Abstract
Background
Genital ulcer disease by virtue of disruption of the mucosal surfaces may enhance HIV acquisition. Genital ulcer disease treatment with resolution of the ulcers may therefore contribute in reducing the sexual acquisition of HIV.
Objectives
To determine the effects of treatment of genital ulcer disease on sexual acquisition of HIV.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, EMBASE, LILACS, NLM Gateway, Web of Science, WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, and reference lists of relevant publications for eligible studies published between 1980 and August 2011.
Selection criteria
Randomized controlled trials of any treatment intervention aimed at curing genital ulcer disease compared with an alternative treatment, placebo, or no treatment. We included only trials whose unit of randomization was the individual with confirmed genital ulcer.
Data collection and analysis
We independently selected studies and extracted data in duplicate; resolving discrepancies by discussion, consensus, and arbitration by third review author. We expressed study results as risk ratios (RR) with 95% confidence intervals (CI).
Main results
There were three randomized controlled trials that met our inclusion criteria recruited HIV‐negative participants with chancroid (two trials with 143 participants) and primary syphilis (one trial with 30 participants). The syphilis study, carried out in the US between 1995 and 1997, randomized participants to receive a single 2.0 g oral dose of azithromycin (11 participants); two 2.0 g oral doses of azithromycin administered six to eight days apart (eight participants); or benzathine penicillin G administered as either 2.4 million units intramuscular injection once or twice seven days apart (11 participants). No participant in the trial seroconverted during 12 months of follow‐up. The chancroid trials, conducted in Kenya by 1990, found no significant differences in HIV seroconversion rates during four to 12 weeks of follow‐up between 400 and 200 mg single oral doses of fleroxacin (one trial, 45 participants; RR 3.00; 95% CI 0.29 to 30.69), or between 400 mg fleroxacin and 800 mg sulfamethoxazole plus 160 mg trimethoprim (one trial, 98 participants; RR 0.33; 95% CI 0.04 to 3.09). Adverse events reported were mild to moderate in severity, and included Jarisch‐Herxheimer reactions and gastrointestinal symptoms. The differences between the treatment arms in the incidence of adverse events were not significant. The quality of this evidence on the effectiveness of genital ulcer disease treatment in reducing sexual acquisition of HIV, according to GRADE methodology, is of very low quality.
Authors' conclusions
At present, there is insufficient evidence to determine whether curative treatment of genital ulcer disease would reduce the risk of HIV acquisition. The very low quality of the evidence implies that the true effect of genital ulcer disease treatment on sexual acquisition of HIV may be substantially different from the effect estimated from currently available data. However, genital ulcer diseases are public health problems in their own right and patients with these conditions should be treated appropriately; whether the treatment reduces the risk of HIV infection or not.
Keywords: Female, Humans, Male, HIV Seronegativity, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Anti‐Infective Agents, Anti‐Infective Agents/therapeutic use, Azithromycin, Azithromycin/therapeutic use, Chancroid, Chancroid/drug therapy, Fleroxacin, Fleroxacin/therapeutic use, HIV Infections, HIV Infections/prevention & control, HIV Infections/transmission, Penicillin G Benzathine, Penicillin G Benzathine/therapeutic use, Randomized Controlled Trials as Topic, Sulfamethoxazole, Sulfamethoxazole/therapeutic use, Syphilis, Syphilis/drug therapy, Trimethoprim, Trimethoprim/therapeutic use
Plain language summary
Genital ulcer disease treatment for reducing sexual acquisition of HIV
The presence of a genital ulcer would provide an entry point for the HIV virus if an HIV‐negative individual with an ulcer has unprotected sexual intercourse with an HIV‐infected person. Treatment of the condition causing the genital ulcer would allow the ulcer to heal and therefore reduce the chances of HIV acquisition. This review assessed whether giving treatment for diseases that present with ulcers in the genital region would reduce sexual acquisition of HIV. Three studies were identified involving 173 HIV‐negative patients with genital ulcers. These studies did not provide sufficient evidence that treatment of genital ulcer diseases reduces sexual acquisition of HIV infection. However, genital ulcer diseases are public health problems in their own right and patients with these conditions should be treated appropriately; whether the treatment reduces the risk of HIV infection or not.
Summary of findings
Summary of findings for the main comparison. Azithromycin (single‐ or two‐dose) compared to penicillin for patients with syphilis.
| Patient or population: patients with syphilis Settings: STD clinics in Birmingham, Alabama, and New Orleans, Louisiana (US) Intervention: azithromycin (single‐ or two‐dose) Comparison: penicillin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Penicillin | Azithromycin (single‐ or two‐dose) | |||||
| HIV seroconversion ‐ penicillin vs single‐dose azithromycin | See comment | See comment | Not estimable | 19 (1 study) | ⊕⊝⊝⊝ very low1,2 | There were no HIV seroconversions in the study |
| HIV seroconversion ‐ penicillin vs two‐dose azithromycin | See comment | See comment | Not estimable | 22 (1 study) | ⊕⊝⊝⊝ very low1,2 | There were no HIV seroconversions in the study |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HIV: human immunodeficiency virus | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 No information on allocation concealment; high attrition rate (19%) 2 Small sample size; no participants with events
Summary of findings 2. Fleroxacin 200 mg or TMP‐SMZ compared to fleroxacin 400 mg for patients with chancroid.
| Patient or population: patients with chancroid Settings: Nairobi City Commission Special Treatment Clinic Intervention: fleroxacin 200 mg or TMP‐SMZ Comparison: fleroxacin 400 mg | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Fleroxacin 400 mg | Fleroxacin 200 mg or TMP‐SMZ | ||||
| HIV seroconversion ‐ fleroxacin 400 mg vs fleroxacin 200 mg | 111 per 1000 | 333 per 1000 (32 to 1000) | RR 3.00 (0.29 to 30.69) | 45 (1 study) | ⊕⊝⊝⊝ very low1,2 |
| HIV seroconversion ‐ fleroxacin 400 mg vs TMP‐SMZ | 61 per 1000 | 20 per 1000 (2 to 189) | RR 0.33 (0.04 to 3.09) | 98 (1 study) | ⊕⊝⊝⊝ very low3,4 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HIV: human immunodeficiency virus; RR: Risk ratio; TMP‐SMZ: trimethoprim‐sulfamethoxazole | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No information on allocation concealment and blinding 2 Small sample size; small number of events; very wide confidence intervals 3 No information on allocation concealment; no information on blinding of outcome assessors; early stoppage of the study 4 Very wide confidence intervals
Background
Description of the condition
Some sexually transmitted infections (STIs) present as a break or discontinuity in the genital epithelium and are collectively referred to as genital ulcer disease (GUD). These include genital herpes (caused by herpes simplex virus type 2 (HSV‐2)), syphilis (Treponema pallidum), chancroid (Haemophilus ducreyi), lymphogranuloma venereum (Chlamydia trachomatis serotypes L1, L2, or L3), and donovanosis or granuloma inguinale (Klebsiella granulomatis). The geographical distribution of the causative agents of GUD is varied (WHO 2007; WHO 2011). HSV‐2 is the leading cause of GUD in low‐ and middle‐income countries; with 30% to 80% of women and 10% to 50% of men in sub‐Saharan Africa, 20% to 40% of women in Central and South America, and 10% to 30% of the general population in developing Asian countries estimated to be infected with the virus, while its prevalence among 14‐ to 49‐year‐old people in the USA is only 19% (WHO 2007). In 2008, the global estimates of new cases of syphilis in adult men and women ranged from zero in the World Health Organization (WHO) Eastern Mediterranean region to 3.4 million in the African region (WHO 2011).
Close to three million people were newly infected with the human immunodeficiency virus (HIV) worldwide in 2010, bring the total number of people living with the virus globally to 34 million (WHO 2007; WHO 2011). The primary mode of HIV acquisition is unprotected sexual intercourse, with the major risk factors being unprotected sex with multiple partners and the presence of other sexually transmitted infections (WHO 2011). It is estimated that 498 million new cases of STIs occur each year worldwide (WHO 2001; WHO 2007; WHO 2011). These include curable STIs, such as syphilis, gonorrhea, chlamydia and trichomoniasis, as well as noncurable STIs caused by viruses such as herpes simplex viruses (HSV) and human papillomavirus (HPV).
HIV and other STIs are interdependent with an overlap in risk behaviors associated with the two infections (Quinn 1996). STIs are commonly associated with breaching the protective mucosal barriers of the genital tract as well as causing genital bleeding; factors that increase the risk of HIV exposure during sexual activity (Boily 2009; Royce 1997). HIV causes immunosuppression, which increases susceptibility to other STIs or may modify the natural history of the infection, its clinical presentation, and its response to treatment (Cohen 2006; McCoy 2009). STIs, both ulcerative and non‐ulcerative, also synergistically increase the risk of HIV transmission by increasing genital tract shedding of HIV and hence infectiousness in HIV‐positive patients (Fleming 1999; Plourde 1994). The impact of an STI on HIV genital shedding is dependent on the level of its inflammatory response; the higher the inflammatory response, the greater the HIV shedding. STIs that present with genital ulcers or genital discharge produce high inflammatory responses and thus have the greatest impact on HIV genital shedding (Buchacz 2004; Kalichman 2011; LeGoff 2007; Plummer 1991; Schaker 1998), for example syphilis and genital herpes (ulcerative STIs) and chlamydia and gonorrhea (non‐ulcerative STIs with genital discharge) each has high genital HIV shedding (Buchacz 2004; Johnson 2008; Paz‐Bailey 2010; Tanton 2010) while HPV, which elicits an insignificant inflammatory response, is not associated with HIV shedding (Muller 2010; Smith 2010). GUD enhances the ability of HIV‐positive people to transmit the virus by increasing HIV shedding, and increases the susceptibility of HIV‐negative individuals to acquire the virus (Cohen 1997; Freeman 2006; Gray 2001; Mayaud 2001; WHO 2001; WHO 2011).
Description of the intervention
Curable GUDs include bacterial conditions such as syphilis, chancroid, lymphogranuloma venereum, and donovanosis. Treatment for these GUDs include benzathine penicillin for syphilis; azithromycin, ceftriaxone, ciprofloxacin, and erythromycin for chancroid; doxycycline and erythromycin for lymphogranuloma venereum; and doxycycline, azithromycin, ciprofloxacin, erythromycin, and trimethoprim‐sulfamethoxazole for donovanosis (CDC 2010). Unlike these bacterial infections, genital herpes is a noncurable, chronic and life‐long viral infection with acute episodes of genital ulcers, which is managed using either short‐duration episodic or long‐duration suppressive therapy with antiviral agents such as acyclovir, famciclovir, and valacyclovir (CDC 2010).
