Abstract
Vernalization-responsive plants use cold weather, or low temperature, as a cue to monitoring the passing of winter. Winter cereals can remember the extent of coldness they have experienced, even when winter is punctuated by warm days. However, in a seemingly unnatural process called “devernalization,” hot temperatures can erase winter memory. Previous studies in bread wheat (Triticum aestivum) have implicated the MADS-box transcription factor VEGETATIVE TO REPRODUCTIVE TRANSITION 2 (VRT2) in vernalization based on transcriptional behavior and ectopic expression. Here, we characterized 3 BdVRT2 loss-of-function alleles in the temperate model grass Brachypodium distachyon. In addition to extended vernalization requirements, mutants showed delayed flowering relative to wild-type plants when exposed only briefly to warm temperatures after partial vernalization, with flowering being unaffected when vernalization was saturating. Together, these data suggest a role for BdVRT2 in both vernalization and in its reinitiation when interrupted by warm temperatures. In controlled constant conditions, BdVRT2 transcription was not strongly affected by vernalization or devernalization. Yet, by monitoring BdVRT2 expression in seasonally varying and fluctuating conditions in an unheated greenhouse, we observed strong upregulation, suggesting that its transcription is regulated by fluctuating vernalizing–devernalizing conditions. Our data suggest that devernalization by hot temperatures is not a peculiarity of domesticated cereal crops but is the extreme of the reversibility of vernalization by warm temperatures and has broader biological relevance across temperate grasses.
A MADS-box transcription factor promotes and stabilizes vernalization in the temperate grass Brachypodium distachyon.
Introduction
Timing the transition to flowering with favorable seasonal conditions is essential for maximum reproductive success and is a key trait that must be modified when plants are adapting to new environments or cultivation zones (Jung and Müller 2009; Díaz et al. 2012). In several temperate cereals (Pooideae, Poaceae), flowering is encouraged by different external conditions, including exposure to prolonged cold temperatures (vernalization) followed by warm spring temperatures. Although impressive advances have been made in understanding the molecular genetic mechanisms involved in temperature-regulated flowering time (Hemming et al. 2012; Ford et al. 2016; Ejaz and von Korff 2017; Kiss et al. 2017; Xu and Chong 2018; Dixon et al. 2019; Mayer et al. 2019), how low- and high-temperature signals are integrated into this pathway is still not well understood. This knowledge gap has implications for predicting how plants will respond to climate change, as well as our ability to engineer crops for sustainable and equitable agriculture.
Many temperate plants utilize vernalization as a signal to distinguish autumn and spring, as both seasons can share similar warm temperature conditions. In non-vernalized autumn plants, warm ambient temperatures prevent precocious flowering, whereas similar temperatures promote reproductive development in vernalized spring plants (Rawson and Richards 1993; Hemming et al. 2012; Ejaz and von Korff 2017). Mechanistically, the vernalization response involves an epigenetic “memory” of winter, and in many plants, the occasional warm winter day will not interfere with saturation of the response. However, the vernalized state of a plant can be, at least in some species, partially or fully reversed if a period of warm to hot (15 to >30 °C), non-vernalizing temperatures is experienced before the vernalization response is saturated or once vernalization is complete (Purvis and Gregory 1952; Chintraruck and Ketellapper 1969; Bouché et al. 2015). Such “devernalization” indicates to the plant that conditions are unpredictable, thus acting to reset the memory until the arrival of a more convincing winter. As global warming increases the likelihood of unseasonal events, devernalization might equally be adaptive during summer–fall frosts to prevent precocious flowering, but maladaptive with winter warming to cause delayed flowering in the spring.
Devernalization has been reported in a range of species including Arabidopsis (Arabidopsis thaliana; Chintraruck and Ketellapper 1969; Shindo et al. 2006; Bouché et al. 2015) and kohlrabi (Brassica oleracea); Gongylodes (Brassicaceae) (Wiebe et al. 1992), leek (Allium porrum) (Wiebe 1994), and onion (Allium cepa) (Amaryllidaceae) (Khokhar et al. 2007); root chicory (Cichorium intybus) (Périlleux et al. 2013; Mathieu et al. 2020) and lettuce (Lactuca serriola) (Asteraceae) (Marks and Prince 1979, 1982); sugar beet (Durrant and Jaggard 1988) and bread wheat (Triticum aestivum) (Slafer 1995; Dixon et al. 2019); and rye (Secale cereale) (grasses, Poaceae) (Gregory and Purvis 1936; Purvis and Gregory 1952). Evidence suggests that a response to vernalization evolved independently in each of these plant families (Preston and Sandve 2013), thus making it likely that devernalization responsiveness has multiple evolutionary origins, too. Despite these independent origins, it appears that factors regulating vernalization are commonly targeted during devernalization. In Arabidopsis, for example, reactivation of the major vernalization-dependent floral repressor FLOWERING LOCUS C (FLC) occurs as a result of devernalization treatment, via decreased histone methylation marks at the promoter (Bouché et al. 2015; Shirakawa et al. 2021). Similarly, in wheat and barley (Hordeum vulgare) exposure to warm temperatures after non-saturating vernalization leads to the reactivation of vernalization-dependent repressors of the floral transition: VERNALIZATION 2 (VRN2) and/or the monocot FLC homolog ODDSOC2 (Dixon et al. 2019). These changes are also associated with lower levels of the floral promoters VRN1, which is a FRUITFULL (FUL)-like MADS-box gene and one of the florigen activation complex genes FLOWERING LOCUS T (FT), manifesting in delayed flowering.
Outside of the wheat tribe (Triticeae), devernalization responsiveness has not been described for grasses, posing the question as to the importance of this trait within vernalization-responsive, undomesticated members of this economically important family. Moreover, while some progress has been made in elucidating the molecular genetic basis of devernalization, much is still to be learned. Here, we show that the model temperate grass species Brachypodium (Brachypodium distachyon), that is sister to the “core” Pooideae cereal clade, can be devernalized by moderate to high temperatures after incomplete vernalization. We find that an ortholog of the wheat flowering promoter VEGETATIVE TO REPRODUCTIVE TRANSITION 2 (VRT2) tempers devernalization and contributes to setting the vernalization requirement. In wheat, VRT2 has been linked to vernalization based on its expression level being responsive to cold (Kane et al. 2005; Xie et al. 2021). However, functional analyses are incomplete for the wheat gene, with a loss-of-function line suggesting it is a weak flowering promoter in a non-vernalization-responsive “spring” variety (Li et al. 2021) and overexpression experiments showing mixed impacts on flowering time (Adamski et al. 2021; Li et al. 2021; Xie et al. 2021). Here, we characterize loss-of-function lines in a facultative vernalization-responsive “winter” accession of Brachypodium, which allows us to functionally link VRT2 to both vernalization and devernalization.
