Summary
Background
There is a significant association between low vitamin D levels at diagnosis of indolent B-cell lymphomas and inferior overall survival (OS). To determine whether supplemental vitamin D improves event-free survival (EFS) in these patients, we conducted a comparative double-blind study of vitamin D3 vs. placebo.
Methods
In this phase 3, randomized, double-blind, placebo-controlled trial, patients with low tumor burden follicular, marginal zone or small lymphocytic lymphoma, age 18 or older, with stage two or greater disease and no prior systemic treatment were enrolled at 7 academic cancer centers. Patients were stratified by histology and FLIPI (Follicular Lymphoma International Prognostic Index) score and randomized 2:1 to receive 2000 IU vitamin D3 or placebo daily beginning on day one with rituximab 375 mg/m2 administered weekly times four. 257 patients were assessed for participation: 24 were not eligible and 22 refused. Patients with stable disease or disease progression at week 13 counted as events; responding patients continued treatment with vitamin D or placebo until progression for up to three years. The primary endpoint was EFS, defined as the time from randomization to lack of response at week 13, initiation of a new treatment, disease progression or death. Secondary endpoints included week 13 response and OS. This trial is registered at clinicaltrials.gov, NCT03078855.
Findings
206 evaluable patients (135 on vitamin D and 71 on placebo) were enrolled between September 2017 and March 2022 with a median EFS follow-up of 19.6 months (IQR, 9.3–33.5). The median age was 62 years (IQR, 54–70); 118 (57%) female; 182 (89%) white. At week 13 the mean vitamin D level increased to 41.6 ng/mL (SD 10.1) in the vitamin D arm vs. remaining stable (31.3 ng/mL, SD 11.2) in the placebo arm. There was insufficient evidence of a difference in EFS between the two arms (P = 0.26): three-year EFS in the vitamin D arm was 47.7% (95% CI, 39.0–58.4) compared to 49.5% (95% CI, 37.6–65.0) in the placebo arm. There was no difference in week 13 response between the arms (both 84%). Adverse events associated with vitamin D supplementation were rare. The median OS follow-up was 35.1 months (IQR, 22.9–45.1), overall survival was 96.6% (95% CI, 93.1–98.6) and there was no significant difference between the vitamin D and placebo arms (P = 0.47).
Interpretation
As tested in this study, there is no benefit to routine vitamin D supplementation in patients with indolent lymphoma treated with rituximab. These results have implications for ongoing and planned studies of vitamin D supplementation in other malignancies.
Funding
This study was funded by the National Institutes of Health, National Cancer Institute grant R01CA214890.
Keywords: Lymphoma, Vitamin D, Cholecalciferol
Research in context.
Evidence before this study
The association between low vitamin D levels and poor outcome in patients with newly diagnosed follicular lymphoma treated with immunochemotherapy has been previously published and validated in two large prospective studies. Laboratory-based studies have also demonstrated that vitamin D supplementation of deficient patients with diffuse large B-cell lymphoma (DLBCL) can improve in vitro antibody-dependent cell-mediated cytotoxicity, the major mechanism of action of rituximab therapy, compared to pre-supplemented samples. We searched PubMed for clinical trials evaluating vitamin D supplementation in patients with leukemia published between Jan 1, 1997 and Jun 1, 2024 using search terms “lymphoma”, “vitamin D” and “cholecalciferol.” No prospective clinical trials tested whether supplementation with vitamin D could improve outcomes in patients with indolent, low tumor burden lymphoma were conducted during this time. One ongoing study, NCT01787409, is recruiting patients with DLBCL and chronic lymphocytic lymphoma (CLL), however these patient populations are distinct, only vitamin D insufficient patients are supplemented, and patients are treated with immunochemotherapy or observation.
Added value of this study
Despite robust evidence that vitamin D deficient patients with follicular lymphoma have worse prognosis compared to patients with sufficient vitamin D levels, this is the first study to evaluate if supplementation with vitamin D3 can improve EFS in patients with low-tumor burden indolent lymphoma.
Implications of all the available evidence
As tested in this double-blind, randomized clinical trial, there is no EFS or overall survival benefit to routine vitamin D supplementation in patients with indolent, low-tumor burden lymphoma treated with single agent rituximab. This could have implications for the management of patients and for ongoing and planned studies of vitamin D supplementation in other malignancies.
Introduction
Indolent B-cell lymphomas, including follicular lymphoma (FL), are the most prevalent lymphomas in the United States (US) and in most circumstances remain incurable with standard treatment approaches. The therapeutic approach to indolent lymphoma is generally individualized and is based on patient and disease factors at presentation. The most common presentation of indolent lymphomas in the US is “low tumor burden” disease, often defined as asymptomatic disease, non-bulky lymphadenopathy and no cytopenias. Two large randomized trials have suggested single agent rituximab is an appropriate therapeutic option for this presentation, supported by expert opinion and consensus guidelines.1 However, a subset of these lymphomas do not respond to rituximab, and there is a continuous rate of treatment failure, so low-cost and low-toxicity strategies to improve outcome of rituximab treatment in this setting are clearly needed.
In an international SWOG/LYSA (Lymphoma Study Association) collaborative analysis of newly diagnosed patients with FL uniformly treated with standard chemotherapy (CHOP: cyclophosphamide, doxorubicin, vincristine and prednisone) plus anti-CD20 therapy, there was a significant association between low vitamin D levels at diagnosis and inferior lymphoma outcomes in two independent cohorts of patients.2 The magnitude of the observed negative survival association with low vitamin D levels in these trials was stronger than the individual clinical prognostic factors within the FLIPI (Follicular Lymphoma International Prognostic Index) score which is used clinically,3 and vitamin D level is the strongest association reported of a pre-therapy prognostic factor in FL. These results have been robustly validated,4 leading some to recommend supplementation with vitamin D after diagnosis of lymphoma.