How the intervention might work
GUDs have been shown to increase the risk of HIV acquisition and transmission several times (Barnabas 2011; Brown 2007; Celum 2004; Corey 2004; Freeman 2006; Mayer 2011; Plourde 1994; van de Wijgert 2009; Venkatesh 2011). The increased risk of HIV infection is probably a consequence of GUD breaching the protective mucosal barriers of the genital tract as well as causing genital bleeding, factors that increase the risk of HIV exposure during unprotected sex (Boily 2009; Royce 1997). Therefore, GUD treatment with resolution of the ulcers may reduce sexual acquisition and transmission of HIV (Baeten 2008; Delany 2009; Dunne 2008; Hook 2002;MacDonald 1989; Mayaud 2009; Phiri 2010; Plourde 1992; Wang 2001; Zuckerman 2009).
Why it is important to do this review
Ng and colleagues have published a systematic review of population‐based biomedical STI interventions on the incidence of HIV infection (Ng 2011). The authors included randomized controlled trials in which the unit of randomization was either a community or a treatment facility (Gregson 2007; Grosskurth 1995; Kamali 2003; Wawer 1998), and found no evidence that presumptive STI treatment reduces the incidence of HIV infection. Other trials have been conducted involving participants with confirmed GUD as well as disease‐specific treatment, in which the individual participant was the unit of randomization (Hook 2002; MacDonald 1989; Plourde 1992). Our aim was to conduct a systematic review of these and similar individually randomized controlled trials that have assessed the effects of curative GUD treatment on HIV acquisition.
Objectives
To determine the effects of treatment of genital ulcer disease on sexual acquisition of HIV.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials in which the unit of randomization was the individual with confirmed genital ulcer.
Types of participants
Sexually active men and women with confirmed GUD caused by a curable STI, who were confirmed to be HIV‐negative at the beginning of the study.
Types of interventions
Any treatment intervention aimed at curing GUD compared with an alternative treatment, a placebo, or no treatment. We excluded studies that assessed the effects of suppressive HSV therapy on HIV acquisition (Celum 2008; Watson‐Jones 2008), because this review is focused on curable GUDs.
Types of outcome measures
Primary outcomes
Incidence of HIV infection.
Secondary outcomes
Incidence of other STIs (clinical, microbiological, or both diagnosis), including gonorrhoea, chlamydia, and trichomoniasis.
Adverse events.
Search methods for identification of studies
We compiled detailed search strategies in consultation with the Trials Search Coordinators of the Cochrane Sexually Transmitted Infection Group (in May 2009) and Cochrane HIV and AIDS Group (in July 2010 and August 2011). These search strategies were based on the comprehensive search strategy for the respective Cochrane Review Group in combination with intervention terms and the Cochrane highly sensitive search strategy for randomized controlled trials as published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The sensitive search strategies consisted of both controlled vocabulary terms and free‐text terms. We made every attempt to identify all relevant studies regardless of publication status or language of publication.
Electronic searches
Searches were undertaken in the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (on 26 May 2009, 19 July 2010, and 5 August 2011; Appendix 1), MEDLINE/PubMed (on 26 May 2009, 19 July 2010, and 5 August 2011; Appendix 2), EMBASE (on 26 May 2009, 19 July 2010, and 5 August 2011; Appendix 3), LILACS (on 26 May 2009 and 19 July 2010; Appendix 4), NLM Gateway (on 26 May 2009; Appendix 5), Web of Science (on 26 May 2009 and 19 July 2010; Appendix 6), and AIDS Education Global Information System (AEGIS) (on 5 August 2011; Appendix 7). The searches were, where applicable, carried out from January 1980 to include all studies that may have been carried out since the advent of HIV. In addition, on 2 May 2012 we searched CENTRAL and PubMed to verify if new relevant studies have been published since August 2011.
Searching other resources
The literature search included gray literature such as conference abstracts; handsearching of journals; reference lists of relevant articles, reviews, and guidelines; and contacting experts.
Conference proceedings
We searched proceedings of the International Conference on AIDS and STDs in Africa (ICASA) (on 5 August 2011), the Biennial meeting of the International Society for Sexually Transmitted Diseases Research (on 5 August 2011), and the International Congress of Sexually Transmitted Infections (on 5 August 2011). We searched the conference records from January 1980, or the date of the first conference if the conference started after 1980, to ensure that we identified all relevant studies carried out since the emergence of HIV as a public health problem.
Researchers, organizations, and pharmaceutical companies
On 26 May 2009, 19 July 2010, and 5 August 2011 we searched the WHO International Clinical Trials Registry Platform (ICTRP) (Appendix 8) and ClinicalTrials.gov (Appendix 9) for any ongoing trials on GUD treatment.
Reference lists
We checked reference lists of all the full‐text articles reviewed for eligibility (Gregson 2007; Grosskurth 1995; Hook 2002; Hook 2010; Kamali 2003; MacDonald 1989; Malonza 1999; Moodley 2003; Plourde 1992; Riedner 2005; Rolfs 1997; Tyndall 1994; Wawer 1998) and subject‐related reviews (Ng 2011) and guidelines (CDC 2010).
Data collection and analysis
Selection of studies
FMM and MJM independently assessed the relevance of all identified titles and abstracts and created a list of potentially eligible studies. The two review authors compared the lists and, once agreement was reached, obtained the full texts of all potentially eligible studies. The two review authors independently assessed the eligibility of each full‐text article using a standardized eligibility form with predefined inclusion criteria. The criteria for relevance were based on the study design, participants, interventions, and outcomes. Following the eligibility assessment, each study was classified as included, excluded, ongoing (if the study was not yet completed), or awaiting assessment (if the study was completed but data had not yet been published and if relevant outcome data were not forthcoming from the trial investigators). The excluded studies were listed with documentation of the reasons for exclusion. We contacted the study authors where information required to classify a study was missing, to provide further clarification. We resolved disagreements by discussion and consensus and, when this failed, arbitration by the third review author (CSW). If we had found duplicate publication of findings of an included study, we would have reported the duplicate data as one study.
Data extraction and management
FMM and MJM independently extracted data using a pretested data extraction form. The following data were extracted from each included study:
study details: study design, location and setting, population size, attrition rate;
participant details: study population, demographic, and risk characteristics, inclusion and exclusion criteria, number of patients randomized to each arm;
intervention details: type of intervention, time period for the intervention, length of follow‐up;
outcome details: incidence of HIV infection, incidence of other STIs, incidence of STI re‐infections, types of laboratory tests used to confirm HIV diagnosis before and after treatment of GUD, types of laboratory tests used to confirm diagnosis of the STIs;
study results: number of participants with the outcome and the total number in each study arm for dichotomous outcomes; mean, standard deviation, and total number of participants for continuous outcomes;
additional notes, such as correspondence with study authors, clarification of queries, language of publication of the study, relevant studies identified in the reference list.
Differences in opinion between the two review authors on data extraction were resolved by discussion and, where this failed, arbitration by the third review author (CSW).
Assessment of risk of bias in included studies
Two review authors (FMM, MJM) independently assessed the risk of bias within each included study by addressing seven specific domains, using a pretested assessment form, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The seven domains were random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and 'other issues'.
We assessed the adequacy of allocation sequence generation and allocation sequence concealment to determine the risk of selection bias in each included study. The presence or absence of blinding of the participants and caregivers was recorded to assess the risk of performance bias. We also determined the risk of detection bias in each study by assessing the method of outcome assessment, whether the same method was used in both the treatment and the control arm, and whether there was blinding of outcome assessors. Finally, we assessed the risk of attrition bias by looking at the percentage of withdrawals and losses to follow‐up and how these were treated in the final analyses (whether the authors performed an intention‐to‐treat or a per‐protocol analysis). For each included study, the two review authors independently described what the study authors reported that they did for each domain and then made a decision relating to the risk of bias for that domain; by assigning a judgment of 'low risk' of bias, 'high risk' of bias, or 'unclear risk' of bias. The two review authors compared the results of their independent assessments of risk of bias and resolved any discrepancies by discussion and consensus. Any differences in opinion between FMM and MJM were resolved by discussion, failing which CSW arbitrated.
Measures of treatment effect
All data were dichotomous, so we used data on the number of participants randomized to each group, the number with the outcome of interest, and the number analyzed. These were summarized in 2 x 2 tables and compared using the risk ratio (RR). All effect estimates were presented with 95% confidence intervals (CI).
Unit of analysis issues
We encountered no unit of analysis issues in this review, since we included only studies in which the unit of randomization was the individual.
Dealing with missing data
All authors of eligible studies were contacted for additional data such as HIV status. For each included study, we have described missing data and drop‐outs in the 'Risk of bias' table and assessed the extent to which the missing data may have introduced systematic errors (i.e. attrition bias) to the study findings.
Assessment of heterogeneity
If two or more studies comparing the same interventions were not found or important heterogeneity was found, the meta‐analysis was not possible. We would then have used the I2 test to quantify the degree of heterogeneity as low (l2 value below 40%), moderate (l2 value of 40% to 75%), or high (l2 value above 75%).
Assessment of reporting biases
The protocols of the included studies were assessed for selective outcome reporting (i.e. reporting bias) by comparing what the investigators set out to do with what they reported in the published articles.
Data synthesis
All participants were analyzed in the groups to which they were randomized (i.e. intention‐to‐treat analysis). If we had more than one study comparing the same interventions, in the absence of significant statistical heterogeneity between study results (i.e. heterogeneity P > 0.1), we would have calculated the weighted treatment effect using a fixed‐effect method; otherwise, we would have used the random‐effects method. Fixed‐effect meta‐analysis assumes that each included study is estimating exactly the same quantity while the random‐effects model assumes that the different included studies are estimating different, yet related, intervention effects.
Subgroup analysis and investigation of heterogeneity
If we had performed a meta‐analysis and found significant statistical heterogeneity, we would have been explored possible causes of the inconsistency in study results using subgroup analyses; with subgroups defined by sex of study participants, type of sexual relationship (heterosexual or homosexual), type of intervention, concomitant STI, and underlying cause of the ulcer.
Sensitivity analysis
If we had obtained sufficient trials, we would have conducted a sensitivity analysis to investigate the effect of risk of bias (high/unclear versus low risk of bias) on the robustness of the results.