Results
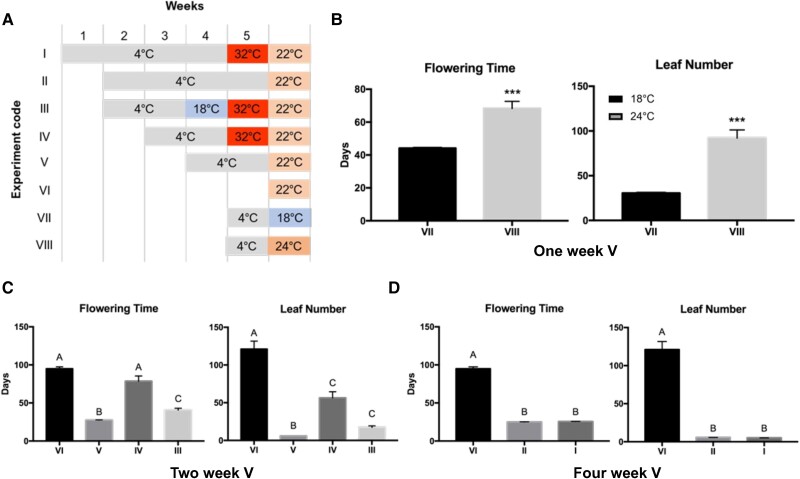
Vernalization in Brachypodium is reversed by moderate to high temperatures
While devernalization has been described for Triticeae crops, the relevance for undomesticated Pooideae grasses remains unclear. We therefore wondered whether and to what extent the process of devernalization is also present in wild-type Brachypodium. To investigate this, we compared the flowering time of Brachypodium accessions Bd21-3 exposed to a variety of temperature conditions as seeds (Fig. 1). Bd21-3 has a facultative vernalization response, with a delay in flowering of approximately 2 mo when not vernalized and grown in 16 h long-day conditions (Ream et al. 2014; Sharma et al. 2017) (Fig. 1, A and C; V vs. VI). The 1-wk partially vernalized plants were devernalized by warm temperature (24 °C) compared with moderate (18 °C) temperature, based on delayed days to emergence of leaves and flowering (Fig. 1, A and B). Significantly delayed flowering in line with non-vernalized plants (Fig. 1, A and C; VI) was also evident after 2 wk of non-saturating vernalization followed by a heat break at 32 °C (Fig. 1, A and C; IV vs. V). Taken together with other observations of devernalization responsiveness (Purvis and Gregory 1952; Chintraruck and Ketellapper 1969; Périlleux et al. 2013; Bouché et al. 2015), this behavior contrasts with the intuition that warm temperatures always accelerate flowering time (e.g. McClung et al. 2016), and highlights the more complex and widespread interaction of temperature on plant development.
Figure 1.
The devernalization response of Brachypodium distachyon 21-3. A) Experimental setup of devernalization treatments. Wild-type Bd21-3 was vernalized at 4 °C as seeds before being moved to various temperature treatments. Temperatures in the final column remained constant for the remainder of the experiment. B) Devernalization phenotypes from 1 wk of vernalization followed by moderate (18 or 24 °C) temperatures. C) Devernalization phenotypes from 2 wk of vernalization. D) Devernalization phenotypes from 4 wk of vernalization. DV, devernalization; V, vernalization. Flowering time is the date of the first sign of heading. Leaf number is total leaf number at flowering time. Error bars represent standard error of the mean (SEM) (n = 25). The letters above the bars represent post hoc test results of ANOVA C and D). The asterisks are results of a Mann–Whitney U test B). Data that do not share a letter are significantly different (P < 0.01). ***P < 0.001.
In contrast to high devernalizing temperatures, reports on Triticeae cereals suggest that lower non-chilling temperatures can actually stabilize the vernalized state (Friend and Purvis 1963). To determine whether the same is true for Bd21-3, seeds were moved to 18 °C post-vernalization for 1 wk before devernalization at 32 °C, and the flowering time was compared with plants that did not receive the 18 °C temperature (Fig. 1, A and C; III vs. IV). As predicted, the 18 °C treatment significantly reduced the delay in flowering time imposed by devernalization of germinating seeds, albeit not to the extent of vernalized control plants (Fig. 1, A and C; V). In contrast to several non-grass taxa but similar to winter rye (Purvis and Gregory 1952; Périlleux et al. 2013), we also observed that Bd21-3 devernalization was no longer possible after 4 wk of saturating seed vernalization (Fig. 1, A and D; I vs. II).
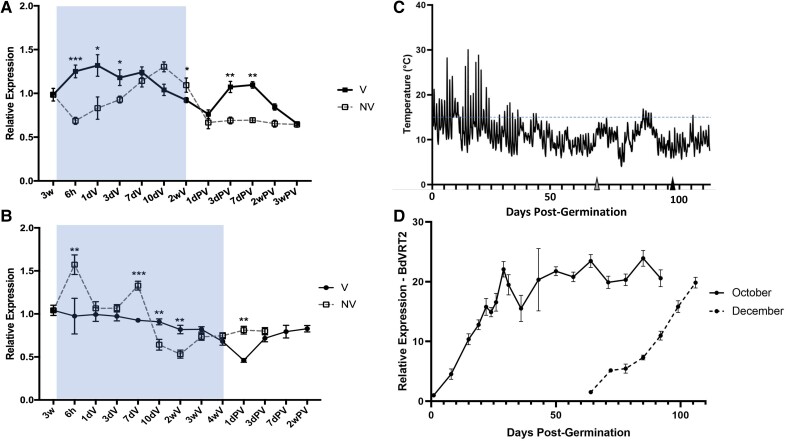
BdVRT2 gene expression is upregulated in natural conditions
Homologs of BdVRT2 are suggested to be involved in vernalization-dependent flowering in cereals based on their gene expression (Kane et al. 2005; Sasani et al. 2009; Xie et al. 2021) and regulate temperature-dependent flowering in Arabidopsis (Lee et al. 2007; Li et al. 2008). Early studies in wheat reported that TaVRT2 expression was downregulated by vernalization and that TaVRT2 might be a floral repressor that negatively regulates TaVRN1 (Kane et al. 2005, 2007). Later studies showed that VRT2 homologs were upregulated by vernalization in wheat and barley, casting doubts on that hypothesis (Trevaskis et al. 2007; Dubcovsky et al. 2008). To investigate whether BdVRT2 expression is regulated by vernalization in Brachypodium, a gene expression time course was conducted (Fig. 2). Wild-type Bd21-3 plants were grown under long days (16 h/8 h) at 22 °C for 3 wk, before being moved to short days (8 h/16 h) at 4 °C to simulate winter. The vernalization treatment here contrasts with the previous experiment as vernalization is occurring in juvenile plants, as opposed to seeds. This method was chosen to obtain more material to assess gene responses to vernalization as vernalization is equally effective in both plants and seeds once cells are mitotically active. Additionally, the transcripts activated across the different tissue types are generally the same (Finnegan and Dennis 2007).
Figure 2.
BdVRT2 expression is strongly upregulated under natural conditions. BdVRT2 relative expression in a temperature-controlled growth chamber during 2 (A) and 4 (B) weeks of vernalization. Bd21-3 plants were grown at 22 °C under a long-day photoperiod (16/8 h) for 3 wk before being moved to 4 °C under short days (8/16 h). The samples were compared with non-vernalized control plants, which remained at 22 °C. Blue boxes delimit the period of vernalization. C) Temperature conditions from October to January in an unheated, naturally lit greenhouse. Gray and black triangles indicate the start of the December- and October-sown time course, respectively. The blue-dashed line denotes the reported upper limit of plant vernalization temperatures. D) Relative expression of BdVRT2 from plants sown in October or December under the conditions in C). Error bars are SEM. Asterisks indicate the P-value from a Bonferroni-corrected Kruskal–Wallis test. ***P < 0.001; **P < 0.01; *P < 0.05. wk, week; h, hour; d, day; V, vernalization at 4 °C; PV, post-vernalization.
Analysis of BdVRT2 expression showed relatively minor changes in these controlled conditions. While some changes are significant when compared with plants grown without vernalization, the changes are so small that it is unclear whether they are likely to be functional. This therefore suggests that neither vernalization, nor a change in photoperiod (as vernalization was in short days), nor warm conditions after partial vernalization strongly affect BdVRT2 gene expression. We reasoned that this could be because temperature regulation could mainly occur at the protein level, similar to its ortholog SHORT VEGETATIVE PHASE in Arabidopsis, which is heat labile (Lee et al. 2013; Jin et al. 2022).
