, Charlat S, and Engelstädter J (2021). Wolbachia host shifts: routes, mechanisms, constraints and evolutionary consequences. Biological Reviews 96, 433–453. doi: 10.1111/brv.12663 [DOI] [PubMed] [Google Scholar]
- Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Tse T, … Sherry ST (2022). Database resources of the national center for biotechnology information. Nucleic acids research, 50(D1), D20–D26. 10.1093/nar/gkab1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TL, Filipović I, Hoffmann AA, and Rašić G (2018). Fine-scale landscape genomics helps explain the slow spatial spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. Heredity (Edinb) 120, 386–395. doi: 10.1038/s41437-017-0039-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, et al. (2020) NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). Jan 1;2020:baaa062. doi: 10.1093/database/baaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard D, Harker A, Halasa-Rappel Y, Rincon C, Villarreal L, and Samantha R (2022). Economic evaluation of Wolbachia deployment in Colombia. Available at: https://www.worldmosquitoprogram.org/sites/default/files/2023-03/Economic%20evaluation%20of%20Wolbachia%20deployment%20in%20Colombia.pdf (Accessed August 11, 2023). [Google Scholar]
- Simmons CP, Donald W, Tagavi L, Tarivonda L, Quai T, Tavoa R, et al. (2024). Successful introgression of wMel Wolbachia into Aedes aegypti populations in Fiji, Vanuatu and Kiribati. PLoS Negl Trop Dis 18, e0012022. doi: 10.1371/journal.pntd.0012022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões PM, Mialdea G, Reiss D, Sagot M-F, and Charlat S (2011). Wolbachia detection: an assessment of standard PCR protocols. Mol Ecol Resour 11, 567–72. doi: 10.1111/j.1755-0998.2010.02955.x [DOI] [PubMed] [Google Scholar]
- Sinkins SP, Walker T, Lynd AR, Steven AR, Makepeace BL, Godfray HC, Parkhill J (2005) Wolbachia variability and host effects on crossing type in Culex mosquitoes. Nature 436, 257–60. doi: 10.1038/nature03629 [DOI] [PubMed] [Google Scholar]
- Skelton E, Rancè E, Frentiu FD, Kusmintarsih ES, Aki Iturbe-Ormaetxe I, Caragata EP, et al. (2016). A Native Wolbachia Endosymbiont Does Not Limit Dengue Virus Infection in the Mosquito Aedes notoscriptus (Diptera: Culicidae). J Med Entomol 53, 401–408. doi: 10.1093/jme/tjv235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snetselaar J, Andriessen R, Suer RA, Osinga AJ, Knols BG, & Farenhorst M (2014). Development and evaluation of a novel contamination device that targets multiple life-stages of Aedes aegypti. Parasites & vectors, 7, 200. 10.1186/1756-3305-7-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks TC, and Nauen R (2015). IRAC: Mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121, 122–8. doi: 10.1016/j.pestbp.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Sri Lanka: (2023). Weekly Epidemiological Report. Epidemiology Unit. Available at: https://www.epid.gov.lk/weekly-epidemiological-report/weekly-epidemiological-report (Accessed August 11, 2023). [Google Scholar]
- Stephens CR, and Juliano SA (2012). Wing Shape as an Indicator of Larval Rearing Conditions for Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J Med Entomol 49, 927–938. doi: 10.1603/ME12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Ibarra AM, and Lowe R (2013). Climate and Non-Climate Drivers of Dengue Epidemics in Southern Coastal Ecuador. Am J Trop Med Hyg 88, 971. doi: 10.4269/AJTMH.12-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard ST, Forshey BM, Morrison AC, Paz-Soldan VA, Vazquez-Prokopec GM, Astete H, et al. (2013). House-to-house human movement drives dengue virus transmission. Proceedings of the National Academy of Sciences 110, 994–999. doi: 10.1073/pnas.1213349110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickman D. (2006). Longevity of Aedes aegypti (Diptera: Culicidae) compared in cages and field under ambient conditions in rural Thailand. Southeast Asian J Trop Med Public Health 37, 456–62. [PubMed] [Google Scholar]
