Abstract
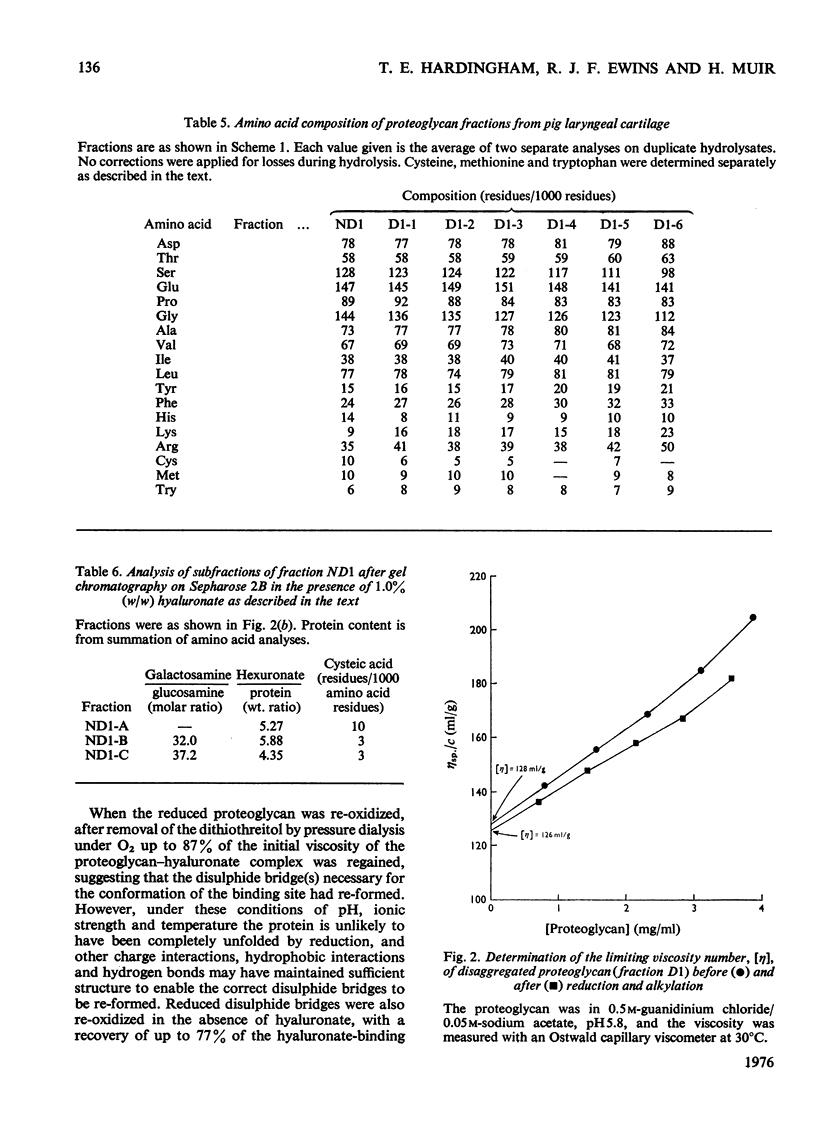
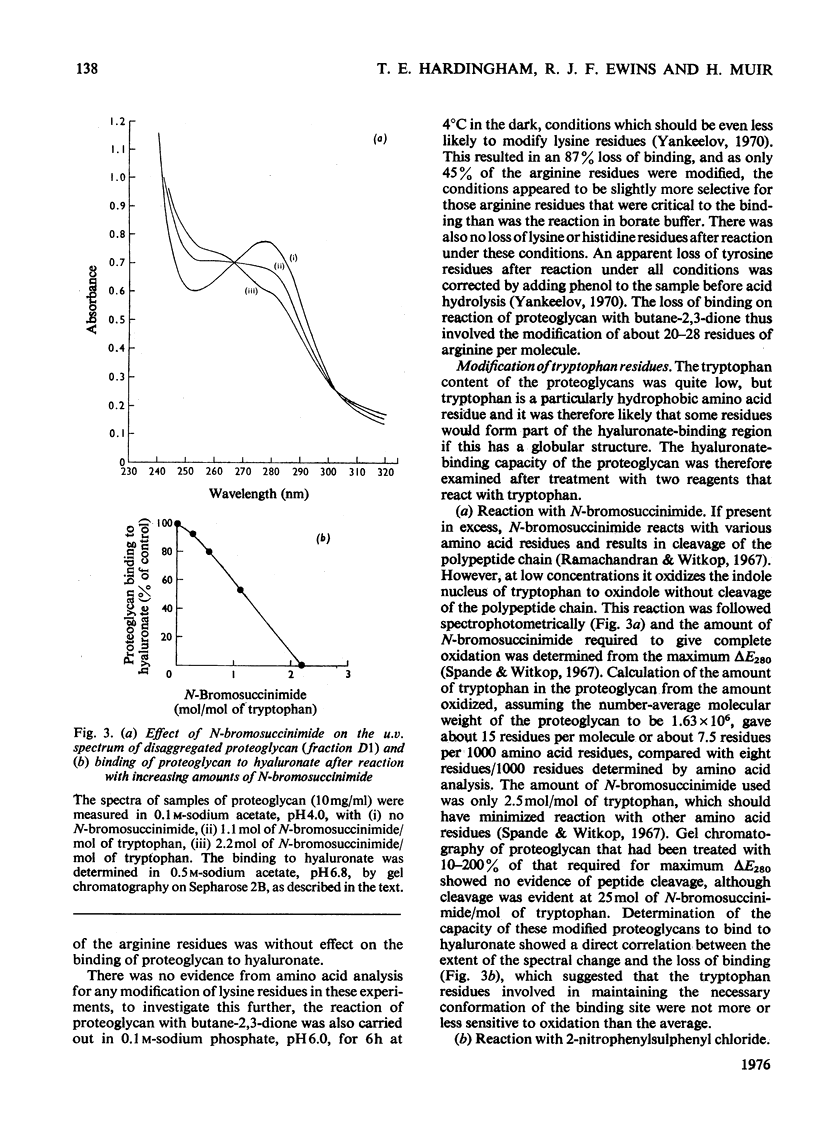
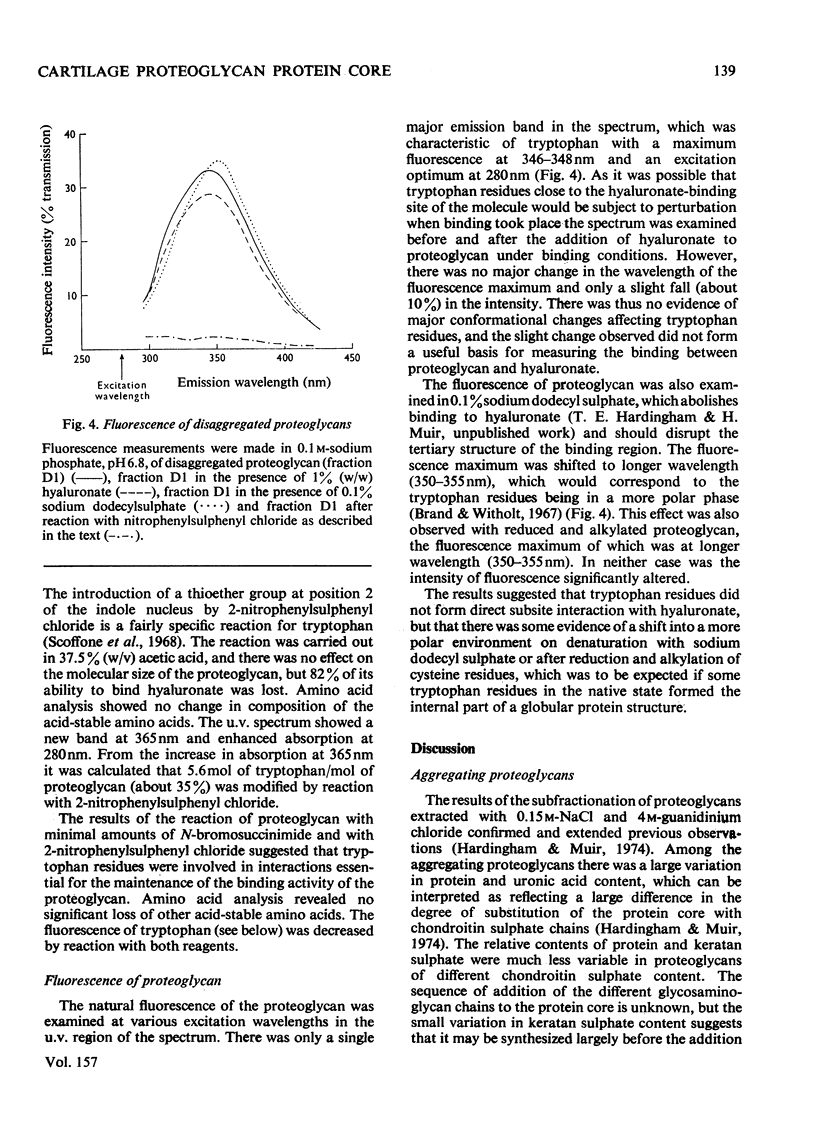
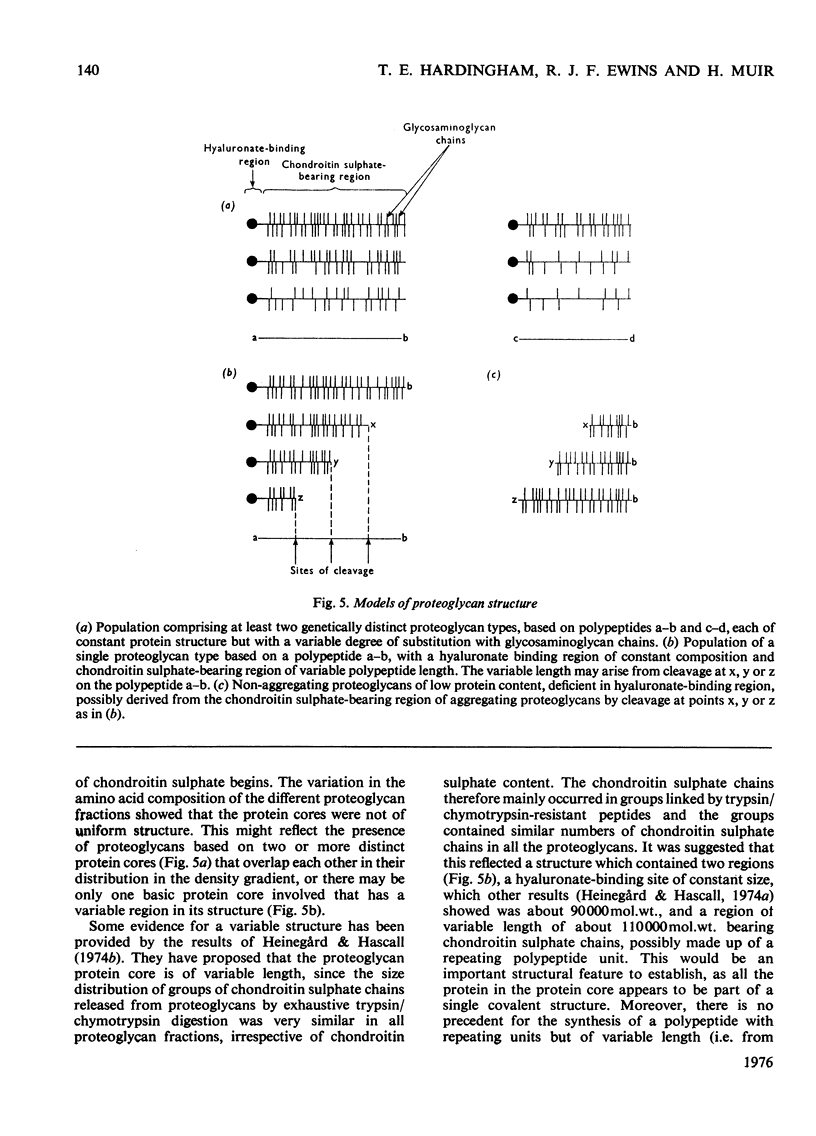
Purified proteoglycans extracted from pig laryngeal cartilage in 0.15 M-NaCl and 4 M-guanidinium chloride were analysed and their amino acid compositions determined. Selective modification of amino acid residues on the protein core confirmed that binding to hyaluronate was a function of the protein core, and was dependent on disulphide bridges, intact arginine and tryptophan residues, and epsilon-amino groups of lysine. Fluorescence measurement suggested that tryptophan was not involved in direct subsite interactions with the hyaluronate. The polydispersity in size and heterogeneity in composition of the aggregating proteoglycan was compatible with a structure based on a protein core containing a globular hyaluronate-binding region