Results
Description of studies
Results of the search
We obtained 2617 citations in May 2009, 289 citations in July 2010, and 539 citations in August 2011. We used the Endnote reference manager to eliminate 202 duplicate records. We then screened the abstracts of the remaining 3243 records and excluded 3229 records that were clearly irrelevant. At this stage we excluded studies that were not relevant to our review because of the type of study (not randomized controlled trials), participants (e.g. HIV positive at start of study, had nonulcerative STI such as gonorrhea, or had a noncurable ulcerative STI such as genital herpes), or interventions (e.g. behavioral interventions). We identified 14 potentially relevant articles (Gregson 2007; Grosskurth 1995; Hook 2002; Hook 2010; Kamali 2003; MacDonald 1989; Malonza 1999; Moodley 2003; Plourde 1992; Riedner 2005; Rolfs 1997; Tyndall 1994; Wawer 1998). Two articles were reports of the Wawer 1998 study. After reviewing the 14 full‐text articles, we determined that three met our inclusion criteria (Hook 2002; MacDonald 1989; Plourde 1992). Figure 1 summarizes the study search and selection process. Appendix 10 outlines the number of citations identified from the different databases in May 2009, July 2010, and August 2011. We did not identify additional studies from nonelectronic searches.
1.
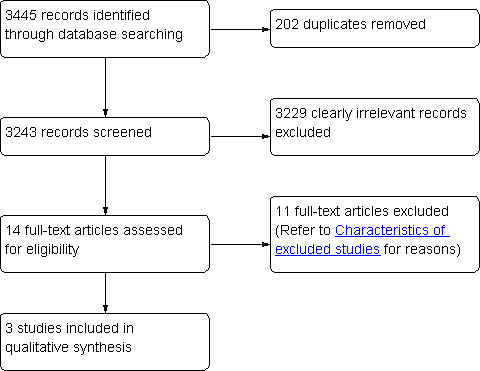
PRISMA flow diagram showing the search and selection of studies.
Included studies
Studies included in this review are Hook 2002, MacDonald 1989, and Plourde 1992. Details of each study are given in the Characteristics of included studies table and are briefly described below:
Hook 2002: Hook and colleagues conducted a randomized, comparative pilot study on HIV‐negative male and female patients aged between 18 and 56 years presenting with primary syphilis at sexually transmitted disease (STD) clinics in Birmingham, Alabama, and New Orleans, Louisiana, US. The study was carried out between October 1995 and December 1997. Thirty participants were randomly assigned using a computer‐generated randomization code to receive one of three regimens: azithromycin 2.0 g administered as a single oral dose (eight participants); two 2.0 g oral doses of azithromycin (11 participants), administered six to eight days apart; benzathine penicillin G (11 participants), administered as either 2.4 million units intramuscularly once in Birmingham or twice, seven days apart, in New Orleans. Three participants were later found to have been HIV‐positive at baseline (two received 2.4 benzathine penicillin G, and one received two 2.0 g azithromycin doses one week apart). HIV‐negative participants were retested at six and 12 months.
MacDonald 1989: MacDonald and colleagues conducted a randomized controlled trial on HIV‐positive and HIV‐negative men aged between 18 and 60 years presenting with GUD due to Haemophilus ducreyi at the Nairobi City Council Special Treatment Clinic in Kenya. The dates of the study were not reported. A total of 45 HIV‐negative participants were randomized to receive either 200 mg (group 1: 27 participants) or 400 mg (group 2: 18 participants) of oral fleroxacin as a single dose. Participants were followed up on days three, seven, 14, and 28 after randomization. HIV testing was done at enrolment and at follow‐up.
Plourde 1992: Plourde and colleagues conducted a randomized controlled trial on HIV‐seronegative men aged between 18 and 65 years presenting with genital ulcers due to Haemophilus ducreyi at the Nairobi City Council Special Treatment Clinic in Kenya. The study was conducted between July 1989 and February 1990. A total of 98 participants were enrolled in the study and randomly assigned in equal numbers to receive either a single 400 mg oral dose of fleroxacin or 800 mg of sulfamethoxazole and 160 mg of trimethoprim (TMP‐SMZ) (Bactrim DS, Roche) orally twice daily for three days. Eight participants in each group were lost to follow‐up. Five participants in the fleroxacin group (three with positive syphilis serology and two with negative Haemophilus ducreyi cultures) were excluded from further analyses; four participants in the TMP‐SMZ group with positive syphilis serology were excluded from further analyses. This study was terminated early due to a high overall clinical failure rate. Only a total of 24 participants were followed up for eight or more weeks, 12 in each treatment arm. These 24 participants were tested for HIV after eight to 12 weeks of follow‐up.
None of the three studies assessed the incidence of other STIs during the follow‐up period.
Excluded studies
See: Characteristics of excluded studies tables.
Eleven potentially eligible studies (Gregson 2007; Grosskurth 1995; Hook 2010; Kamali 2003; Malonza 1999; Moodley 2003; Riedner 2005; Rolfs 1997; Tyndall 1994; Wawer 1998) were excluded. Two articles were reports of the Wawer 1998 study. The Moodley 2003 study was excluded because it was not a randomized controlled trial. Five individually randomized controlled trials (Hook 2010; Malonza 1999; Riedner 2005; Rolfs 1997; Tyndall 1994) were excluded because they did not report HIV incidence as an outcome and the remaining four studies (Gregson 2007; Grosskurth 1995; Kamali 2003; Wawer 1998) were excluded because their unit of randomization was a community or health facility, rather than an individual with confirmed GUD.
Risk of bias in included studies
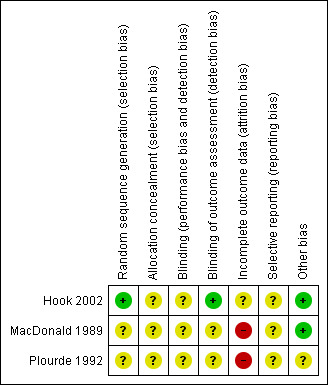
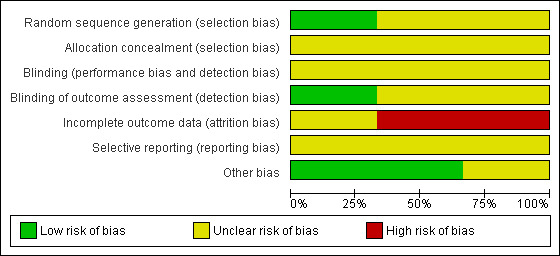
We have summarized the risk of bias in included studies in Figure 2 and Figure 3, and provide a brief description for each study below.
2.
3.
Hook 2002: allocation sequence was adequately done using a computer‐generated randomization code. It is unclear how the allocation concealment was carried out. There is no information on blinding of the participants and the personnel although this would not have been easy given that one treatment arm received an injection while the other received oral medication. The outcome assessors were blinded to all clinical data. The attrition rate of patients with primary syphilis was not reported. Overall there are differences in the attrition rate between the treatment arms (follow‐up at 12 months was 48% in the benzathine penicillin; 67% in the single‐dose azithromycin; and 69% in the two‐dose azithromycin). It was unclear to us whether there was selective reporting in this study or not, since we did not have access to the study protocol. In this trial, we did not have any reason to believe that biases other than those listed above could have been introduced.
MacDonald 1989: allocation sequence generation was unclear. The authors stated that individuals were randomly assigned to the treatment arms but it is unclear how randomization was achieved. It is also unclear whether allocation concealment was carried out. There was no information on blinding of the participants, personnel, and outcome assessors. There was a high risk of attrition bias in this study, as loss to follow‐up was significantly higher in one treatment arm (17% versus 0%). It was unclear to us whether there was selective reporting in this study or not, since we did not have access to the study protocol. In this trial, we did not have any reason to believe that biases other than those listed above could have been introduced.
Plourde 1992: allocation sequence generation was unclear. The authors state that eligible participants were randomized but it is unclear how this randomization was achieved. It is also unclear whether allocation concealment was carried out. Packaging of the drugs was such that the participants all completed an identical three‐day course. However, there is no information as to whether the drugs were identical in all other aspects (appearance and taste). It is also unclear whether the personnel and outcome assessors were blinded. There were 49 participants assigned to each treatment arm but only 12 in each arm were followed up for eight weeks or longer giving an attrition rate of 76% in each treatment arm. The trial was terminated early because of data‐dependent reasons. It was unclear to us whether there was selective reporting in this study or not, since we did not have access to the study protocol.
Effects of interventions
Three randomized controlled trials that met our inclusion criteria, recruited participants with chancroid (two trials with 143 participants; MacDonald 1989; Plourde 1992) and primary or secondary syphilis (one trial with 30 participants; Hook 2002). These three trials evaluated HIV seroconversions at the end of the trial period.
Treatment of syphilis
There were three treatment arms in the trial by Hook et al (Hook 2002), one that received benzathine penicillin G and the other two received azithromycin in two different doses. There were no HIV seroconversions by the end of the 12‐month study period (Analysis 1.1; Analysis 1.2; Table 1).
1.1. Analysis.
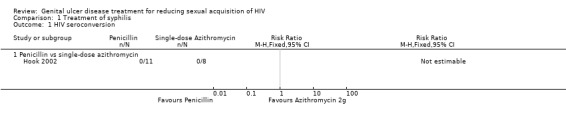
Comparison 1 Treatment of syphilis, Outcome 1 HIV seroconversion.
1.2. Analysis.
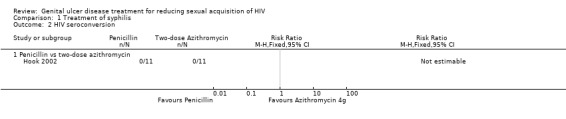
Comparison 1 Treatment of syphilis, Outcome 2 HIV seroconversion.
The study authors did not report data on the incidence of other STIs in the follow‐up period. All adverse events in this trial were described as being mild to moderate in severity (Hook 2002). Five (24%) penicillin recipients and nine (17%) azithromycin recipients had Jarisch‐Herxheimer reactions. These differences in Jarisch‐Herxheimer reactions were not significant. Gastrointestinal adverse events were also found in both treatment arms but were more common in the azithromycin treatment group. Fifty‐two participants on azithromycin and 19 on penicillin returned for one‐ or two‐week follow‐up visits. Of these, gastrointestinal events reported included vomiting (one event), nausea (seven events (13%)) and diarrhea (five events (10%)) for the azithromycin group and nausea (one event (5%)) for penicillin recipients. These differences in gastrointestinal events were not significant (RR 4.75; 95% CI 0.67 to 33.9).
Treatment of chancroid
Two trials focused on treatment of chancroid (MacDonald 1989; Plourde 1992).
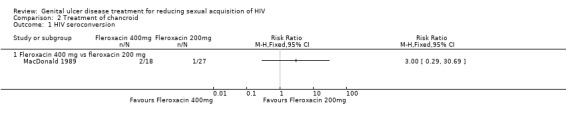
In the MacDonald 1989 study, two of the 18 participants on 400 mg fleroxacin and one of the 27 participants receiving 200 mg fleroxacin tested HIV positive by the last follow‐up visit on day 28 (RR 3.00; 95% CI 0.29 to 30.69; Analysis 2.1; Table 2).