To further investigate Bd21-3 gene expression in Brachypodium, we also grew plants in an unheated greenhouse in Leuven, Belgium, and monitored hourly temperature and expression of BdVRT2 over the course of winter and spring until flowering (Fig. 2, C and D). BdVRT2 expression now strongly increased at the start of the winter season and subsequently plateaued (Fig. 2D), suggesting that its transcriptional regulation either responds to fluctuating environmental conditions (Fig. 2C) or to a gradual decrease in the photoperiod. While photoperiod has been described to regulate VRT2 in wheat and barley (Kane et al. 2005), in the above controlled experiment, a switch between long days, short days in combination with cold or extended long days post-vernalization did not affect BdVRT2 gene expression to the extent observed in the unheated greenhouse experiment. Therefore, it remains undetermined whether the key determinant of changing BdVRT2 transcript expression is variable temperature, gradually changing photoperiod, humidity, or their interaction.
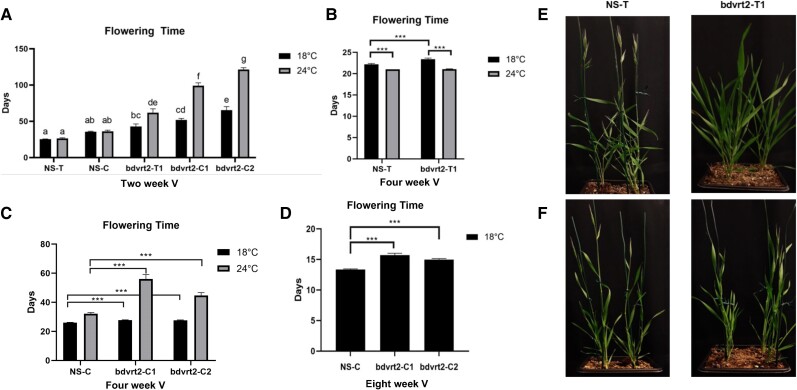
BdVRT2 knock-outs show delayed flowering in warm temperatures after vernalization
To functionally test whether BdVRT2 gene function is affected by temperature in temperate grasses, we investigated flowering in a Bd21-3 accession T-DNA line of BdVRT2 (bdvrt2-T1), with an insertion in the first intron and barely detectable expression levels of BdVRT2 (Supplementary Fig. S1). The 2-wk vernalized bdvrt2 T-DNA insertion mutants were significantly delayed in flowering relative to null sibling controls (NS-T) in a temperature-dependent manner (Fig. 3A). Mutants grown at 18 °C post-vernalization flowered significantly later than NS-T plants, with a concomitant increase in leaf and tiller number (Supplementary Fig. S2). With a 6 °C increase in temperature from 18 to 24 °C, flowering time of bdvrt2 mutants was further delayed by an average of 61.2 d with more leaves and tillers (Fig. 3A; Supplementary Fig. S2). By contrast, in the null siblings, increased temperature had no significant effect on days to flowering, but did increase leaf and tiller number at flowering, which is comparable to previous reports in Brachypodium (Boden et al. 2013; An et al. 2015). To confirm these findings using independent loss-of-function lines, we generated 2 bdvrt2 Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) lines (bdvrt2-C1 and bdvrt2-C2) and again compared flowering to null siblings (NS-C) obtained through segregation of the CRISPR lesion mutants. Under the same conditions, both CRISPR lines flowered later than controls as well as the T-DNA line, suggesting that the former lines have stronger loss-of-function alleles than bdvrt2-T1 (Fig. 3A). Together, these mutant phenotypes provide strong evidence that BdVRT2 plays a role in promoting flowering under warm long-day conditions following vernalization. The bdvrt2 mutants appear to be more sensitive to devernalizing conditions, at least at the moderate temperature of 24 °C (Fig. 3A).
Figure 3.
Effect of temperature on bdvrt2 mutants under incomplete or complete vernalization. A and E). Mutants exhibit delayed flowering in a moderate temperature-dependent manner under incomplete 2-wk vernalization. B and F). When given 4 wk of vernalization, the flowering delay between NS and bdvrt2-T1 vanishes. C) Flowering is still delayed in CRISPR lines treated with 4 wk of cold temperature. D) An 8-wk vernalization period greatly reduces the flowering delay between NS and bdvrt2-C mutants; however, a small delay remains. E and F) Images show exemplar phenotypes under 24 °C. The asterisks indicate the adjusted P-value from a Bonferroni-corrected Kruskal–Wallis test: ***P < 0.0001. Data bars in A) that do not share common letters are significantly different (P < 0.05) based on Tukey HSD test. Error bars represent SEM. V, vernalized; T, T-DNA; C, CRISPR.
BdVRT2 contributes to establishing the length of the vernalization response
Given that the moderate temperature-dependent delay in bdvrt2 mutant flowering was generally absent in control plants, we hypothesized that bdvrt2 has a role in vernalization and/or devernalization. Although devernalization is a possible explanation, the typical temperature range in which this process is reported to occur is high (e.g. >27 °C), potentially failing to explain the observed late flowering of bdvrt2 mutants at moderate (18 and 24 °C) temperatures. In the null siblings, no ambient temperature effect was observed, suggesting that their vernalization response was already saturated at the end of 2 wk of low temperatures (Fig. 3, A and E). By contrast, the temperature-dependent delay on bdvrt2 mutant flowering suggested that their vernalization was incomplete (Fig. 3, A and E). To confirm this, we repeated the above experiment, increasing seed vernalization to 4 wk to ensure that the vernalization requirement was satisfied for all plants. As predicted, after what was presumably full vernalization, the extreme delay in 24 °C relative to the 18 °C flowering of the bdvrt2 T-DNA mutants was alleviated (Fig. 3, B and F). In fact, both control and mutant plants flowered slightly but significantly earlier with warmer temperatures. This observation has not previously been reported for Brachypodium possibly due to daylength differences between studies (Boden et al. 2013; An et al. 2015), but is congruent with research in cereal grasses (Fischer 1985; Rawson and Richards 1993; Porter and Gawith 1999; Karsai et al. 2013). We applied the same 4-wk vernalization treatment to the homozygous CRISPR lines to confirm these findings. However, these lines retained their late flowering phenotype in 24 °C relative to 18 °C plants (Fig. 3C), again suggesting that the CRISPR edits cause complete null alleles that take longer than both control and T-DNA mutant plants to saturate their vernalization response. As such, we further extended the vernalization treatment for the CRISPR lines to 8 wk (Fig. 3D). Although the significant delay in flowering remained for the CRISPR lines at 18 °C (Fig. 3D), the difference with controls was only 2 to 3 d. Together, these results demonstrate that BdVRT2 contributes to the saturation point of the vernalization requirement in Brachypodium Bd21-3, a trait that has been found to vary across naturally occurring accessions of Brachypodium and Arabidopsis in response to different seasonal environments (Stinchcombe et al. 2005).
