Abstract
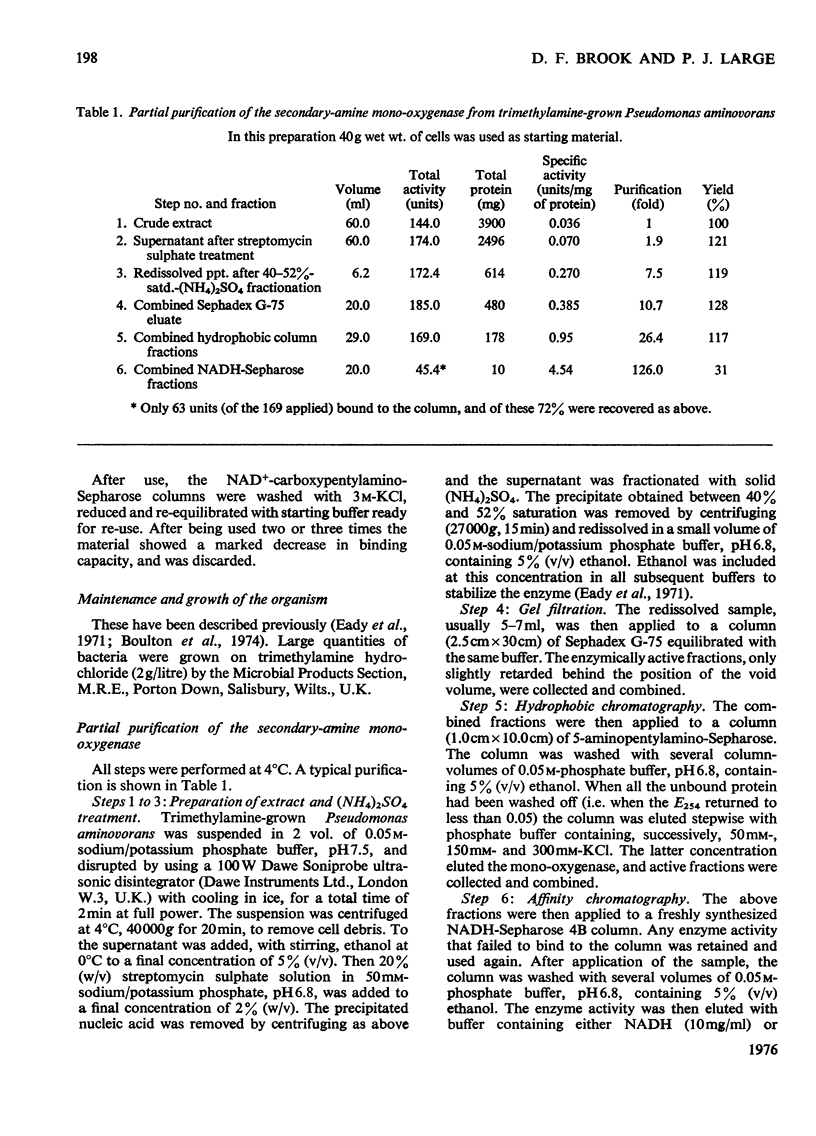
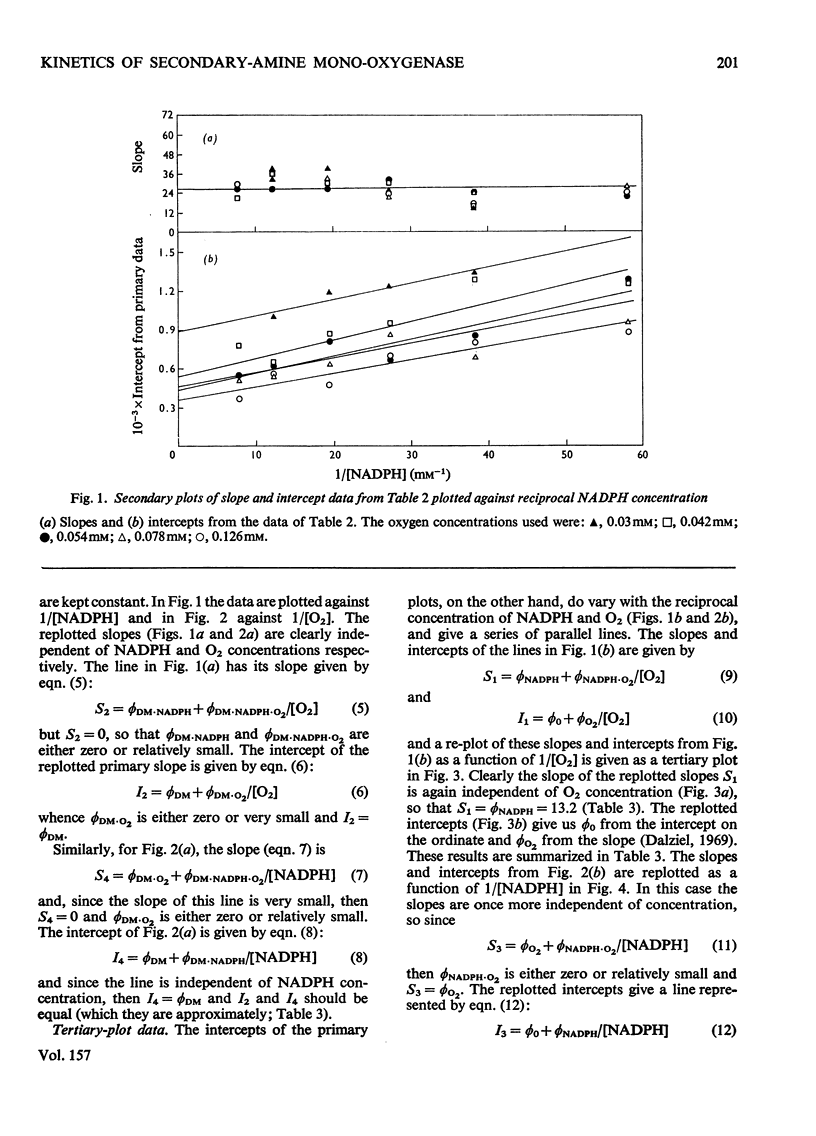
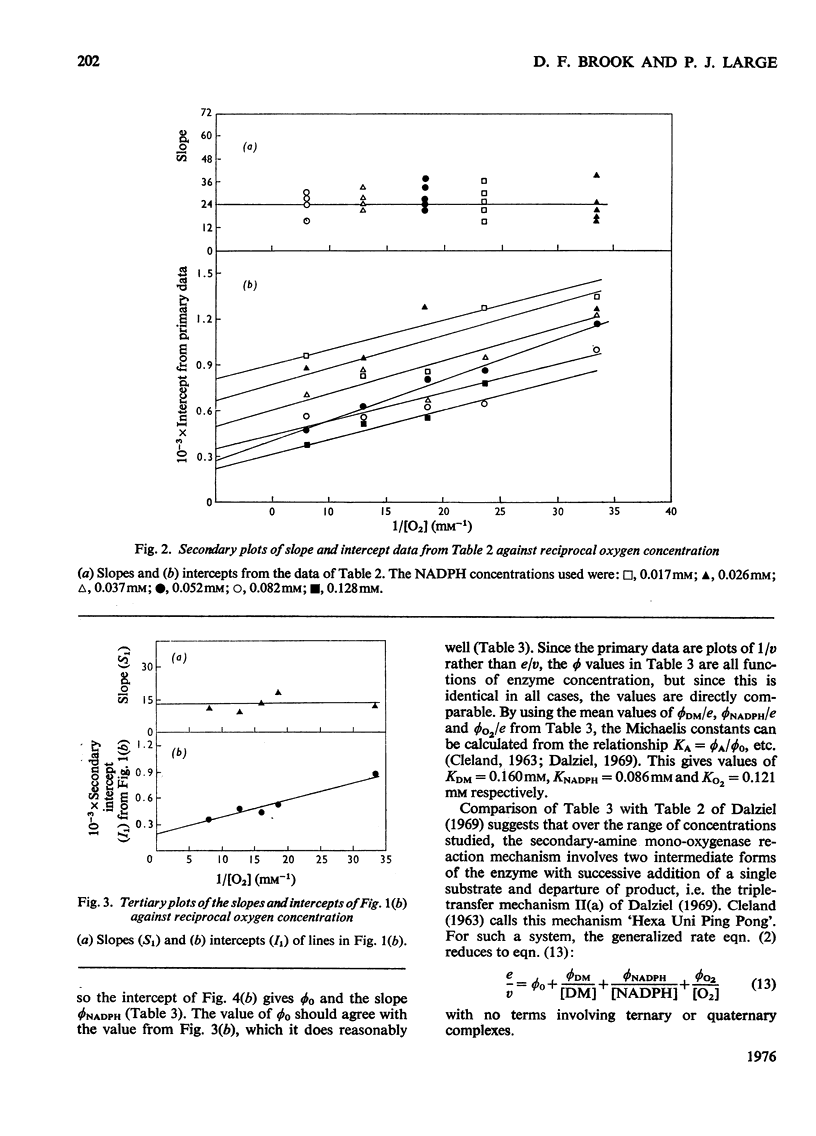
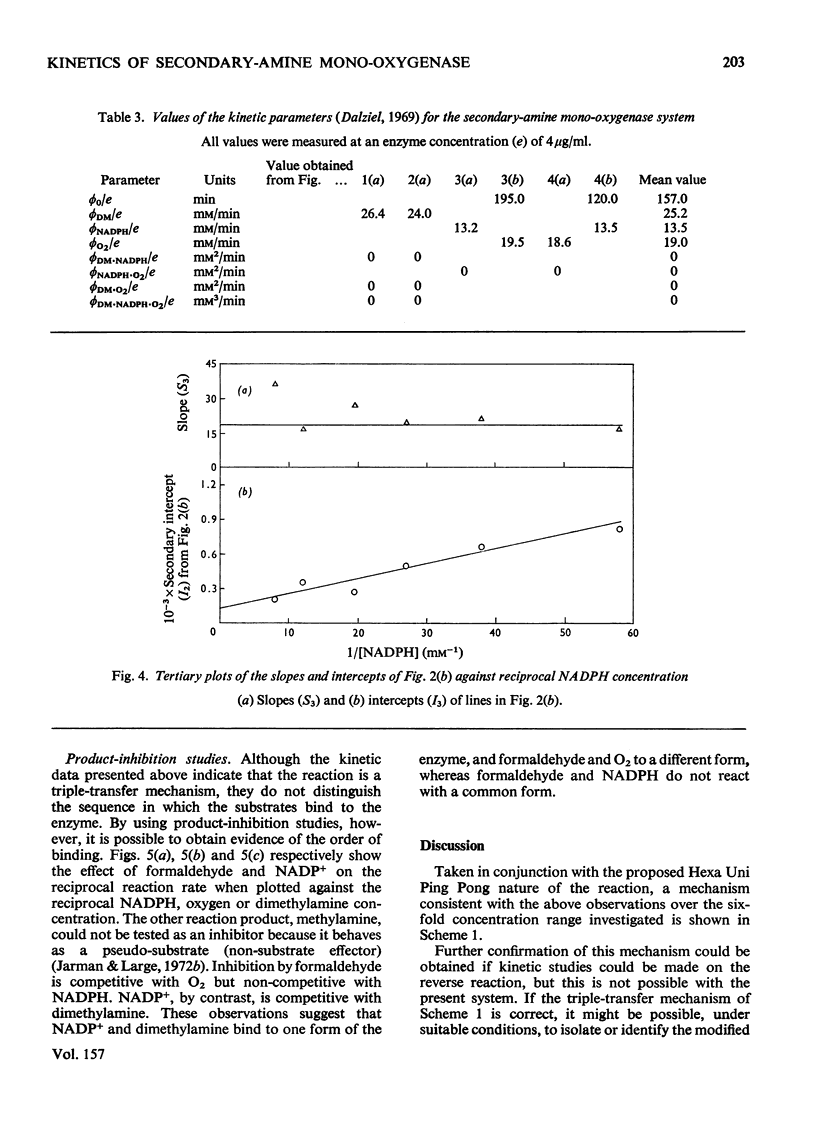
1. Secondary-amine mono-oxygenase (proposed EC group 1.14.99.-) was partially purified from trimethylamine-grown Pseudomonas aminovorans by (NH4)2SO4 fractionation, gel filtration, hydrophobic chromatography on 5-aminopentylamino-Sepharose, and affinity chromatography on Sepharose-bound NADH. 2. Some problems in the affinity-chromatography step are discussed. 3. A steady-state kinetic analysis varying substrate, oxygen and electron-donor concentrations was performed, which, over the concentration range studied, gave a series of families of approximately parallel double-reciprocal plots. From secondary and tertiary plots, Michaelis constants of 0.160 mM, 0.086 mM and 0.121 mM were obtained for dimethylamine, NADPH and oxygen respectively. 