2.1. Analysis.
Comparison 2 Treatment of chancroid, Outcome 1 HIV seroconversion.
The MacDonald 1989 study investigators did not report data on adverse events. In addition, the authors did not report the effects of the treatment on the incidence of other STIs.
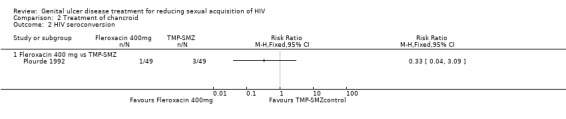
Of the 12 participants included in the final analyses in the Plourde 1992 study, one in the fleroxacin arm and three in the TMP‐SMZ arm seroconverted by the end of eight weeks. There were no significant differences in the rate of HIV seroconversion at eight to 12 weeks in the two treatment arms (RR 0.33; 95% CI 0.04 to 3.09; Analysis 2.2; Table 2).
2.2. Analysis.
Comparison 2 Treatment of chancroid, Outcome 2 HIV seroconversion.
The authors did not report the effects of the treatment on the incidence of other STIs in the follow‐up period (Plourde 1992). In the fleroxacin group, four of 41 (10%) participants reported adverse events. These included nonspecific gastrointestinal complaints (one event), fever (one event), and moderate dizziness and insomnia (two events). In the TMP‐SMZ group, two of 41 (5%) participants reported adverse events. These included serum‐like sickness (one event) and mild nausea (one event). The differences in adverse events between the two groups were not significant.
Using GRADE methodology, we rated the quality of this evidence on the effects of GUD treatment on sexual acquisition of HIV to be of very low quality (Table 1; Table 2).
Discussion
Summary of main results
We found only three individually randomized controlled trials on the treatment of GUD that have assessed the effects of such treatment on HIV acquisition. The trials together provide insufficient evidence to evaluate the effects of treatment of GUD on the sexual acquisition of HIV.
Overall completeness and applicability of evidence
The strength of this review lies in our adherence to standardized guidelines, including a focused question, clearly defined selection criteria, duplicate study selection and data extraction, and an a priori procedure for resolution of conflicts (Higgins 2011).
Two of the three trials assessed the effect of treatment of chancroid (MacDonald 1989;Plourde 1992) but compared the effect of different antimicrobial agents therefore a meta‐analysis was not possible, while the third trial (Hook 2002) assessed the treatment of syphilis. Two of the trials were carried out in the same clinic in a low‐income country (MacDonald 1989;Plourde 1992) and included male participants only. The other trial was carried out in two settings in a high‐income country and included both men and women (Hook 2002). The HIV testing method included an enzyme‐linked immunosorbent assay (ELISA) and Western Blot for two of the trials (MacDonald 1989;Plourde 1992) and is not reported for the third (Hook 2002). The duration of follow‐up for the MacDonald 1989 and Plourde 1992 studies may have made it difficult to identify participants who may have seroconverted after the follow‐up period since the diagnostic tests that were used were antibody‐based and take a longer time to detect seroconverters. This may also have been the case in the participant recruitment in that those who seroconverted in the course of the trial may have acquired the virus prior to enrolment in the trial. The use of more specific laboratory tests would have been useful to make a clear‐cut selection of HIV‐negative participants and ensure that seroconverters had only acquired the infection during the study period. The quality of the evidence from the two trials (MacDonald 1989;Plourde 1992) in the low‐income country was very low (Balshem 2011;Guyatt 2008) hence the results of these trials may not be generalizable to the rest of the country in which they were carried out or to other low‐ or middle‐income countries. The quality of evidence for the trial carried out in a high‐income country (Hook 2002) was also very low, hence may not be generalizable to other settings in industrialized countries.
The interaction between GUD and nonulcerating inflammatory STIs and HIV infection is biologically plausible with strong associations having been demonstrated (Cameron 1989; Cohen 1997; Fleming 1999; Laga 1993; Wasserheit 1992). There is also evidence that the presence of an STI is accompanied by increased HIV shedding, a feature that reduces with treatment of the STI (Baeten 2008; Delany 2009; Dunne 2008; Wang 2001; Zuckerman 2009). However, Ng and colleagues conducted a systematic review of cluster‐randomized trials on presumptive treatment of STIs and found no evidence that STI treatment is an effective HIV prevention strategy (Ng 2011).
Quality of the evidence
We rated the quality of the evidence reported in this review according to the recommendations of the GRADE Working Group which have been adopted by The Cochrane Collaboration (Guyatt 2008; Higgins 2011). At present, the quality of the evidence on the effectiveness of genital ulcer treatment to reduce the risk of HIV acquisition is very low (Table 1; Table 2); implying that we are uncertain about the estimated treatment effect (Balshem 2011).
Potential biases in the review process
We minimized potential biases in the process of preparing this review by adhering to standard Cochrane procedures outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Agreements and disagreements with other studies or reviews
Ng and colleagues conducted a systematic review to determine the impact of population‐based biomedical STI interventions on the incidence of HIV infection (Ng 2011). The authors looked for "randomised controlled trials involving one or more biomedical interventions in general populations (as opposed to occupationally or behaviourally‐defined groups, such as sex workers) in which the unit of randomisation was either a community or a treatment facility and in which the primary outcome was incident HIV infection". The term 'community' was interpreted to include a group of villages, an arbitrary geographical division, or the catchment population of a group of health facilities." (Ng 2011) These selection criteria are different from ours i.e. individually‐randomized controlled trials that assessed the effects of curative treatment of confirmed GUD on sexual HIV acquisition. Despite the differences in selection criteria, the finding by Ng and colleagues that STI treatment may not reduce the risk of HIV infection is in agreement with ours. However, the evidence from our review is weakened by the paucity of relevant data.
Authors' conclusions
Implications for practice.
At present, there is insufficient evidence to determine whether treatment of GUD would reduce the risk of HIV acquisition. The very low quality of the evidence implies that the true effect of GUD treatment on sexual acquisition of HIV may be substantially different from the effect estimated from currently available data. However, GUDs are public health problems in their own right and patients with these conditions should be treated appropriately; whether the treatment reduces the risk of HIV infection or not.
Implications for research.
Further randomized controlled trials on the treatment of GUD should be of high quality and include HIV transmission or acquisition (as appropriate) as outcomes. The studies can also assess the rate of reduction in the HIV ribonucleic acid (RNA) concentration in the genital fluid (in case of studies involving HIV‐infected people) during the treatment period.
Acknowledgements
Dr. Florence M. Mutua was awarded a Reviews for Africa Programme Fellowship (www.mrc.ac.za/cochrane/rap.htm), funded by a grant from the Nuffield Commonwealth Programme, through the Nuffield Foundation. We would like to acknowledge the support provided by the staff of the Cochrane Sexually Transmitted Infections Group, the South African Cochrane Centre, and the Cochrane HIV Review Group.
Appendices
Appendix 1. CENTRAL*: date range: 1 January 1980 ‐ 5 August 2011
| Search set | |
| 1 | ("Sexually Transmitted Diseases"[Mesh] OR "Sexually Transmitted Diseases, Bacterial"[Mesh] OR "Sexually Transmitted Diseases, Viral"[Mesh]) OR (sexually transmitted disease*:ti,ab,kw OR sexually transmissible disease*:ti,ab,kw OR sexually transmitted infection*:ti,ab,kw OR sexually transmissible infection*:ti,ab,kw OR sexually transmitted infectious disease*:ti,ab,kw OR sexually transmissible infectious disease*:ti,ab,kw OR sexually transmitted disorder*:ti,ab,kw OR sexually transmissible disorder*:ti,ab,kw OR STI:ti,ab,kw OR STD:ti,ab,kw OR genital ulcer*:ti,ab,kw OR genital ulcer disease*:ti,ab,kw OR ulcerative sexually transmitted*:ti,ab,kw OR genital infection*:ti,ab,kw OR genital disorder*:ti,ab,kw OR venereal disease*:ti,ab,kw OR venereal infection*:ti,ab,kw OR venereal disorder*:ti,ab,kw OR herpes simplex:ti,ab,kw OR herpes genitalis:ti,ab,kw OR genital herpes:ti,ab,kw OR herpes virus:ti,ab,kw OR HSV‐1:ti,ab,kw OR HSV‐2:ti,ab,kw OR donovanosis:ti,ab,kw OR granuloma inguinale:ti,ab,kw OR calymmatobacterium granulomatis:ti,ab,kw OR donovania:ti,ab,kw OR klebsiella granulomatis:ti,ab,kw OR syphilis:ti,ab,kw OR treponema pallidum:ti,ab,kw OR chancre:ti,ab,kw OR primary syphilis:ti,ab,kw OR secondary syphilis:ti,ab,kw OR condylomata lata:ti,ab,kw OR chancroid:ti,ab,kw OR haemophilus ducreyi:ti,ab,kw OR soft chancre:ti,ab,kw OR lymphogranuloma venereum:ti,ab,kw OR chlamydia trachomatis:ti,ab,kw OR chlamydia infections:ti,ab,kw OR LGV:ti,ab,kw OR genital ulcer*:ti,ab,kw OR anogenital ulcer*:ti,ab,kw OR anorectal ulcer*:ti,ab,kw OR anorectal ulcer*:ti,ab,kw OR penile ulcer*:ti,ab,kw) OR ("Herpes Genitalis"[Mesh]) OR ("Granuloma Inguinale"[Mesh] OR "Calymmatobacterium"[Mesh]) OR ("Syphilis"[Mesh]) OR ("Chlamydia trachomatis"[Mesh] OR "Lymphogranuloma Venereum"[Mesh]) OR |
| 2 | (HIV infections:ti,ab,kw) OR HIV:ti,ab,kw OR HIV‐1*:ti,ab,kw OR HIV‐2*:ti,ab,kw OR HIV1:ti,ab,kw OR HIV2:ti,ab,kw OR (HIV infect*:ti,ab,kw) OR (human immunodeficiency virus:ti,ab,kw) OR (human immunedeficiency virus:ti,ab,kw) OR (human immuno‐deficiency virus:ti,ab,kw) OR (human immune‐deficiency virus:ti,ab,kw) OR ((human immun*:ti,ab,kw) and (deficiency virus:ti,ab,kw)) OR (acquired immunodeficiency syndrome:ti,ab,kw) OR (acquired immunedeficiency syndrome:ti,ab,kw) OR (acquired immuno‐deficiency syndrome:ti,ab,kw) OR (acquired immune‐deficiency syndrome:ti,ab,kw) OR ((acquired immun*:ti,ab,kw) AND (deficiency syndrome:ti,ab,kw)) OR (HIV Infections[MeSH] OR HIV[MeSH]) |