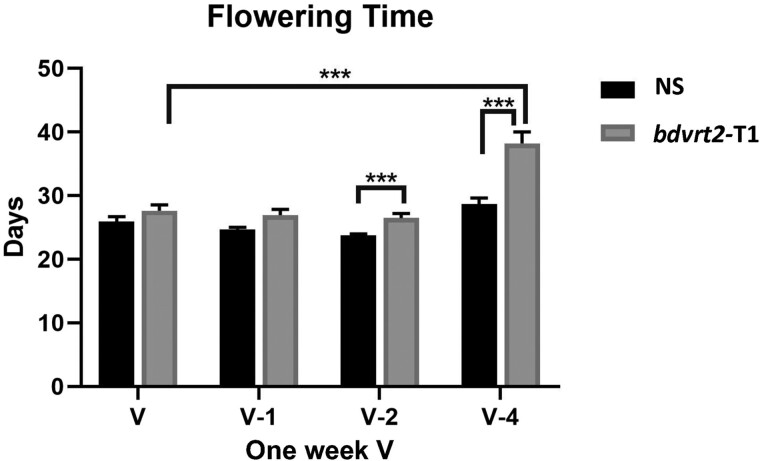
BdVRT2 restarts vernalization after devernalizing temperatures
We have shown that exposure to moderate to hot temperatures after a period of low temperatures can delay flowering time in Brachypodium. However, a more natural situation is where intermittent warm periods disrupt cool, vernalizing periods. Previous reports in winter rye have shown that disrupting the vernalization process can delay flowering time, despite the total sum of vernalizing days being equal to the vernalization requirement (Purvis and Gregory 1952). To determine if an 18 °C disruption to seed vernalization accelerates flowering in Bd21-3, and if bdvrt2 mutants are differently impacted, we vernalized mutant and null sibling seeds for 1 wk at 4 °C before moving them to 18 °C for 1, 2, or 4 d (Fig. 4). The seeds were then returned to 4 °C so that the total amount of time at vernalizing temperatures was 4 wk. With a disruption of 2 or 4 d at 18 °C, mutants exhibited delayed flowering compared with NS after 2 and 4 d, respectively (Fig. 4), of disrupted vernalization. This provides further evidence that BdVRT2 is important in the vernalization process, but specifically to restart vernalization after devernalizing temperatures have been experienced. These results also emphasize that the phenotype of bdvrt2 mutants is a devernalization phenotype, and not a moderate temperature response.
Figure 4.
Influence of disrupted vernalization on flowering time. Null siblings (NS) and bdvrt2-T1 mutants were vernalized for 1 wk at 4 °C before being moved to 18 °C for 1 (v-1), 2 (v-2) or 4 d (v-4). The seeds were then returned to 4 °C to complete a total of 4 wk under vernalizing conditions. The seeds vernalized for 4 wk without a disruption were used as a control (V). Asterisks indicate the adjusted P-value from a Bonferroni-corrected Kruskal–Wallis test: ***P < 0.001. Error bars represent SEM (n = 20).
Key floral promoters and repressors are differentially expressed in bdvrt2 mutants
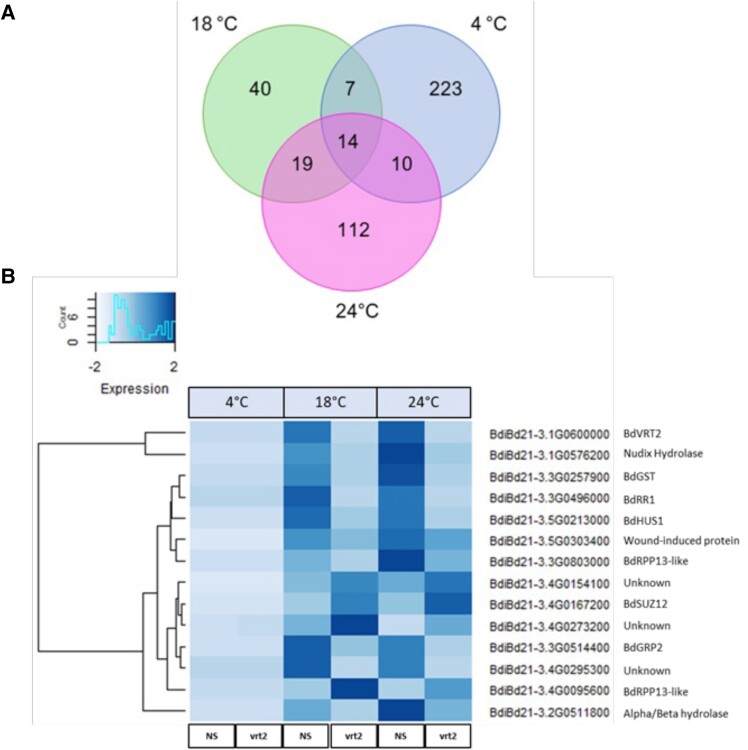
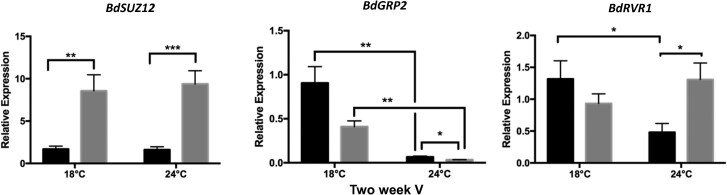
To obtain a global view on Brachypodium devernalization at the transcriptomic level, we performed 2 RNA-seq experiments. Comparing 3-wk-old bdvrt2-T1 mutants to NS plants after 3 d at 4 °C revealed 254 differentially expressed genes (DEGs), whereas the same genotypic comparisons after the 2-wk incomplete seed vernalization followed by devernalization revealed 80 and 155 DEGs at 18 and 24 °C, respectively (Fig. 5A). Altogether 14 DEGs were common to all 3 treatments, one of which was BdVRT2, which was reduced in the mutant line as expected (Fig. 5B). We also identified the Polycomb Repressive Complex 2 (PRC2) gene BdSUPPRESSOR OF ZESTE12 (BdSUZ12) (Fig. 5B), which was upregulated under all conditions in the mutant, as confirmed by reverse transcription quantitative polymerase chain reaction (RT-qPCR) (Fig. 6). BdSUZ12 is homologous to EMBRYONIC FLOWER2 (EMF2) in Arabidopsis, a major regulator of plant development, particularly during the floral transition, where it negatively regulates floral homeotic genes like AGAMOUS and APETALA1 (Chen et al. 1997; Yoshida et al. 2001; Kim et al. 2010). Additionally, we identified an RNA-binding protein BdGRP2, an ortholog of wheat GLYCINE-RICH PROTEIN2 (TaGRP2) that negatively regulates TaVRN1 before vernalization (Xiao et al. 2014). BdGRP2 is downregulated in bdvrt2 mutants (Figs. 5B and 6), suggesting that its role in Brachypodium is different to that in wheat, perhaps acting to target a negative regulator of flowering or affecting BdVRN1 mRNA processing. Another protein known to regulate BdVRN1 is BdRVR1. This protein represses BdVRN1 prior to vernalization by increasing repressive chromatin modifications at the BdVRN1 locus (Woods et al. 2017). In line with this, BdRVR1 expression was significantly higher in bdvrt2 mutants during devernalization at 24 °C (Fig. 6). These results point toward BdVRT2 being involved in an epigenetic mechanism to regulate vernalization and flowering.
Figure 5.
DEGs shared across temperature treatments between control (NS) and bdvrt2 mutant plants. A) Venn diagram showing the number and overlap of DEGs after 3 d of 4 °C vernalization, an 18 °C warm treatment after 2-wk of non-saturating vernalization, and a 24 °C devernalization treatment after 2-wk of non-saturating vernalization. Pairwise comparisons are NS control plants versus bdvrt2 mutants. B) Heatmap showing the differential expression and identity of the 14 overlapping genes from the center of the Venn diagram in A). The scale is based on scaled Fragments Per Kilobase of exon model per Million mapped values.
Figure 6.
Expression validation for genes implicated in epigenetic modifications during vernalization–devernalization. Material used for RNA-seq was also used for RT-qPCR. Error bars are SEM (n = 5). The asterisks indicate the P-value of ANOVA with a post hoc Tukey HSD test: ***P < 0.001; **P < 0.01; *P < 0.05. V, vernalized.