and an extended region of variable length also containing a variable degree of substitution with chondroitin sulphate chains. The non-aggregated proteoglycan extracted preferentially in 0.15 M-NaCl, which was unable to bind to hyaluronate, contained less cysteine and tryptophan than did other aggregating proteoglycans and may be deficient in the hyaluronate-binding region. Its small average size and low protein and keratan sulphate contents suggest that it may be a fragment of the chondroitin sulphate-bearing region of aggregating proteoglycan produced by proteolytic cleavage of newly synthesized molecules before their secretion from the cell.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Specific chemical modification of proteins. Annu Rev Biochem. 1970;39:101–130. doi: 10.1146/annurev.bi.39.070170.000533. [DOI] [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg A. L., Pressman D. Modification of arginine in the active sites of antibodies. Biochemistry. 1968 Jan;7(1):272–279. doi: 10.1021/bi00841a033. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Preparation of enzymically active, water-insoluble derivatives of trypsin. Arch Biochem Biophys. 1967 Mar;119(1):264–268. doi: 10.1016/0003-9861(67)90453-5. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Moore S. Determination of the tryptophan content of proteins by ion exchange chromatography of alkaline hydrolysates. J Biol Chem. 1972 May 10;247(9):2828–2834. [PubMed] [Google Scholar]
- Kemp R. G., Forest P. B. Reactivity of the sulfhydryl groups of muscle phosphofructokinase. Biochemistry. 1968 Jul;7(7):2596–2602. doi: 10.1021/bi00847a022. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Riordan J. F. Functional arginyl residues in carboxypeptidase A. Modification with butanedione. Biochemistry. 1973 Sep 25;12(20):3915–3923. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Pal S., Beale R. J. Proteoglycans from bovine proximal humeral articular cartilage. J Biol Chem. 1973 May 25;248(10):3681–3690. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Scoffone E., Fontana A., Rocchi R. Sulfenyl halides as modifying reagents for polypeptides and proteins. I. Modification of tryptophan residues. Biochemistry. 1968 Mar;7(3):971–979. doi: 10.1021/bi00843a014. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Lohmander S., Heinegård D. Proteoglycans of hyaline cartilage: Electron-microscopic studies on isolated molecules. Biochem J. 1975 Oct;151(1):157–166. doi: 10.1042/bj1510157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Yankeelov J. A., Jr Modification of arginine in proteins by oligomers of 2,3-butanedione. Biochemistry. 1970 Jun 9;9(12):2433–2439. doi: 10.1021/bi00814a007. [DOI] [PubMed] [Google Scholar]