| 3 | ("Azithromycin"[Mesh] OR "Ceftriaxone"[Mesh] OR "Ciprofloxacin"[Mesh] OR "Erythromycin"[Mesh] OR "Acyclovir"[Mesh] OR "famciclovir "[Substance Name] OR "valacyclovir [Substance Name]" OR "Penicillin G"[Mesh] OR "Penicillin G, Benzathine"[Mesh] OR "Doxycycline"[Mesh] OR "Trimethoprim‐Sulfamethoxazole Combination"[Mesh]) OR (azithromycin:ti,ab,kw OR ceftriaxone:ti,ab,kw OR ciprofloxacin:ti,ab,kw OR erythromycin base:ti,ab,kw OR acyclovir:ti,ab,kw OR famciclovir:ti,ab,kw OR valacyclovir:ti,ab,kw OR penicillin G:ti,ab,kw OR benzathine penicillin:ti,ab,kw OR doxycycline:ti,ab,kw OR trimethoprim sulphamethoxazole:ti,ab,kw) |
| 4 | 1 AND 2 AND 3 AND 4 |
| *Cochrane Central Register of Controlled Trials |
Appendix 2. Medline (PubMed): date range: 1 January 1980 ‐ 5 August 2011
| Search set | |
| 1 | ("Sexually Transmitted Diseases"[Mesh] OR "Sexually Transmitted Diseases, Bacterial"[Mesh] OR "Sexually Transmitted Diseases, Viral"[Mesh]) OR (sexually transmitted disease*[tiab] OR sexually transmissible disease*[tiab] OR sexually transmitted infection*[tiab] OR sexually transmissible infection*[tiab] OR sexually transmitted infectious disease*[tiab] OR sexually transmissible infectious disease*[tiab] OR sexually transmitted disorder*[tiab] OR sexually transmissible disorder*[tiab] OR STI[tiab] OR STD[tiab] OR genital ulcer*[tiab] OR genital ulcer disease*[tiab] OR ulcerative sexually transmitted*[tiab] OR genital infection*[tiab] OR genital disorder*[tiab] OR venereal disease*[tiab] OR venereal infection*[tiab] OR venereal disorder*[tiab]) OR ("Herpes Simplex"[Mesh] OR "Herpes Genitalis"[Mesh] OR herpes simplex[tiab] OR herpes genitalis[tiab] OR genital herpes[tiab] OR herpes virus[tiab] OR HSV‐1[tiab] OR HSV‐2[tiab]) OR ("Granuloma Inguinale"[Mesh] OR "Calymmatobacterium"[Mesh] OR donovanosis[tiab] OR granuloma inguinale[tiab] OR calymmatobacterium granulomatis[tiab] OR donovania[tiab] OR klebsiella granulomatis[tiab]) OR ("Syphilis"[Mesh] OR syphilis[tiab] OR treponema pallidum[tiab] OR chancre[tiab] OR primary syphilis[tiab] OR secondary syphilis[tiab] OR condylomata lata[tiab]) OR (chancroid [tiab] OR haemophilus ducreyi[tiab] OR soft chancre[tiab]) OR ("Chlamydia trachomatis"[Mesh] OR "Lymphogranuloma Venereum"[Mesh] OR lymphogranuloma venereum[tiab] OR chlamydia trachomatis[tiab] OR chlamydia infections[tiab] OR LGV[tiab]) OR (genital ulcer*[tiab] OR anogenital ulcer*[tiab] OR anorectal ulcer*[tiab] OR anorectal ulcer*[tiab] OR penile ulcer*[tiab]) |
| 2 | HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR “sexually transmitted diseases, viral” [MESH:NoExp] |
| 3 | ("Azithromycin"[Mesh] OR azithromycin[tiab] OR "Ceftriaxone"[Mesh] OR ceftriaxone[tiab] OR "Ciprofloxacin"[Mesh] OR ciprofloxacin[tiab] OR "Erythromycin"[Mesh] OR erythromycin base[tiab] OR "Acyclovir"[Mesh] OR acyclovir[tiab] OR "famciclovir "[Substance Name] OR famciclovir[tiab] OR "valacyclovir [Substance Name]" OR valacyclovir[tiab] OR "Penicillin G"[Mesh] OR penicillin G[tiab] OR "Penicillin G, Benzathine"[Mesh] OR benzathine penicillin[tiab] OR "Doxycycline"[Mesh] OR doxycycline[tiab] OR "Trimethoprim‐Sulfamethoxazole Combination"[Mesh] OR trimethoprim sulphamethoxazole[tiab]) |
| 4 | ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) AND (humans[mh]) |
| 5 | 1 AND 2 AND 3 AND 4 Limits: Publication Date from 1980/01/01 to 2011/08/05 |
| **Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term |
Appendix 3. EMBASE: date range: 1 January 1980 ‐ 5 August 2011
| Search set | |
| 1 | 'human immunodeficiency virus infection' /exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus'OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| 2 | 'sexually transmitted diseases'/exp OR 'sexually transmitted diseases, bacterial'/exp OR 'sexually transmitted diseases, viral'/exp OR (sexually AND transmitted AND disease*:ti OR sexually AND transmitted AND disease*:ab) OR (sexually AND transmissible AND disease*:ti OR sexually AND transmissible AND disease*:ab) OR (sexually AND transmitted AND infection*:ti OR sexually AND transmitted AND infection*:ab) OR (sexually AND transmissible AND infection*:ti OR sexually AND transmissible AND infection*:ab) OR (sexually AND transmitted AND infectious AND disease*:ti OR sexually AND transmitted AND infectious AND disease*:ab) OR (sexually AND transmissible AND infectious AND disease*:ti OR sexually AND transmissible AND infectious AND disease*:ab) OR (sexually AND transmitted AND disorder*:ti OR sexually AND transmitted AND disorder*:ab) OR (sexually AND transmissible AND disorder*:ti OR sexually AND transmissible AND disorder*:ab) OR sti:ti OR sti:ab OR std:ti OR std:ab OR (genital AND ulcer*:ti OR genital AND ulcer*:ab) OR (genital AND 'ulcer'/exp AND disease*:ti OR genital AND 'ulcer'/exp AND disease*:ab) OR (ulcerative AND sexually AND transmitted*:ti OR ulcerative AND sexually AND transmitted*:ab) OR (genital AND infection*:ti OR genital AND infection*:ab) OR (genital AND disorder*:ti OR genital AND disorder*:ab) OR (venereal AND disease*:ti OR venereal AND disease*:ab) OR (venereal AND infection*:ti OR venereal AND infection*:ab) OR (venereal AND disorder*:ti OR venereal AND disorder*:ab) OR 'herpes simplex'/exp OR 'herpes genitalis'/exp OR ('herpes'/exp AND simplex:ti OR 'herpes'/exp AND simplex:ab) OR ('herpes'/exp AND genitalis:ti OR 'herpes'/exp AND genitalis:ab) OR (genital AND herpes:ti OR genital AND herpes:ab) OR ('herpes'/exp AND virus:ti OR 'herpes'/exp AND virus:ab) OR 'hsv 1':ti OR 'hsv 1':ab OR 'hsv 2':ti OR 'hsv 2':ab OR donovanosis:ti OR donovanosis:ab OR ('granuloma'/exp AND inguinale:ti OR 'granuloma'/exp AND inguinale:ab) OR ('calymmatobacterium'/exp AND granulomatis:ti OR 'calymmatobacterium'/exp AND granulomatis:ab) OR donovania:ti OR donovania:ab OR ('klebsiella'/exp AND granulomatis:ti OR 'klebsiella'/exp AND granulomatis:ab) OR 'syphilis'/exp OR syphilis:ti OR syphilis:ab OR ('treponema'/exp AND pallidum:ti OR 'treponema'/exp AND pallidum:ab) OR chancre:ti OR chancre:ab OR (primary AND syphilis:ti OR primary AND syphilis:ab) OR (secondary AND syphilis:ti OR secondary AND syphilis:ab) OR ('condylomata'/exp AND lata:ti OR 'condylomata'/exp AND lata:ab) OR chancroid:ti OR chancroid:ab OR ('haemophilus'/exp AND ducreyi:ti) OR (soft AND chancre:ti OR soft AND chancre:ab) OR 'chlamydia trachomatis'/exp OR 'lymphogranuloma venereum'/exp OR (lymphogranuloma AND venereum:ti OR lymphogranuloma AND venereum:ab) OR ('chlamydia'/exp AND trachomatis:ti OR 'chlamydia'/exp AND trachomatis:ab) OR ('chlamydia'/exp AND infections:ti OR 'chlamydia'/exp AND infections:ab) OR lgv:ti OR lgv:ab OR (vaginal AND ulcer*:ti OR vaginal AND ulcer*:ab) OR (anogenital AND ulcer*:ti OR anogenital AND ulcer*:ab) OR (anorectal AND ulcer*:ti OR anorectal AND ulcer*:ab) OR (penile AND ulcer*:ti OR penile AND ulcer*:ab) |
| 3 | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomized controlled trial'/exp OR 'randomized controlled trial'/de OR 'randomized controlled trial' |
| 4 | 'azithromycin'/de OR azithromycin OR 'ceftriaxone'/de OR ceftriaxone OR 'ciprofloxacin'/de OR ciprofloxacin OR 'erythromycin base'/de OR 'erythromycin base' OR 'acyclovir'/de OR acyclovir OR 'famciclovir'/de OR famciclovir OR 'valacyclovir'/de OR valacyclovir OR 'penicillin g'/de OR 'penicillin g' OR 'penicillin g, benzathine'/de OR 'penicillin g, benzathine' OR 'benzathine penicillin'/de OR 'benzathine penicillin' OR 'doxycycline'/de OR doxycycline OR 'trimethoprim‐sulfamethoxazole combination'/de OR 'trimethoprim‐sulfamethoxazole combination' OR 'trimethoprim sulphamethoxazole'/de OR 'trimethoprim sulphamethoxazole' |
| 5 | #1 AND #2 AND #3 AND #4 |
| 6 | #1 AND #2 AND #3 AND #4 AND [humans]/lim AND [embase]/lim AND [1980‐2011]/py |
Appendix 4. LILACS: date range: 1 January 1980 ‐ 19 July 2010
| Search set | |
| 1 | azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole [Palavras do resumo] OR azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole [Palavras do título] AND ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Palavras] |
Appendix 5. NLM Gateway: date range: 1 January 1980 ‐ 26 May 2009
| Search set | |
| 1 | azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole |
| 2 | herpes genitalis OR genital herpes OR herpes virus OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR condylomata lata OR chancroid OR haemophilus ducreyi OR lymphogranuloma venereum OR chlamydia OR genital ulcer OR anogenital ulcer OR anorectal ulcer OR anorectal ulcer OR penile ulcer |
| 3 | HIV OR HIV‐1 OR HIV‐2 OR human immunodeficiency virus OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome |
| 4 | (azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole) AND (herpes genitalis OR genital herpes OR herpes virus OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR condylomata lata OR chancroid OR haemophilus ducreyi OR lymphogranuloma venereum OR chlamydia OR genital ulcer OR anogenital ulcer OR anorectal ulcer OR anorectal ulcer OR penile ulcer) AND (HIV OR HIV‐1 OR HIV‐2 OR human immunodeficiency virus OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome) |
| 5 | herpes genitalis OR genital herpes OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR condylomata lata OR haemophilus ducreyi OR lymphogranuloma venereum OR chlamydia OR genital ulcer |
| 6 | (azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole) AND (HIV OR HIV‐1 OR HIV‐2 OR human immunodeficiency virus OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome) AND (herpes genitalis OR genital herpes OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR condylomata lata OR haemophilus ducreyi OR lymphogranuloma venereum OR chlamydia OR genital ulcer) |
Appendix 6. Web of Science: date range: 1 January 1980 ‐ 19 July 2010
| Search set | |