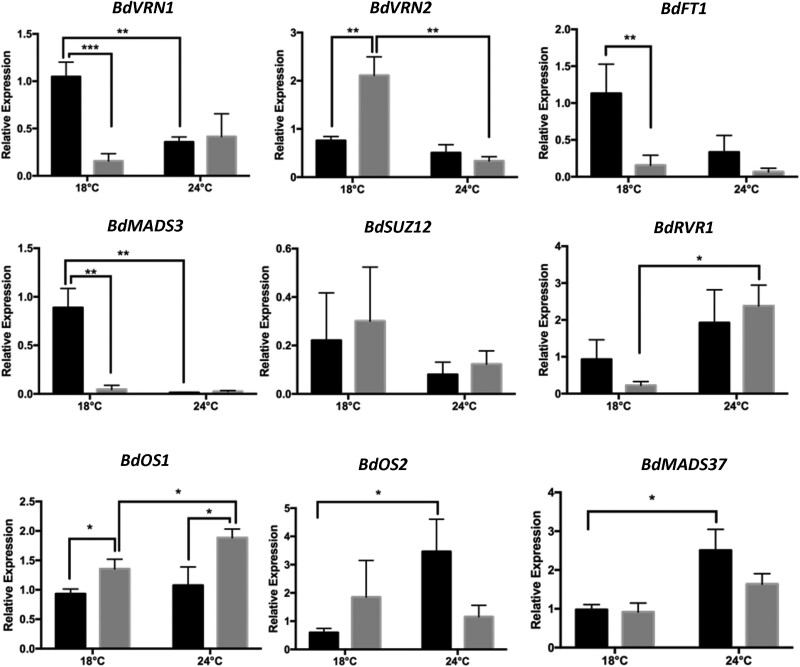
To investigate the overlap of DEGs in our bdvrt2-T1 mutant to the bdrvr1 mutant reported by Woods et al. (2017), we conducted a Fisher's exact test, which revealed that 29 genes were shared across datasets with 17 genes showing opposite expression patterns within the 2 mutants (P < 0.0001). This suggests that BdVRT2 and BdRVR1 are involved in the same genetic pathway and may act antagonistically to regulate BdVRN1 or other floral promoters under devernalizing conditions. To identify other genes within this genetic pathway that were not identified by RNA-seq, we conducted qPCR on genes known to play roles in vernalization-dependent flowering time regulation (Fig. 7, Supplementary Fig. S3). In 2-leaf stage seedlings at 18 °C following incomplete vernalization (Fig. 7), known floral promoters such as BdVRN1 and BdFT1 were significantly downregulated in the mutant compared with the NS, consistent with their upregulation in TaVRT2 overexpression wheat lines (Xie et al. 2021). By contrast, repressors of the floral transition like BdVRN2 and the FLC homolog BdOS1 were upregulated in the mutant, also potentially explaining the delayed flowering phenotype. Interestingly, BdOS2 and BdMADS37 were upregulated in the NS at 24 °C compared with 18 °C, which implicates them in the devernalization process.
Figure 7.
Key regulators of flowering time are differentially expressed in bdvrt2 mutants at the 2-leaf stage. Black bars, segregating null siblings; gray bars, bdvrt2 T-DNA mutants. Whole shoots were used. The asterisks indicate the P-value from Student's t tests of normalized data: *P < 0.05; **P < 0.01; ***P < 0.001. Error bars represent SEMs (n = 5).
In contrast to 18 °C, no differences in gene expression between the NS and mutants at the 2-leaf stage were observed at 24 °C, except for BdOS1 (Fig. 7). We speculated that devernalization of the NS at 24 °C resulted in differential regulation of these genes relative to the 18 °C treatment, concealing differences between the NS and the mutant, and that it was during this stage that transcriptional reprogramming in response to the environment was occurring. Subsequent analysis of gene expression in the 3-leaf stage seedlings revealed that this may have been the case (Supplementary Fig. S3). At the 3-leaf stage, although there was no significant difference in BdVRN1 expression between the NS and bdvrt2 mutants, the expression of BdVRN1 in the mutants at 24 °C was still on average lower than the NS (Supplementary Fig. S3). The modified expression of key vernalization genes suggests that bdvrt2 mutants are not in a comparable state of vernalization to the NS, and the low/high levels of promoters/repressors may explain the delay in flowering time of the bdvrt2 mutants.
Discussion
We provide evidence that BdVRT2 contributes to the length and stability of the vernalization requirement for timely flowering in Brachypodium, a role likely conserved in cereal grasses (Purvis and Gregory 1945). While there are clearly different observations of gene expression that suggest that not all is necessarily conserved between this model species and the related domesticated crops, we think it is unlikely that these signify major functional differences. Rather, the observed differences seem to occur between different varieties or accessions within the same species and because of specific conditions in which gene expression is measured. This is the case for TaVRT2 in wheat, with seemingly opposite results between varieties (Kane et al. 2005, 2007; Trevaskis et al. 2007; Dubcovsky et al. 2008). Also, the gene function of TaVRT2 has thus far only been studied in a spring accession and through ectopic expression or gain of function alleles (Xie et al. 2021; Adamski et al. 2021). The latter results do not necessarily reflect gene function accurately as the expression is established at unnatural levels in unusual times and tissue locations. Hence, we feel that the loss-of-function characterization of BdVRT2 significantly contributes to our understanding of its biological role, both in this model species and related crop species.
The interpretation of our results as evidence of the devernalization occurring in Brachypodium may come as a surprise because the temperatures we used are both in the moderate (18 to 24 °C) and the hot range (e.g. 32 °C). Devernalization is most often considered as unusual and occurring from unlikely hot temperatures that reverse vernalization. We think, however, that there is a continuum between reversibility of vernalization at ambient temperatures and the more extreme process of devernalization, as extensively studied in Arabidopsis with a focus on FLOWERING LOCUS C (Antoniou-Kourounioti et al. 2018). In both processes, partial vernalization is reversed and while devernalization is mostly known as a phenomenon in crop breeding to be avoided, it is probably just a more extreme version of reversing partial vernalization. The more the vernalization progresses, the more extreme the temperatures that are required to reverse it. This range of temperatures that result in reversibility of vernalization or devernalization appears to be more moderate in the bdvrt2 mutants. We therefore consider the role of BdVRT2 to be in reinitiating the process of vernalization after it has been interrupted, possibly directly through an epigenetic process. High levels of epigenetic regulators like BdSUZ12 and BdRVR1 in bdvrt2 mutants suggest that, in wild-type plants, negative regulation of these epigenetic factors by BdVRT2 stabilizes the vernalization state.
An outstanding question resulting from our data is at what level or levels BdVRT2 is regulated. We only monitored regulation at the transcript level and while the data suggest fluctuating temperatures as the driving factor for BdVRT2 gene expression, it seems likely to us that additional regulation at the protein level contributes to its function. This is the case for Arabidopsis SVP and would follow from more general trends of protein biochemical functions being relatively conserved. We intend to study this further, as this could potentially explain the variable effects of ectopic expression on flowering time observed in wheat (Adamski et al. 2021; Li et al. 2021; Xie et al. 2021).
Despite its occurrence in several disparate plant species, the process of devernalization remains relatively under-studied in grasses and crops and its ecological implications have not been fully explored. We have gathered evidence showing that devernalization depends on several factors including the length of time at vernalizing temperatures prior to devernalizing ones and the temperature being experienced. We also confirm that the vernalized state becomes more stable with increasing exposure to cold temperatures, similar to what has been observed for other species (Purvis and Gregory 1945). In our observations, a 4-d 18 °C disruption in vernalization can lead to a delay in flowering despite exposure to enough days typically required to saturate the vernalization response. With autumn/winter temperatures projected to increase and become more variable, reversibility of vernalization or “devernalization” should be taken into consideration when modeling for impacts on flowering time due to warming climates for our important grass crops.