- Tantowijoyo W, Andari B, Arguni E, Budiwati N, Nurhayati I, Fitriana I, et al. (2020). Stable establishment of wMeI Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS Negl Trop Dis 14, 1–13. doi: 10.1371/journal.pntd.0008157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Conyer R, Méndez-Galván J, and Burciaga-Zúñiga P (2012). Community participation in the prevention and control of dengue: the patio limpio strategy in Mexico. Paediatr Int Child Health 32, 10–13. doi: 10.1179/2046904712Z.00000000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-López E, Bardach A, Ciapponi A, Alcaraz A, García-Perdomo HA, Ruvinsky S, et al. (2019). [Experiences, barriers and facilitators to the implementation of interventions for controlling Aedes aegypti in Latin America and the Caribbean: a qualitative study]. Cad Saude Publica 35, e00092618. doi: 10.1590/0102-311X00092618 [DOI] [PubMed] [Google Scholar]
- Teixeira L, Ferreira Á, and Ashburner M (2008). The Bacterial Symbiont Wolbachia Induces Resistance to RNA Viral Infections in Drosophila melanogaster. PLoS Biol 6, e1000002. doi: 10.1371/journal.pbio.1000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terradas G, and McGraw EA (2017). Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr Opin Insect Sci 22, 37–44. doi: 10.1016/j.cois.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Thi Hue Kien D, Edenborough K, Da Silva Goncalves D, Thuy Vi T, Casagrande E, Thi Le Duyen H, et al. (2023). Genome evolution of dengue virus serotype 1 under selection by Wolbachia pipientis in Aedes aegypti mosquitoes. Virus Evol 9. doi: 10.1093/VE/VEAD016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GD, and Dutton B (2003). Insecticide Resistance Action Committee (IRAC). Pesticide Outlook 14, 146. doi: 10.1039/b308501p [DOI] [Google Scholar]
- Tian N, Zheng J-X, Guo Z-Y, Li L-H, Xia S, Lv S, et al. (2022). Dengue Incidence Trends and Its Burden in Major Endemic Regions from 1990 to 2019. Trop Med Infect Dis 7, 180. doi: 10.3390/tropicalmed7080180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Imanian B (2023) VBCG: 20 validated bacterial core genes for phylogenomic analysis with high fidelity and resolution. Microbiome. Nov 8;11(1):247. doi: 10.1186/s40168-023-01705-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver BE, Anderson MAE, and Adelman ZN (2009). Homing endonucleases catalyze double-stranded DNA breaks and somatic transgene excision in Aedes aegypti. Insect Mol Biol 18, 623–633. doi: 10.1111/j.1365-2583.2009.00905.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley AP, Moreira LA, O’Neill SL, and McGraw EA (2009). Wolbachia Infection Reduces Blood-Feeding Success in the Dengue Fever Mosquito, Aedes aegypti. PLoS Negl Trop Dis 3, e516. doi: 10.1371/journal.pntd.0000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich JN, Beier JC, Devine GJ, and Hugo LE (2016). Heat Sensitivity of wMel Wolbachia during Aedes aegypti Development. PLoS Negl Trop Dis 10. doi: 10.1371/JOURNAL.PNTD.0004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdaneta-Marquez L, and Failloux A-B (2011). Population genetic structure of Aedes aegypti, the principal vector of dengue viruses. Infection, Genetics and Evolution 11, 253–261. doi: 10.1016/j.meegid.2010.11.020 [DOI] [PubMed] [Google Scholar]
- Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. (2021). Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. New England Journal of Medicine 384, 2177–2186. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Panhuis WG, Choisy M, Xiong X, Chok NS, Akarasewi P, Iamsirithaworn S, et al. (2015). Region-wide synchrony and traveling waves of dengue across eight countries in Southeast Asia. Proceedings of the National Academy of Sciences 112, 13069–13074. doi: 10.1073/pnas.1501375112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe V, Villegas E, Oviedo M, Baly A, Lenhart A, McCall PJ, et al. (2011). Evaluation of the Effectiveness of Insecticide Treated Materials for Household Level Dengue Vector Control. PLoS Negl Trop Dis 5, e994. doi: 10.1371/journal.pntd.0000994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez ID, Tanamas SK, Arbelaez MP, Kutcher SC, Duque SL, Uribe A, et al. (2023). Reduced dengue incidence following city-wide wMel Wolbachia mosquito releases throughout three Colombian cities: Interrupted time series analysis and a prospective case-control study. PLoS Negl Trop Dis 17. doi: 10.1371/JOURNAL.PNTD.0011713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez-Bernal ID (2023). Establecimiento de la infección con Wolbachia en los mosquitos Aedes aegyti de Bello, Medellín e Itaguí (Colombia), y su impacto en el control de la transmisión del dengue y otros arbovirus. Diagnóstico 62, e460. doi: 10.33734/diagnostico.v62i2.460 [DOI] [Google Scholar]
- Walker J, Pyke A, Florian P, Moore F, Smoll N, Adegbija O, et al. (2021). Re-emergence of dengue virus in regional Queensland: 2019 dengue virus outbreak in Rockhampton, Central Queensland, Australia. Commun Dis Intell (2018) 45. doi: 10.33321/CDI.2021.45.31 [DOI] [PubMed] [Google Scholar]
- Wang GH, Gamez S, Raban RR, Marshall JM, Alphey L, Li M, Rasgon JL, & Akbari OS (2021). Combating mosquito-borne diseases using genetic control technologies. Nature communications, 12(1), 4388. 10.1038/s41467-021-24654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xiong X, Cao W, Zhang C, Werren JH, and Wang X (2020). Phylogenomic analysis of Wolbachia strains reveals patterns of genome evolution and recombination. Genome Biol Evol 12, 2508–2520. doi: 10.1093/GBE/EVAA219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Charlier C, Vasilakis N, and Lecuit M (2018). Zika, Chikungunya, and Other Emerging Vector-Borne Viral Diseases. Annu Rev Med 69, 395–408. doi: 10.1146/annurev-med-050715-105122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, and Clark ME (2008). Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6, 741–751. doi: 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- Werren JH, Zhang W, and Guo LR (1995). Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proceedings of the Royal Society B: Biological Sciences 261, 55–63. doi: 10.1098/rspb.1995.0117 [DOI] [PubMed] [Google Scholar]
- West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, Mosha FW, et al. (2014). Indoor Residual Spraying in Combination with Insecticide-Treated Nets Compared to Insecticide-Treated Nets Alone for Protection against Malaria: A Cluster Randomised Trial in Tanzania. PLoS Med 11, e1001630. doi: 10.1371/journal.pmed.1001630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017a). How to design vector control efficacy trials, guidance on phase III vector control field trial design. Geneve. Available at: https://iris.who.int/bitstream/handle/10665/259688/WHO-HTM-NTD-VEM-2017.03-eng.pdf;jsessionid=D1D2BFC1F8E69335837E6C4D561C362B?sequence=1 (Accessed August 18, 2024). [Google Scholar]
- WHO (2017b). Global vector control response 2017–2030. ., 1st Edn, ed. World Health Organization. Genova. Available at: https://apps.who.int/iris/bitstream/handle/10665/259205/9789241512978-eng.pdf (Accessed July 29, 2022). [Google Scholar]
- WHO (2023). Update on the Dengue situation in the Western Pacific Region. Available at: https://cdn.who.int/media/docs/default-source/wpro---documents/emergency/surveillance/dengue/dengue-20230803.pdf?sfvrsn=5160e027_115 (Accessed August 11, 2023).