| 1 | TI=(sexually transmitted disease* OR sexually transmissible disease* OR sexually transmitted infection* OR sexually transmissible infection* OR sexually transmitted infectious disease* OR sexually transmissible infectious disease* OR sexually transmitted disorder* OR sexually transmissible disorder* OR STI OR STD OR genital ulcer* OR genital ulcer disease* OR ulcerative sexually transmitted* OR genital infection* OR genital disorder* OR venereal disease* OR venereal infection* OR venereal disorder* OR herpes simplex OR herpes genitalis OR genital herpes OR herpes virus OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR donovania OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR primary syphilis OR secondary syphilis OR condylomata lata OR chancroid OR haemophilus ducreyi OR soft chancre OR lymphogranuloma venereum OR chlamydia trachomatis OR chlamydia infections OR LGV OR genital ulcer* OR anogenital ulcer* OR anorectal ulcer* OR anorectal ulcer* OR penile ulcer*) |
| 2 | TS=(sexually transmitted disease OR sexually transmissible disease OR sexually transmitted infection OR sexually transmissible infection OR sexually transmitted infectious disease OR sexually transmissible infectious disease OR sexually transmitted disorder OR sexually transmissible disorder OR STI OR STD OR genital ulcer disease OR ulcerative sexually transmitted OR genital infection OR genital disorder OR venereal disease OR venereal infection OR venereal disorder OR herpes simplex OR herpes genitalis OR genital herpes OR herpes virus OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR donovania OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR primary syphilis OR secondary syphilis OR condylomata lata OR chancroid OR haemophilus ducreyi OR soft chancre OR lymphogranuloma venereum OR chlamydia trachomatis OR chlamydia infections OR LGV OR genital ulcer OR anogenital ulcer OR anorectal ulcer OR anorectal ulcer OR penile ulcer) |
| 3 | TS=(sexually transmitted diseases OR sexually transmissible diseases OR sexually transmitted infections OR sexually transmissible infections OR sexually transmitted infectious diseases OR sexually transmissible infectious diseases OR sexually transmitted disorders OR sexually transmissible disorders OR genital ulcer diseases OR genital infections OR genital disorders OR venereal diseases OR venereal infections OR venereal disorders OR genital ulcers OR anogenital ulcers OR anorectal ulcers OR anorectal ulcers OR penile ulcers) |
| 4 | 1 OR 2 OR 3 |
| 5 | TI=(HIV infections OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR HIV infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome) |
| 6 | TI=(human immun* AND deficiency virus) |
| 7 | TI=(acquired immun* AND deficiency syndrome) |
| 8 | TS=(HIV infections OR HIV OR HIV‐1 OR HIV‐2 OR HIV1 OR HIV2 OR HIV infection OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome) |
| 9 | 5 OR 6 OR 7 OR 8 |
| 10 | TI=(azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole) |
| 11 | TS=(azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole) |
| 12 | 10 OR 11 |
| 13 | TI=((randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups) AND (humans)) |
| 14 | TS=((randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups) AND (humans)) |
| 15 | 13 OR 14 |
| 16 | 4 AND 9 AND 12 AND 15 |
| *Cochrane Central Register of Controlled Trials |
Appendix 7. AEGIS: date range: 1 January 1980 ‐ 5 August 2011
| Search set | |
| 1 | (genital ulcer* OR sexually transmitted or sti*) AND (azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole) |
Appendix 8. WHO ICTRP PORTAL: date range: 1 January 1980 ‐ 5 August 2011
| Search set | |
| 1 | (azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin OR acyclovir OR famciclovir OR valacyclovir OR penicillin OR doxycycline OR trimethoprim sulphamethoxazole) in the INTERVENTION AND ulcer* OR sexually transmit* OR STI* OR STD* OR venereal OR herpes OR HSV‐1 OR HSV‐2 OR donovanosis OR granuloma inguinale OR calymmatobacterium granulomatis OR donovania OR klebsiella granulomatis OR syphilis OR treponema pallidum OR chancre OR syphilis OR syphillis OR condylomata lata OR chancroid OR haemophilus ducreyi OR soft chancre OR lymphogranuloma venereum OR chlamydia trachomatis OR chlamydia infections in the CONDITION; RECRUITMENT STATUS = ALL; DATE OF REGISTRATION = BETWEEN 1 JANUARY 2010 – 5 AUGUST 2011 |
| **Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term |
Appendix 9. ClinicalTrials.gov: date range: 1 January 1980 ‐ 5 August 2011
| Search set | |
| 1 | (sexually transmitted infections OR ulcer) AND (azithromycin OR ceftriaxone OR ciprofloxacin OR erythromycin base OR acyclovir OR famciclovir OR valacyclovir OR penicillin G OR benzathine penicillin OR doxycycline OR trimethoprim sulphamethoxazole) | Interventional Studies | received from 01/01/1980 to 08/05/2011 |
Appendix 10. Citations
| Electronic database |
Number of citations in 2009 |
Number of citations in 2010 |
Number of citations in 2011 |
| CENTRAL | 179 | 15 | 17 |
| MEDLINE (PubMed) | 2016 | 7 | 114 |
| EMBASE | 4 | 197 | 45 |
| LILACS | 37 | 5 | |
| NLM Gateway | 152 | ||
| Web of Science | 24 | 3 | |
| WHO ICTRP PORTAL | 77 | 45 | 28 |
| ClinicalTrials.gov | 128 | 17 | 166 |
| AEGIS | 169 | ||
| TOTAL | 2617 | 289 | 539 |
Data and analyses
Comparison 1. Treatment of syphilis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV seroconversion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Penicillin vs single‐dose azithromycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 HIV seroconversion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Penicillin vs two‐dose azithromycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Treatment of chancroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HIV seroconversion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fleroxacin 400 mg vs fleroxacin 200 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 HIV seroconversion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fleroxacin 400 mg vs TMP‐SMZ | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hook 2002.
| Methods | Randomized comparative pilot study, carried out at STD clinics in Birmingham, Alabama, and New Orleans, Louisiana (US) between October 1995 and December 1997 Participants were randomly allocated by means of a computer‐generated randomization code to receive 1 of 3 treatment regimens |
|
| Participants | HIV seropositive and seronegative patients were eligible if they had early (primary, secondary, or early latent) syphilis Primary syphilis was defined on the basis of positive dark‐field microscopy for Treponema pallidum on lesion exudate Secondary syphilis was defined as a clinically typical or dark‐field‐positive cutaneous eruption and RPR titer ≥ 1:8, and a reactive MHA‐TP test or FTA‐ABS test. Enrolled patients had to be willing to return for study‐related follow‐up visits for 1 year, and aged ≥ 18 years Exclusion criteria for the study were pregnancy; breastfeeding; allergy to beta‐lactam or macrolide antibiotics; history of intravenous drug abuse; history of use of antibiotics active against T pallidum or use of an investigational drug in the 30 days preceding enrolment (use of quinolone, sulfonamide, trimethoprim, metronidazole, and spectinomycin antibiotics was allowed); known or suspected coexistent STDs requiring treatment with drugs effective against T pallidum; advanced HIV infection manifested by a history of opportunistic infection; known severe liver or renal disease; and unreliability (as considered likely by the investigators) for participating in the study procedures and prompt follow‐up Urine pregnancy tests were performed for all women at the initial visit |
|
| Interventions | Group 1: azithromycin, 2.0 g administered as a single oral dose Group 2: two 2.0 g oral doses of azithromycin, administered 6 to 8 days apart Group 3: benzathine penicillin G, administered as either 2.4 million units intramuscularly once in Birmingham or twice, 7 days apart, in New Orleans Participants were seen at 7 and 14 days after initiation of treatment and then 1, 3, 6, 9, and 12 months after initiation of therapy. At each visit patients provided an interval history of sexual exposure, were clinically evaluated for persistent or recurrent syphilis, and had a serum specimen obtained for syphilis serological testing. Consenting patients who were initially HIV‐seronegative were retested for HIV at 6 and 12 months |
|
| Outcomes | Main outcome: cure of early syphilis Secondary outcome: HIV seroconversion rates |
|
| Notes | The study was supported by the Centers for Disease Control and Prevention through grants to the Alabama and Louisiana State Health Departments and by donations of medication by Pfizer, Inc., and Ortho‐McNeil Pharmaceuticals Drs. Hook and Martin each received research grant support and honoraria from Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated by means of a computer‐generated randomisation code to receive one of three treatment regimens" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided, but we do not think that it would have been possible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "For study‐related evaluation of therapeutic response, sera were stored frozen and shipped to Birmingham, where all sera for each patient were tested at the same time by a technician masked to all clinical data in order to minimize any potential effect of day‐to‐day variation in serological test performance" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Sixty patients (81%) were followed up for 3 months and could be evaluated for response to therapy (benzathine penicillin, 14; azithromycin, single dose, 17; azithromycin, two doses, 29)" Overall follow‐up at 12 months (Table 2): benzathine penicillin, 10; single‐dose azithromycin, 14; 2‐dose azithromycin, 22 No information on the loss to follow‐up in patients with primary syphilis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information ‐ study protocol not available |
| Other bias | Low risk | No other apparent problem that could introduce bias |
MacDonald 1989.