Materials and methods
Plant material
Wild-type Brachypodium distachyon accession Bd21-3 was originally obtained from the US Department of Energy (DOE) Joint Genome Institute (JGI) and bulked-up at the University of Leuven under standard greenhouse conditions. T-DNA insertional mutant seed of BdVRT2 (Bradi1g45812) (line JJ7712) in the Bd21-3 background was also ordered from the DOE-JGI, with plants being genotyped and propagated for at least 3 generations before use. The segregating lines that no longer contained the T-DNA insertion were used as null sibling controls. Genotyping was conducted as described on the JGI website (https://jgi.doe.gov/our-science/science-programs/plant-genomics/brachypodium/brachypodium-t-dna-collection/). For CRISPR editing of BdVRT2, a single guide RNA was designed targeting the first exon of BdVRT2 and cloned into vector pZMUBI-BdCas9-BdU6Pro-gRNA-NOS under the care of John Vogel. Embryogenic callus was cultured and transformed as described in Alves et al. (2009). T0 and T1 generation plants were genotyped by sequencing the sgRNA target region of BdVRT2: 5′-GAACAGCCCGCGCCGCCGCT-3′. The genotyping primers are listed in Supplementary Table S1.
Experimental conditions and phenotyping
Wild-type Bd21-3 seeds were sown into a pot containing a 3:1 mix of soil and vermiculite. A total of 25 seeds were used per condition with 5 seeds sown per pot. For vernalization, the pots were placed into a Conviron growth chamber at 4 °C under short days (8 h light/16 h dark, 50 µmol m−2 s−1) before being moved to a Lovibond growth chamber at various temperatures under long days (16 h light/8 h dark, 100 µmol m−2 s−1). Non-vernalized controls were sown and moved directly to a Lovibond growth chamber at 22 °C under long days. To determine how they behaved under more natural conditions, wild-type Bd21-3 plants were also grown in an unheated, naturally lit greenhouse experiment starting in October or December 2021 in Leuven, Belgium: 50.8823° N, 4.7138° E, with a time-series of aboveground tissues collected for qPCR.
For the moderate temperature experiments comparing Bd21-3 VRT2 mutants to their respective controls, 40 plants per treatment per genotype were sown as described previously and vernalized in darkness at 4 °C for 2 wk. The plants were placed in a Lovibond growth chamber (16 h light/8 h dark, 100 µmol m−2 s−1) at either 18 or 24 °C for the duration of the experiment. The experiments were repeated twice. To analyze the effect of the duration of devernalizing temperatures during vernalization, 20 seeds were sown per condition and placed into a Conviron growth chamber at 4 °C under short days (8 h light/16 h dark, 50 µmol m−2 s−1). The pots were covered with aluminum foil to prevent exposure to light and growth of germinated seedlings. A total of 4 conditions were tested: the seeds were vernalized fully for 4 wk (control); after 1 wk, the pots were moved to a Lovibond at 18 °C under long days (16 h light/8 h dark, 100 µmol m−2 s−1) for devernalization for either 1 d (V-1), 2 d (V-2), or 4 d (V-4). After the devernalization treatment, the pots were returned to 4 °C to complete vernalization. After vernalization, the pots were moved to a Lovibond growth chamber at 22 °C under long-day conditions (16 h light/8 h dark, 100 µmol m−2 s−1) until flowering. Non-vernalized controls were sown and moved directly to 22 °C for growth. The experiments were ended after 120 d. Any non-flowering plants were given a flowering time of 120 d as a standard to assist with data analysis.
For the transcriptomic experiments during vernalization, the plants were grown for 3 wk in a growth room under long days (16 h light/8 h dark, 115 µmol m−2 s−1) at 22 ± 2 °C before being moved to 4 °C under short days (8 h light/16 h dark, 50 µmol m−2 s−1). Subsequently, 3 d later, 6 replicates of whole shoot tissues were harvested for RNA. For devernalization transcriptomic analyses, the seeds were incompletely vernalized for 2 wk at 4 °C and then moved to a Lovibond chamber at either 18 or 24 °C under long days (16 h light/8 h dark, 100 µmol m−2 s−1) for germination. Whole shoot samples were harvested for RNA when plants reached the 3-leaf stage using 6 replicates per condition.
RNA sequencing
The aboveground tissue was harvested for total RNA extraction using the TRIsure method (Bioline), and DNase treatment was given using TURBO DNAase (Invitrogen). Totally, 5 biological replicates were used for both RNA-seq studies. Sequencing and bioinformatic analysis were performed by Novogene, UK. Over 15 gigabytes of transcriptomic data for each sample were obtained from the Illumina HiSeq 4000 platform. The R package DESeq2 was used to analyze differential gene expression. The genes with adjusted P-value <0.05 and logFC >1 were annotated using descriptions from Phytozome and Ensemble plants. NCBI Blast was used to identify previously annotated proteins. Data were deposited in the SRA database with the project number PRJNA1118079.
Quantitative PCR
Precisely 500 ng of whole shoot RNA was used to synthesize cDNA using the SensiFast cDNA synthesis kit (Bioline). Quantitative RT-PCR was performed with 3 technical and 5 biological replicates using the StepOne Real-Time PCR system and the relative expression levels were calculated using the ΔΔCt method with BdUBC18 (Ubiquiting Conjugating enzyme 18) as a reference gene. Transcript abundance of known flowering time genes, BdVRN1, BdVRN2, BdFT1, and BdVRT2 (Distelfeld et al. 2009; Ream et al. 2014; Woods et al. 2016), was assessed, as well as FLC relatives, BdODDSOC1 (BdOS1), BdOS2, and BdMADS37. TaODDSOC2 was previously shown to be differentially regulated in wheat by incomplete vernalization (Dixon et al. 2019). We analyzed gene expression in 2- and 3-leaf stage seedlings, as this is around the time of the floral transition (Hong et al. 2011; Ream et al. 2014).
Statistical analyses
Significant differences between temperature treatments or genotypes within experiments were determined based on analysis of variance (ANOVA) using the aov function in R version 4.0.3, followed by post hoc tests. Student’s t tests were conducted for simple pairwise comparisons, whereas Bonferroni-corrected Kruskal–Wallis or Tukey honestly significant difference (HSD) tests were conducted to take into account multiple comparisons.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession number PRJNA1118079.
Supplementary Material
Acknowledgments
The authors would like to thank Cristobal Uauy (JIC, UK) and John Vogel (UC Berkeley, USA) for constructs and lines.
Contributor Information
Alice Kennedy, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Meixia Li, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Anja Vandeperre, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Muhammad Usama Hameed, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Michelle Van Dyck, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Sarah Engelen, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Jill C Preston, Department of Plant Biology, College of Agriculture and Life Sciences, University of Vermont, Burlington, VT 05405, USA.
Koen Geuten, Department of Biology, Leuven Plant Institute, KU Leuven, 3000 Leuven, Belgium.
Author contributions
A.K., M.L., and K.G. conceived the experiments; A.K., M.L., A.V., M.U.H., M.V.D., and S.E. performed the experiments; A.K., J.C.P., and K.G. wrote the article; and all authors approved of the article.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1. T-DNA insertion model and expression of BdVRT2 after 3 wk of growth.
Supplementary Figure S2. Leaf and tiller numbers of bdvrt2 mutants and NS lines grown at 18 or 24 °C after incomplete 2-wk vernalization or complete 4-wk vernalization.
Supplementary Figure S3. Key regulators of flowering time are differentially expressed in bdvrt2 mutants at the 3-leaf stage.
Supplementary Table S1. Primers for CRISPR edit genotyping and qPCR.