4. Product-inhibition studies supported the postulated Hexa Uni Ping Pong (triple-transfer) reaction mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry S., O'Carra P. Affinity chromatography of nicotinamide-adenine dinucleotide-linked dehydrogenases on immobilized derivatives of the dinucleotide. Biochem J. 1973 Dec;135(4):595–607. doi: 10.1042/bj1350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton C. A., Crabbe M. J., Large P. J. Microbial oxidation of amines. Partial purification of a trimethylamine mono-oxygenase from Pseudomonas aminovorans and its role in growth on trimethylamine. Biochem J. 1974 May;140(2):253–263. doi: 10.1042/bj1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook D. F., Large P. J. Inhibition by carbon monoxide of the secondary-amine mono-oxygenase of Pseudomonas aminovorans and the photochemical action spectrum for its reversal. Eur J Biochem. 1975 Jul 15;55(3):601–609. doi: 10.1111/j.1432-1033.1975.tb02197.x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dalziel K. The interpretation of kinetic data for enzyme-catalysed reactions involving three substrates. Biochem J. 1969 Sep;114(3):547–556. doi: 10.1042/bj1140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eady R. R., Jarman T. R., Large P. J. Microbial oxidation of amines. Partial purification of a mixed-function secondary-amine oxidase system from Pseudomonas aminovorans that contains an enzymically active cytochrome-P-420-type haemoprotein. Biochem J. 1971 Nov;125(2):449–459. doi: 10.1042/bj1250449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M., Joh T. H., Garvey T. Q., 3rd Kinetic studies of the enzymatic dopamine beta-hydroxylation reaction. Biochemistry. 1968 Aug;7(8):2724–2730. doi: 10.1021/bi00848a005. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Tipton K. F. The reaction pathway of membrane-bound rat liver mitochondrial monoamine oxidase. Biochem J. 1973 Dec;135(4):735–750. doi: 10.1042/bj1350735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman T. R., Large P. J. Distribution of the enzymes oxidizing secondary and tertiary amines in Pseudomonas aminovorans grown on various substrates. J Gen Microbiol. 1972 Nov;73(1):205–208. doi: 10.1099/00221287-73-1-205. [DOI] [PubMed] [Google Scholar]