| Methods | Randomized clinical trial undertaken at the Nairobi City Commission Special Treatment Clinic, Nairobi, Kenya. Dates of study not given in the article | |
| Participants | Participants were HIV‐infected and uninfected men between the age of 18 and 60 years, with genital ulcer(s) "whose cultures were positive by dark‐field microscopy, who had positive cultures for H. ducreyi, and who had no treatment in the preceding week" | |
| Interventions | Group 1: single 200 mg oral dose of fleroxacin Group 2: single 400 mg oral dose of fleroxacin Clinical evaluations were repeated on days 3, 7, 14, and 28; cultures for Haemophilus ducreyi repeated on each visit as long as the ulcer remained unhealed; serologic testing for Treponema pallidum on days 14, and 28, and HIV‐1 status re‐evaluated on follow‐up |
|
| Outcomes | Stated main outcome: efficacy of fleroxacin in clinical H. ducreyi infections Secondary outcome: HIV seroconversion rate |
|
| Notes | Funding: not indicated 53 H. ducreyi culture‐positive men were enrolled; 30 were assigned to group 1 and 23 to group 2. Of these 27 men in group 1 and 18 men in group 2 were HIV‐uninfected. The age (year ± SD) of the participants was 24.3 ± 4.0 for group 1 and 26.7 ± 6.5 for group 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Individuals whose cultures were positive by dark‐field microscopy, who had positive cultures for H.ducreyi, and who had no treatment in the preceding week were randomly assigned to receive either 200mg or 400mg of oral fleroxacin as a single dose" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on 4 participants not evaluated in group 1. All participants enrolled in group 2 were evaluated |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information ‐ study protocol not available |
| Other bias | Low risk | No other problem apparent that could have introduced bias |
Plourde 1992.
| Methods | Randomized, double‐blind study undertaken at the Nairobi City Commission Special Treatment Clinic, Nairobi, Kenya, between July 1989 and February 1990 | |
| Participants | HIV‐1‐seronegative men between the ages of 18 and 65 years. The participants were enrolled into the study if they had a genital ulcer negative by dark‐field microscopy, positive cultures for H. ducreyi, and had received no treatment in preceding 72 hours 98 men were enrolled, and 49 randomly assigned to each group. The age of the participants was (mean ± SD) 25.7 ± 7.1 years for the fleroxacin group and 26.1 ± 6.8 years for the TMP‐SMZ group |
|
| Interventions | Group 1: single 400 mg oral dose of fleroxacin Group 2: 800 mg of sulfamethoxazole and 160 mg of trimethoprim (Bactrim DS, Roche) orally twice daily for 3 days Follow‐up visits were on days 8, 15, 29, 57, and 85; detailed clinical evaluations were repeated on each follow‐up visit. Cultures for H. ducreyi were done at each follow‐up visit as long as the ulcer remained unepithelialized, serologic testing for syphilis on days 0 and 29, and HIV‐1 status was evaluated at each follow‐up visit. Pills were counted on the first follow‐up visit to document compliance 8 men in each group were lost to follow‐up. 5 men in the fleroxacin group (3 with positive syphilis serology and 2 with negative H. ducreyi cultures) were excluded from further analysis; 4 men in the TMP‐SMZ group with positive syphilis serology were excluded from further analysis |
|
| Outcomes | Stated main outcome: cure rate of chancroid/efficacy of fleroxacin vs TMP‐SMZ Secondary outcome: HIV seroconversion rate |
|
| Notes | For ethical reasons, the study was terminated and the code broken after 98 patients were enrolled owing to an unexpectedly high overall clinical failure rate Funding: Medical Research Council of Canada; Hoffman‐La Roche; Seller’s Foundation Award, University of Manitoba |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible men were then randomised to receive either a single 400mg oral dose of fleroxacin or 800mg of SMZ and 160mg of TMP orally twice daily for 3 days" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Eligible men were then randomised to receive either a single 400mg oral dose of fleroxacin or 800mg SMZ and 160mg of TMP (Bactrim DS; Roche, Basel, Switzerland) orally twice daily for 3 days. Packaging of the drugs was such that all patients completed an identical 3‐day course" No report as to whether the drugs were identical in appearance and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Eight men in each group were lost to follow‐up. In addition, five men in the fleroxacin group (three with positive syphilis serology and two inadvertently enrolled with positive H.ducreyi cultures) and four in the TMP‐SMZ group (all with positive syphilis serology) were excluded from further analysis" "Four of 24 men followed up for ≥ 8 weeks seroconverted to HIV‐1" Table 1: A total of 24 men were followed up for ≥ 8 weeks (12 in each treatment arm) "The follow‐up rates were 63% and 45% at 2 and 4 weeks respectively" |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information ‐ study protocol not available |
| Other bias | Unclear risk | "For ethical reasons, the study was terminated and the code broken after 98 patients were enrolled because of an unexpected high overall clinical failure rate" |
FTA‐ABS: fluorescent treponemal antibody–absorption; HIV: human immunodeficiency virus; MHA‐TP: microhemagglutination Treponema pallidum; RPR: rapid plasma reagin; SD: standard deviation; STD: sexually transmitted disease; TMP‐SMZ: trimethoprim‐sulfamethoxazole.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gregson 2007 | Cluster‐randomized controlled trial that assessed syndromic management of STIs |
| Grosskurth 1995 | Cluster‐randomized controlled trial that assessed syndromic management of STIs |
| Hook 2010 | HIV incidence not reported |
| Kamali 2003 | Cluster‐randomized controlled trial that assessed syndromic management of STIs |
| Malonza 1999 | HIV incidence not reported |
| Moodley 2003 | Cluster‐randomized controlled trial that assessed syndromic management of STIs |
| Riedner 2005 | HIV incidence not reported |
| Rolfs 1997 | HIV incidence not reported |
| Tyndall 1994 | HIV incidence not reported |
| Wawer 1998 | Cluster‐randomized controlled trial that assessed syndromic management of STIs |
HIV: human immunodeficiency virus; STI: sexually transmitted infection.
Differences between protocol and review
In the protocol we stated the type of studies as randomized controlled trials and controlled clinical trials. In the review we have revised the type of studies to randomized controlled trials with the individual as the unit of randomization.
In the review we have made the definition of participants more specific by adding that the diagnosis of GUD should be confirmed at the beginning of the study.
The type of interventions specified in the protocol were "any intervention aimed at treating genital ulcer disease compared with a placebo or no treatment." In the review, we have modified this definition of interventions to "any treatment intervention aimed at curing genital ulcer disease compared with an alternative treatment, a placebo, or no treatment. We excluded studies that assessed the effects of suppressive HSV therapy on HIV acquisition, because this review is focused on curable genital ulcer diseases."
In the review we have included adverse events as a secondary outcome, and this was not the case in the protocol.
In the review, we have specified the direction of transmission as "acquisition" since the participants were HIV‐negative at the start of the trial.
Contributions of authors
All the authors participated in the development of the protocol and preparation of the review.
Sources of support
Internal sources
University of Nairobi (FMM, MJM), Kenya.
University of Cape Town (CSW), South Africa.
External sources
-
Reviews for Africa Programme, South African Medical Research Council (FMM), South Africa.
FM Mutua was awarded a Reviews for Africa Programme Fellowship, funded by a grant from the Nuffield Commonwealth Programme, through the Nuffield Foundation
-
Wellcome Trust (CSW), UK.
CS Wiysonge receives funding from the Wellcome Trust, through the Clinical Infectious Diseases Research Initiative
Declarations of interest
None known.
New
References
References to studies included in this review
Hook 2002 {published data only}
- Hook EW, Martin DH, Stephens J, Smith BS, Smith K. A randomised, comparative pilot study of azithromycin versus benzathine penicillin G for treatment of early syphilis. Sexually Transmitted Diseases 2002;29:486‐90. [DOI] [PubMed] [Google Scholar]
MacDonald 1989 {published data only}
- MacDonald KS, Cameron DW, D'Costa L, Ndinya‐Achola JO, Plummer FA, Ronald AR. Evaluation of fleroxacin (RO 23‐6240) as single‐oral‐dose therapy of culture‐proven chancroid in Nairobi, Kenya. Antimicrobial Agents and Chemotherapy 1989;33:612‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plourde 1992 {published data only}
- Plourde PJ, D'Costa LJ, Agoki E, Ombette J, Ndinya‐Achola JO, Slaney LA, et al. A randomised, double‐blind study of the efficacy of fleroxacin versus trimethoprim‐sulfamethoxazole in men with culture‐proven chancroid. Journal of Infectious Diseases 1992; Vol. 165:949‐52. [CN‐00083475] [DOI] [PubMed]
References to studies excluded from this review
Gregson 2007 {published data only}
- Gregson S, Adamson S, Papaya S, Mundondo J, Nyamukapa CA, Mason PR, et al. Impact and process evaluation of integrated community and clinic‐based HIV‐1 control: a cluster‐randomised trial in eastern Zimbabwe. PLoS Medicine 2007;4(3):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grosskurth 1995 {published data only}
- Grosskurth H, Mosha F, Todd J, Mwijarubi E, Klokke A, Senkoro K, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet 1995;346:530‐6. [DOI] [PubMed] [Google Scholar]
Hook 2010 {published data only}
- Hook EW, Behets F, Damme K, Ravelomanana N, Leone P, Sena AC, et al. A phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. Journal of Infectious Diseases 2010;201:1729‐35. [DOI] [PubMed] [Google Scholar]
Kamali 2003 {published data only}
- Kamali A, Quigley M, Nakiyingi J, Kinsman J, Kengeya‐Kayondo J, Gopal R, et al. Syndromic management of sexually‐transmitted infections and behaviour change interventions on transmission of HIV‐1 in rural Uganda: a community randomised trial. Lancet 2003;361:645‐52. [DOI] [PubMed] [Google Scholar]
Malonza 1999 {published data only}
- Malonza IM, Tyndall MW, Ndinya‐Achola JO, Maclean I, Omar S, MacDonald KS, et al. A randomised, double‐blind, placebo‐controlled trial of single‐dose ciprofloxacin versus erythromycin for the treatment of chancroid in Nairobi, Kenya. Journal of Infectious Diseases 1999;180:1886‐93. [DOI] [PubMed] [Google Scholar]
Moodley 2003 {published data only}
- Moodley P, Sturm PDJ, Vanmali T, Wilkinson D, Connolly C, Sturm AW. Association between HIV‐1 infection, the etiology of genital ulcer disease, and response to syndromic management. Sexually Transmitted Diseases 2003;30:241‐5. [DOI] [PubMed] [Google Scholar]
Riedner 2005 {published data only}
- Riedner G, Rusizoka M, Todd J, Maboko L, Hoelscher M, Mmbando D, et al. Single‐dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. New England Journal of Medicine 2005;353:1236‐44. [DOI] [PubMed] [Google Scholar]
Rolfs 1997 {published data only}