Funding
This work was funded by KU Leuven grant no. C24/17/037 to K.G., the National Institute of Health (VT INBRE-Data Science Core, RRID:SCR_017686, P20GM103449), and the National Science Foundation (IOS2120732 to J.C.P.).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Adamski NM, Simmonds J, Brinton JF, Backhaus AE, Chen Y, Smedley M, Hayta S, Florio T, Crane P, Scott P, et al. Ectopic expression of Triticum polonicum VRT-A2 underlies elongated glumes and grains in hexaploid wheat in a dosage-dependent manner. Plant Cell. 2021:33(7):2296–2319. 10.1093/plcell/koab119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves SC, Worland B, Thole V, Snape JW, Bevan MW, Vain P. A protocol for Agrobacterium-mediated transformation of Brachypodium distachyon community standard line Bd21. Nat Protoc. 2009:4(5):638–649. 10.1038/nprot.2009.30 [DOI] [PubMed] [Google Scholar]
- An Y, Guo Y, Liu C, An H. BdVIL4 regulates flowering time and branching through repressing MiR156 in ambient temperature dependent way in Brachypodium distachyon. Plant Physiol Biochem. 2015:89:92–99. 10.1016/j.plaphy.2015.02.013 [DOI] [PubMed] [Google Scholar]
- Antoniou-Kourounioti RL, Hepworth J, Heckmann A, Duncan S, Qüesta J, Rosa S, Säll T, Holm S, Dean C, Howard M. Temperature sensing is distributed throughout the regulatory network that controls FLC epigenetic silencing in vernalization. Cell Syst. 2018:7(6):643–655.e9. 10.1016/j.cels.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden S, Kavanová M, Finnegan E, Wigge PA. Thermal stress effects on grain yield in Brachypodium distachyon occur via H2A.Z-nucleosomes. Genome Biol. 2013:14(6):R65. 10.1186/gb-2013-14-6-r65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché F, Detry N, Périlleux C. Heat can erase epigenetic marks of vernalization in Arabidopsis. Plant Signal Behav. 2015:10(3):1–4. 10.4161/15592324.2014.990799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Cheng J-C, Castle L, Sung ZR. EMF genes regulate Arabidopsis inflorescence development. Plant Cell. 1997:9(11):2011–2024. 10.2307/3870561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintraruck B, Ketellapper HJ. Interaction of vernalization, photoperiod and high temperature in flowering of Arabidopsis thaliana (L.) HEYNH. Plant Cell Physiol. 1969:10(2):271–276. 10.1093/oxfordjournals.pcp.a074405 [DOI] [Google Scholar]
- Díaz A, Zikhali M, Turner AS, Isaac P, Laurie DA. Copy number variation affecting the photoperiod-B1 and vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One. 2012:7(3):3. 10.1371/journal.pone.0033234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J. Regulation of flowering in temperate cereals. Curr Opin Plant Biol. 2009:12(2):178–184. 10.1016/j.pbi.2008.12.010 [DOI] [PubMed] [Google Scholar]
- Dixon LE, Karsai I, Kiss T, Adamski NM, Liu Z, Ding Y, Allard V, Boden S, Griffiths S. VERNALIZATION1 controls developmental responses of winter wheat under high ambient temperatures. Development. 2019:146(3):dev172684. 10.1242/DEV.172684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Li C, Distelfeld A, Pidal B, Tranquilli G, Appels R, Eastwood R, Lagudah E, Langridge P, Mackay M, McIntyre L, Sharp P. 2008. Genes and gene networks regulating wheat development. Proceedings of 11th International Wheat Genetics Symposium; 2008 Aug 24–29; Brisbane, Australia: Sydney University Press. http://hdl.handle.net/2123/3211 [Google Scholar]
- Durrant MJ, Jaggard KW. Sugar-beet seed advancement to increase establishment and decrease bolting. J Agric Sci. 1988:110(2):367–374. 10.1017/S0021859600081405 [DOI] [Google Scholar]
- Ejaz M, von Korff M. The genetic control of reproductive development under high ambient temperature. Plant Physiol. 2017:173(1):294–306. 10.1104/pp.16.01275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan EJ, Dennis ES. Vernalization-induced trimethylation of histone H3 lysine 27 at FLC is not maintained in mitotically quiescent cells. Curr Biol. 2007:17(22):1978–1983. 10.1016/j.cub.2007.10.026 [DOI] [PubMed] [Google Scholar]
- Fischer RA. Number of kernels in wheat crops and the influence of solar radiation and temperature. J Agric Sci. 1985:105(2):447–461. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Ford B, Deng W, Clausen J, Oliver S, Boden S, Hemming MN, Trevaskis B. Barley (Hordeum vulgare) circadian clock genes can respond rapidly to temperature in an EARLY FLOWERING 3-dependent manner. J Exp Bot. 2016:67(18):5517–5528. 10.1093/jxb/erw317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend DJC, Purvis ON. Studies in vernalisation of cereals XIV. The thermal reactions in vernalisation. Ann Bot. 1963:27(4):553–579. 10.1093/oxfordjournals.aob.a083870 [DOI] [Google Scholar]
- Gregory FG, Purvis ON. Devernalization of winter rye by high temperature. Nature. 1936:138(3502):1013–1014. 10.1038/1381013b0 [DOI] [Google Scholar]
- Hemming MN, Walford SA, Fieg S, Dennis ES, Trevaskis B. Identification of high-temperature-responsive genes in cereals. Plant Physiol. 2012:158(3):1439–1450. 10.1104/pp.111.192013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Park JH, Cho SH, Yang MS, Park CM. Phenological growth stages of Brachypodium distachyon: codification and description. Weed Res. 2011:51(6):612–620. 10.1111/j.1365-3180.2011.00877.x [DOI] [Google Scholar]
- Jin S, Kim SY, Susila H, Nasim Z, Youn G, Ahn JH. FLOWERING LOCUS M isoforms differentially affect the subcellular localization and stability of SHORT VEGETATIVE PHASE to regulate temperature-responsive flowering in Arabidopsis. Mol Plant. 2022:15(11):1696–1709. 10.1016/j.molp.2022.08.007 [DOI] [PubMed] [Google Scholar]
- Jung C, Müller AE. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009:14(10):563–573. 10.1016/j.tplants.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Kane NA, Agharbaoui Z, Diallo AO, Adam H, Tominaga Y, Ouellet F, Sarhan F. TaVRT2 represses transcription of the wheat vernalization gene TaVRN1. Plant Journal. 2007:51(4):670–680. 10.1111/j.1365-313X.2007.03172.x [DOI] [PubMed] [Google Scholar]
- Kane NA, Danyluk J, Tardif G, Limin AE, Fowler DB, Sarhan F. TaVRT-2, a member of the StMADS-11 clade of flowering repressors, is regulated by vernalization and photoperiod in wheat. Plant Physiol. 2005:138(4):2354–2363. 10.1104/pp.105.061762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsai I, Igartua E, Casas AM, Kiss T, Soós V, Balla K, Bedo Z, Veisz O. Developmental patterns of a large set of barley (Hordeum vulgare) cultivars in response to ambient temperature. Ann Appl Biol. 2013:162(3):309–323. 10.1111/aab.12023 [DOI] [Google Scholar]
- Khokhar KM, Hadley P, Pearson S. Effect of reciprocal transfers of onion sets between inductive and non-inductive temperatures on the incidence of bolting and bulbing and seed yield. Sci Hortic. 2007:112(3):245–250. 10.1016/j.scienta.2006.12.034 [DOI] [Google Scholar]
- Kim SY, Zhu T, Sung ZR. Epigenetic regulation of gene programs by EMF1 and EMF2 in Arabidopsis. Plant Physiol. 2010:152(2):516–528. 10.1104/pp.109.143495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Dixon LE, Soltész A, Bányai J, Mayer M, Balla K, Allard V, Galiba G, Slafer GA, Griffiths S, et al. Effects of ambient temperature in association with photoperiod on phenology and on the expressions of major plant developmental genes in wheat (Triticum aestivum L.). Plant Cell Environ. 2017:40(8):1629–1642. 10.1111/pce.12971 [DOI] [PubMed] [Google Scholar]