- Jarman T. R., Large P. J. Primary amines as uncouplers of electron transport from hydroxylation in the secondary-amine mono-oxygenase system of Pseudomonas aminovorans. Biochem Biophys Res Commun. 1972 Nov 1;49(3):740–747. doi: 10.1016/0006-291x(72)90473-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J. The oxidative cleavage of alkyl-nitrogen bonds in micro-organisms. Xenobiotica. 1971 Jul-Oct;1(4):457–467. doi: 10.3109/00498257109041511. [DOI] [PubMed] [Google Scholar]
- Larsson P. O., Mosbach K. Preparation of a NAD(H)-polymer matrix showing coenzyme function of the bound pyridine nucleotide. Biotechnol Bioeng. 1971 May;13(3):393–398. doi: 10.1002/bit.260130306. [DOI] [PubMed] [Google Scholar]
- McIntyre R. J., Vaughan P. F. Kinetic studies on the hydroxylation of p-coumaric acid to caffeic acid by spinach-beet phenolase. Biochem J. 1975 Aug;149(2):447–461. doi: 10.1042/bj1490447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S., Massey V. The mechanism of action of the flavoprotein melilotate hydroxylase. J Biol Chem. 1973 Apr 25;248(8):2953–2962. [PubMed] [Google Scholar]