- Rolfs RT, Joesoef MR, Hendershot EF, Rompalo AM, Augenbraun MH, Chiu M, et al. A randomised trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. New England Journal of Medicine 1997;337:307‐14. [DOI] [PubMed] [Google Scholar]
Tyndall 1994 {published data only}
- Tyndall MW, Agoki E, Plummer FA, Malisa W, Ndinya‐Achola JO, Ronald AR. Single dose azithromycin for the treatment of chancroid: a randomised comparison with erythromycin. Sexually Transmitted Diseases 1994;21:231‐4. [DOI] [PubMed] [Google Scholar]
Wawer 1998 {published data only}
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Paxton L, Berkley S, et al. A randomised, community‐based trial of intense sexually transmitted diseases control for AIDS prevention, Rakai, Uganda. AIDS 1998;12:1211‐25. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Sewankambo NK, Serwadda D, Quinn TC, Paxton LA, Kiwanuka N, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet 1999;353:525‐35. [DOI] [PubMed] [Google Scholar]
Additional references
Baeten 2008
- Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)‐suppressive therapy decreases plasma and genital HIV‐1 levels in HSV‐2/HIV‐1 co‐infected women: a randomised, placebo controlled, cross‐over trial. Journal of Infectious Diseases 2008;198:1804‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem B, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Broze J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64:401‐6. [DOI] [PubMed] [Google Scholar]
Barnabas 2011
- Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, et al. Impact of herpes simplex virus type 2 on HIV‐1 acquisition and progression in an HIV vaccine trial (the Step Study). Journal of Acquired Immune Deficiency Syndromes 2011;57:238‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boily 2009
- Boily M‐C, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV‐1 infection per sexual act: systematic review and meta‐analysis of observational studies. Lancet Infectious Diseases 2009;9:118‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2007
- Brown JM, Wald A, Hubbard A, Rungruengthanakit K, Chipato T, Rugpao S, et al. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS 2007;21:1515‐23. [DOI] [PubMed] [Google Scholar]
Buchacz 2004
- Buchacz K, Patel P, Taylor M, Kerndt PR, Byers RH, Holmberg SD, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV‐infected patients with new syphilis infections. AIDS 2004;18:2075‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 1989
- Cameron DW, Simonsen JN, D'Costa LJ, Ronald AR, Maitha GM, Gakinya MN, et al. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet 1989;2:403‐07. [DOI] [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. Morbidity and Mortality Weekly Report 2010;59:No. RR‐12. [PubMed] [Google Scholar]
Celum 2004
- Celum C, Levine R, Weaver M, Wald A. Genital herpes and human immunodeficiency virus: double trouble. Bulletin of the World Health Organization 2004;82:447‐53. [PMC free article] [PubMed] [Google Scholar]
Celum 2008
- Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany‐Moretlwe S, et al. Effect of aciclovir on HIV‐1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;371:2109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1997
- Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV‐1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV‐1. Lancet 1997;349:1868‐73. [DOI] [PubMed] [Google Scholar]
Cohen 2006
- Cohen MS. When people with HIV get syphilis: triple jeopardy. Sexually Transmitted Diseases 2006;33:149‐50. [DOI] [PubMed] [Google Scholar]
Corey 2004
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus‐2 on HIV‐1 acquisition and transmission: a review of two overlapping epidemics. Journal of Acquired Immune Deficiency Syndrome 2004;35:435‐45. [DOI] [PubMed] [Google Scholar]
Delany 2009
- Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, LeGoff, et al. Impact of aciclovir on genital and plasma HIV‐1 RNA in HSV‐2/HIV‐1 co‐infected women: a randomised placebo‐controlled trial in South Africa. AIDS 2009;23:461–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunne 2008
- Dunne EF, Whitehead S, Sternberg M, Thepamnuay S, Leelawiwat W, McNicholl JM, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV‐ and HSV‐2‐infected women, Chiang Rai, Thailand. Journal of Acquired Immune Deficiency Syndrome 2008;49:77‐83. [DOI] [PubMed] [Google Scholar]
Fleming 1999
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections 1999;75:3‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 2006
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ, et al. Herpes simplex virus infection increases HIV acquisition in men and women: systematic review and meta‐analysis of longitudinal studies. AIDS 2006;20:73‐83. [DOI] [PubMed] [Google Scholar]
Gray 2001
- Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire‐Mangen F, et al. Probability of HIV‐1 transmission per coital act in monogamous, heterosexual, HIV‐1 discordant couples in Rakai, Uganda. Lancet 2001;357:1149‐53. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 2008
- Johnson LF, Lewis DA. The effect of genital tract infections on HIV‐1 shedding in the genital tract: a systematic review and meta‐analysis. Sexually Transmitted Disease 2008;35:946‐59. [DOI] [PubMed] [Google Scholar]
Kalichman 2011
- Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co‐infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sexually Transmitted Infection 2011;87:183‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laga 1993
- Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non‐ulcerative sexually transmitted diseases as risk factors for HIV‐1 transmission in women: results from a cohort study. AIDS 1993;7:95‐102. [DOI] [PubMed] [Google Scholar]
LeGoff 2007
- LeGoff J, Weiss HA, Gresenguet G, Nzambi K, Frost E, Hayes RJ, et al. Cervciovaginal HIV‐1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 2007;21:1569‐78. [DOI] [PubMed] [Google Scholar]
Mayaud 2001
- Mayaud P, McCormick D. Interventions against sexually transmitted infections (STIs) to prevent HIV infections. British Medical Bulletin 2001;58:129‐53. [DOI] [PubMed] [Google Scholar]
Mayaud 2009
- Mayaud P, LeGoff J, Weiss HA, Grésenguet G, Nzambi K, Bouhlal H, et al. Impact of acyclovir on genital and plasma HIV‐1RNA, genital hHerpes simplex virus type 2 DNA, and ulcer healing among HIV‐1–infected African women with herpes ulcers: a randomized placebo‐controlled trial. Journal of Infectious Diseases 2009;200:216‐26. [DOI] [PubMed] [Google Scholar]
Mayer 2011
- Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. American Journal of Reproductive Immunology 2011;65:308‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCoy 2009
- McCoy SI, Eron JJ, Kuruc JD, Strauss RP, MacDonald PDM, Fiscus SA, et al. Sexually transmitted infections among patients with acute HIV in North Carolina. Sexually Transmitted Diseases 2009;36:372‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muller 2010
- Muller EE, Chirwa TF, Lewis DA. Human papillomavirus (HPV) infection in heterosexual South African men attending sexual health services: associations between HPV and HIV serostatus. Sexually Transmitted Infections 2010;86:175‐80. [DOI] [PubMed] [Google Scholar]
Ng 2011
- Ng BE, Butler LM, Horvath T, Rutherford GW. Population‐based biomedical sexually transmitted infection control interventions for reducing HIV infection. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001220.pub3] [DOI] [PubMed] [Google Scholar]
Paz‐Bailey 2010
- Paz‐Bailey G, Sternberg M, Puren AJ, Steele L, Lewis DA. Determinants of HIV type 1 shedding from genital ulcers among men in South Africa. Clinical Infectious Diseases 2010;50:1060‐7. [DOI] [PubMed] [Google Scholar]
Phiri 2010
- Phiri S, Hoffman IF, Weiss HA, Martinson F, Nyirenda N, Kamwendo D, et al. Impact of aciclovir on ulcer healing, lesional, genital and plasma HIV‐1 RNA among patients with genital ulcer disease in Malawi. Sexually Transmitted Infections 2010;86:345‐52. [DOI] [PubMed] [Google Scholar]
Plourde 1994
- Plourde PJ, Pepin J, Agoki E, Ronald AR, Ombette J, Tyndall M, et al. Human immunodeficiency virus type 1 seroconversion in women with genital ulcers. Journal of Infectious Diseases 1994;170:313‐7. [DOI] [PubMed] [Google Scholar]
Plummer 1991
- Plummer FA, Simonsen JN, Cameron DW, Ndinya‐Achola JO, Kreiss JK, Gakinya MN, et al. Cofactors in male‐female sexual transmission of human immunodeficiency virus type 1. Journal of Infectious Diseases 1991;163:233‐9. [DOI] [PubMed] [Google Scholar]
Quinn 1996
- Quinn TC. Association of sexually transmitted diseases and infection with the human immunodeficiency virus: biological cofactors and markers of behavioural interventions. International Journal of STD & AIDS 1996;7 Suppl 2:17‐24. [DOI] [PubMed] [Google Scholar]
Royce 1997
- Royce R, Seńa A, Cates W, Cohen MS. Sexual transmission of HIV. New England Journal of Medicine 1997;336:1072‐8. [DOI] [PubMed] [Google Scholar]
Schaker 1998
- Schaker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Frequent recovery of HIV‐1 from genital herpes simplex virus lesions in HIV‐1 infected men. JAMA 1998;280:61‐6. [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith JS, Moses S, Hudgens MG, Parker CB, Agot K, Maclean I, et al. Increased risk of HIV acquisition among Kenyan men with human papillomavirus infection. Journal of Infectious Diseases 2010;201:1677‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanton 2010
- Tanton C, Weiss HA, Goff J, Changalucha J, Rusizoka M, Baisley K, et al. Correlates of HIV‐1 genital shedding in Tanzanian women. PLoS ONE 2010;6(3):e17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
van de Wijgert 2009
- Wijgert JHHM, Morrison CS, Brown J, Kwok C, Pol B, Chipato T, et al. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sexually Transmitted Diseases 2009;36:357‐64. [DOI] [PubMed] [Google Scholar]
Venkatesh 2011
- Venkatesh KK, Straten A, Cheng H, Montgomery ET, Lurie MN, Chipato T, et al. The relative contribution of viral and bacterial sexually transmitted infections on HIV acquisition in southern African women in the Methods for Improving Reproductive Health in Africa study. International Journal of STD & AIDS 2011;22:218‐24. [DOI] [PubMed] [Google Scholar]
Wang 2001
- Wang CC, McClelland RS, Reilly M, Overbaugh J, Emery SR, Mandaliya K, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. Journal of Infectious Diseases 2001;183:1017‐22. [DOI] [PubMed] [Google Scholar]
Wasserheit 1992
- Wasserheit JN. Epidemiological synergy: Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sexually Transmitted Diseases 1992;19:61‐77. [PubMed] [Google Scholar]
Watson‐Jones 2008
- Watson‐Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. New England Journal of Medicine 2008;358:1560‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. World Health Organization, 2001. [Google Scholar]
WHO 2007
- World Health Organization. Global strategy for the prevention and control of sexually transmitted infections: 2006 ‐ 2015: breaking the chain of transmission. World Health Organization, 2007. [Google Scholar]
WHO 2011
- World Health Organization. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. World Health Organization, 2011. [Google Scholar]
Zuckerman 2009
- Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Magaret A, et al. HSV suppression reduces seminal HIV‐1 levels in HIV‐1/HSV‐2 co‐infected men who have sex with men. AIDS 2009;23:479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