- Lee JH, Ryu H-S, Chung KS, Posé D, Kim S, Schmid M, Ahn JH. Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science. 2013:342(6158):628–632. 10.1126/science.1241097 [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev. 2007:21(4):397–402. 10.1101/gad.1518407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Debernardi JM, Li C, Lin H, Zhang C, Jernstedt J, von Korff M, Zhong J, Dubcovsky J. Interactions between SQUAMOSA and SHORT VEGETATIVE PHASE MADS-box proteins regulate meristem transitions during wheat spike development. Plant Cell. 2021:33(12):3621–3644. 10.1093/plcell/koab243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell. 2008:15(1):110–120. 10.1016/j.devcel.2008.05.002 [DOI] [PubMed] [Google Scholar]
- Marks MK, Prince SD. Induction of flowering in wild lettuce (Lactuca serriola L.). II. Devernalisation. New Phytol. 1979:82(2):357–363. 10.1111/j.1469-8137.1979.tb02661.x [DOI] [Google Scholar]
- Marks MK, Prince SD. Induction of flowering in wild lettuce (Lactuca serriola L.). III. Vernalisation-devernalisation cycles in buried seeds. New Phytol. 1982:91(4):661–668. 10.1111/j.1469-8137.1979.tb02661.x [DOI] [Google Scholar]
- Mathieu AS, Périlleux C, Jacquemin G, Renard ME, Lutts S, Quinet M. Impact of vernalization and heat on flowering induction, development and fertility in root chicory (Cichorium intybus L. var. sativum). J Plant Physiol. 2020:254:153272. 10.1016/j.jplph.2020.153272 [DOI] [PubMed] [Google Scholar]
- Mayer BF, Bertrand A, Charron J-B. Treatment analogous to seasonal change demonstrates the integration of cold responses in Brachypodium distachyon. Plant Physiol. 2019:182(2):1022–1038. 10.1104/pp.19.01195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR, Lou P, Hermand V, Kim JA. The importance of ambient temperature to growth and the induction of flowering. Front Plant Sci. 2016:7. 10.3389/fpls.2016.01266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périlleux C, Pieltain A, Jacquemin G, Bouché F, Detry N, D’Aloia M, Thiry L, Aljochim P, Delansnay M, Mathieu AS, et al. A root chicory MADS box sequence and the Arabidopsis flowering repressor FLC share common features that suggest conserved function in vernalization and de-vernalization responses. Plant J. 2013:75(3):390–402. 10.1111/tpj.12208 [DOI] [PubMed] [Google Scholar]
- Porter JR, Gawith M. Temperatures and the growth and development of wheat: a review. Eur J Agron. 1999:10(1):23–36. 10.1016/S1161-0301(98)00047-1 [DOI] [Google Scholar]
- Preston JC, Sandve SR. Adaptation to seasonality and the winter freeze. Front Plant Sci. 2013:4. 10.3389/fpls.2013.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis ON, Gregory FG. Devernalization by high temperature. Nature. 1945:155(3926):113–114. 10.1038/155113a0 [DOI] [Google Scholar]
- Purvis ON, Gregory FG. Studies in vernalisation: XII. The reversibility by high temperature of the vernalised condition in petkus winter rye. Ann Bot. 1952:16(1):1–21. 10.1093/oxfordjournals.aob.a083297 [DOI] [Google Scholar]
- Rawson HM, Richards RA. Effects of high temperature and photoperiod on floral development in wheat isolines differing in vernalisation and photoperiod genes. Field Crops Res. 1993:32(3-4):181–192. 10.1016/0378-4290(93)90030-Q [DOI] [Google Scholar]
- Ream TS, Woods DP, Schwartz CJ, Sanabria CP, Mahoy JA, Walters EM, Kaeppler HF, Amasino R. Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 2014:164(2):694–709. 10.1104/pp.113.232678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasani S, Hemming MN, Oliver S, Greenup AG, Tavakkol-Afshari R, Mahfoozi S, Poustini K, Sharifi HR, Dennis ES, Peacock WJ, et al. The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare). J Exp Bot. 2009:60(7):2169–2178. 10.1093/jxb/erp098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Ruelens P, D'hauw M, Maggen T, Dochy N, Torfs S, Kaufmann K, Rohde A, Geuten K. A flowering locus C homolog is a vernalization-regulated repressor in Brachypodium and is cold regulated in wheat. Plant Physiol. 2017:173(2):1301–1315. 10.1104/pp.16.01161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo C, Lister C, Crevillen P, Nordborg M, Dean C. Variation in the epigenetic silencing of FLC contributes to natural variation in Arabidopsis vernalization response. Genes Dev. 2006:20(22):3079–3083. 10.1101/gad.405306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa M, Morisaki Y, Gan E-S, Sato A, Ito T. Identification of a devernalization inducer by chemical screening approaches in Arabidopsis thaliana. Front Plant Sci. 2021:12:634068. 10.3389/fpls.2021.634068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slafer GA. Reproductive development of wheat under different thermal and photoperiodic environments [PhD thesis]. [Melbourne (Australia)]: University of Melbourne; 1995.
- Stinchcombe JR, Caicedo AL, Hopkins R, Mays C, Boyd EW, Purugganan MD, Schmitt J. Vernalization sensitivity in Arabidopsis thaliana (Brassicaceae): the effects of latitude and FLC variation. Am J Bot. 2005:92(10):1701–1707. 10.3732/ajb.92.10.1701 [DOI] [PubMed] [Google Scholar]
- Trevaskis B, Tadege M, Hemming MN, Peacock WJ, Dennis ES, Sheldon C. Short vegetative phase-like MADS-box genes inhibit floral meristem identity in barley. Plant Physiol. 2007:143(1):225–235. 10.1104/pp.106.090860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe HJ. Effects of temperature and daylength on bolting of leek (Allium porrum L.). Sci Hortic. 1994:59(3-4):177–185. 10.1016/0304-4238(94)90011-6 [DOI] [Google Scholar]
- Wiebe HJ, Habegger R, Liebig HP. Quantification of vernalization and devernalization effects for kohlrabi (Brassica oleracea convar. Acephala var. gongylodes L.). Sci Hortic. 1992:50(1-2):11–20. 10.1016/S0304-4238(05)80004-4 [DOI] [Google Scholar]
- Woods DP, McKeown M, Dong Y, Preston JC, Amasino R. Evolution of VRN2/Ghd7-like genes in vernalization-mediated repression of grass flowering. Plant Physiol. 2016:170(4):2124–2135. 10.1104/pp.15.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DP, Ream TS, Bouché F, Lee J, Thrower N, Wilkerson C, Amasino RM. Establishment of a vernalization requirement in Brachypodium distachyon requires REPRESSOR OF VERNALIZATION1. Proc Natl Acad Sci U S A. 2017:114(25):6623–6628. 10.1073/pnas.1700536114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Xu S, Li C, Xu Y, Xing L, Niu Y, Huan Q, Tang Y, Zhao C, Wagner D, et al. O-GlcNAc-mediated interaction between VER2 and TaGRP2 elicits TaVRN1 MRNA accumulation during vernalization in winter wheat. Nat Commun. 2014:5(1):4572. 10.1038/ncomms5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Zhang Y, Wang K, Luo X, Xu D, Tian X, Li L, Ye X, Xia X, Li W, et al. Tavrt2, an SVP-like gene, cooperates with TaVrn1 to regulate vernalization-induced flowering in wheat. New Phytol. 2021:231(2):834–848. 10.1111/nph.16339 [DOI] [PubMed] [Google Scholar]
- Xu S, Chong K. Remembering winter through vernalisation. Nat Plants. 2018:4(12):997–1009. 10.1038/s41477-018-0301-z [DOI] [PubMed] [Google Scholar]
- Yoshida N, Yanai Y, Chen L, Kato Y, Hiratsuka J, Miwa T, Sung ZR, Takahashi S. EMBRYONIC FLOWER2, a novel polycomb group protein homolog, mediates shoot development and flowering in Arabidopsis. Plant Cell. 2001:13(11):2471–2481. 10.1105/tpc.13.11.2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.