- Whyard S, Erdelyan C, Partridge AL, Singh AD, Beebe NW, and Capina R (2015). Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasit Vectors 8, 96. doi: 10.1186/s13071-015-0716-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AL, Boelaert M, Kleinschmidt I, Pinder M, Scott TW, Tusting LS, et al. (2015). Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol 31, 380–390. doi: 10.1016/j.pt.2015.04.015 [DOI] [PubMed] [Google Scholar]
- WMP (2023). World mosquito program. Available at: https://www.worldmosquitoprogram.org/ (Accessed August 11, 2023).
- Wu P, Yu X, Wang P, and Cheng G (2019). Arbovirus lifecycle in mosquito: acquisition, propagation and transmission. Expert Rev Mol Med 21, e1. doi: 10.1017/erm.2018.6 [DOI] [PubMed] [Google Scholar]
- Xi Z, Khoo CCH, and Dobson SL (2005). Wolbachia establishment and invasion in an Aedes Aegypti laboratory population. Science (1979) 310, 326–328. doi: 10.1126/SCIENCE.1117607/SUPPL_FILE/XI.SOM.PDF [DOI] [PubMed] [Google Scholar]
- Yan G, Chadee DD, and Severson DW (1998). Evidence for Genetic Hitchhiking Effect Associated With Insecticide Resistance in Aedes aegypti. Genetics 148, 793–800. doi: 10.1093/genetics/148.2.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye YH, Carrasco AM, Frentiu FD, Chenoweth SF, Beebe NW, van den Hurk AF, et al. (2015). Wolbachia Reduces the Transmission Potential of Dengue-Infected Aedes aegypti. PLoS Negl Trop Dis 9, e0003894. doi: 10.1371/journal.pntd.0003894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye YH, Chenoweth SF, Carrasco AM, Allen SL, Frentiu FD, van den Hurk AF, et al. (2016). Evolutionary potential of the extrinsic incubation period of dengue virus in Aedes aegypti. Evolution (N Y) 70, 2459–2469. doi: 10.1111/evo.13039 [DOI] [PubMed] [Google Scholar]
- Yen JH, and Barr AR (1973). The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22, 242–250. doi: 10.1016/0022-2011(73)90141-9 [DOI] [PubMed] [Google Scholar]
- Yoon M, Kim J, Kim E, Cheong H, Utiera M, Corbett X, et al. (2019). Spatio-temporal Analyses for Dengue Outbreak in Tarawa, Kiribati. Available at: https://www.fnu.ac.fj/college-of-medicine/research-cmnhs/e-prints/ (Accessed January 22, 2024). [Google Scholar]
- Zélé F, Nicot A, Berthomieu A, Weill M, Duron O, and Rivero A (2014). Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proceedings of the Royal Society B: Biological Sciences 281, 20132837. doi: 10.1098/rspb.2013.2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, She L, Yuan H, Luo Y, Wang R, Mao W, et al. (2022). A standalone incompatible insect technique enables mosquito suppression in the urban subtropics. Commun Biol 5, 1419. doi: 10.1038/s42003-022-04332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gao J, Ma Z, Liu Y, Wang G, Liu Q, et al. (2022). Wolbachia infection in field-collected Aedes aegypti in Yunnan Province, southwestern China. Front Cell Infect Microbiol 12. doi: 10.3389/fcimb.2022.1082809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Hui Y, and Hu L (2023). The impact of predators of mosquito larvae on Wolbachia spreading dynamics. J Biol Dyn 17. doi: 10.1080/17513758.2023.2249024 [DOI] [PubMed] [Google Scholar]
- Zulfa R, Lo W-C, Cheng P-C, Martini M, and Chuang T-W (2022). Updating the Insecticide Resistance Status of Aedes aegypti and Aedes albopictus in Asia: A Systematic Review and Meta-Analysis. Trop Med Infect Dis 7, 306. doi: 10.3390/tropicalmed7100306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