Despite the strong evidence suggesting that low vitamin D is negatively correlated with outcome in patients in many cancers,5 rigorous prospective randomized evaluation of vitamin D as an anti-neoplastic agent is lacking. We designed the Indolent Lymphoma and Vitamin D (ILyAD) randomized, placebo-controlled, double-blind trial to definitely address whether modification of vitamin D levels through supplementation with vitamin D3 can improve outcomes in the setting of rituximab therapy for indolent lymphomas. In addition, using whole exome sequencing, we investigated potentially relevant polymorphisms in vitamin D receptor, vitamin D binding protein and putative biomarkers of rituximab response.
Methods
Study design and patients
Eligible patients had biopsy-proven, indolent lymphoma with one of the WHO-defined histologies (Grade 1, 2 or 3a FL, small lymphocytic lymphoma [SLL, but not chronic lymphocytic leukemia] marginal zone lymphoma [MZL]), measurable disease defined by Lugano criteria,6 no prior systemic lymphoma therapy, age ≥18 years, Ann Arbor stage ≥ II. Previous radiation therapy was permitted but radiation on study was considered an event. Patients with FL must have had FDG-avid disease and low tumor burden according to Groupe D'Etude des Lymphomes Folliculaires (GELF) criteria.7 Patients with any of the following parameters were excluded to minimize risk of vitamin D: osteoporosis requiring prescription treatment, known symptomatic primary hyperparathyroidism, hypercalcemia defined as calcium above the institutional normal range, history of calcium-related kidney stones, creatinine >1.5× institutional normal range. Supplemental vitamin D was discouraged but allowed at a maximum total dose of 1000 IU daily. Sex information was obtained from medical records. Study data were managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Rochester.
Randomization and masking
Randomization was stratified by histology (follicular vs. non-follicular) and by the FLIPI score within FL, resulting in three strata: FL-high, FL-intermediate/low, non-FL. If a patient's histology was miscategorized at enrollment, their randomization strata remained unaltered, but their corrected histology was used for secondary subgroup analyses. Within each stratum, participants were 2:1 block-randomized with random block sizes of three or six to maintain treatment:control balance and preserve blinding. All study personnel were blinded to the treatment assignment except one statistician. Vitamin D3 and placebo were packaged, labeled and distributed by the University of Rochester's Clinical Materials Service Unit. Randomization and subsequent drug dispensing were performed at each site within the customized REDCap Randomization Module.
Procedures
Randomization occurred prior to initiation of any treatment, and baseline PET/CT imaging was required within eight weeks of randomization. Local laboratory tests to determine eligibility and FLIPI score were performed within four weeks of randomization and included CBC, creatinine, LDH, hepatitis B, calcium and albumin. Baseline measurement of parathyroid hormone (PTH) and 25(OH) vitamin D2 and D3, were performed centrally at the University of Rochester Laboratory (see Supplemental Methods). Saliva was collected for the isolation of germline DNA and polymorphism analysis as described in the Supplemental Methods. Hepatitis C, HIV and phosphate data were not collected.
Treatment included intravenous rituximab, or approved biosimilar, at 375 mg/m2 (or subcutaneous equivalent) once per week for a total of four doses. Patients were randomized in a double-blind, 2:1 fashion to receive 2000 IU vitamin D3 (cholecalciferol) or placebo daily (BioTech Pharmacal Inc.) beginning on day one of rituximab. Restaging PET/CTs were performed at week 13, a standard timepoint for evaluation of maximal rituximab response.8 Patients with a partial or complete response continued on study with daily dosing of vitamin D3 or placebo for up to 36 months or until disease progression or a new treatment. Stable disease or disease progression at week 13 counted as events and patients were then off study. Study visits were conducted every three months and included physical exam, adverse event assessment, drug distribution and drug reconciliation. Imaging by CT was performed annually. In response to the COVID pandemic, telemedicine remote visits were permitted when deemed appropriate by investigators. Serum was collected at 13 weeks, 12 months, and 36 months for central measurement of vitamin D; investigators were blinded to results.
Outcomes
The primary endpoint was EFS defined as the time from randomization to lack of response at week 13, initiation of a new treatment, disease progression defined by Lugano criteria, or death. Secondary outcomes included EFS by histology, EFS by baseline vitamin D level, overall survival and response at week 13. Correlative studies evaluated polymorphisms in genes involved in the vitamin D pathway and biomarkers of response to rituximab using whole exome sequencing of germline DNA. Adverse events were assessed beginning on Day 1 through 30 days after the last dose of vitamin D/placebo.
Statistics
Clinical characteristics and adverse events were summarized via counts and proportions for categorical variables and mean and standard deviation (SD) or median and interquartile range for continuous variables. Longitudinal vitamin D levels by treatment arm were visualized using side-by-side boxplots. The primary endpoint was Event-Free Survival (EFS), defined as the time from randomization to lack of response at week 13, initiation of a new treatment, disease progression defined by Lugano criteria, or death, right-censored at the time of last follow-up. Since participants were monitored by clinical evaluation for progression every three months, yet some visits may have been early while others may have been delayed, EFS data were coarsened to a discrete time grid of 1.5-month increments prior to fitting a grouped relative risk model with ties broken via the exact conditional probability, equivalent to summing all terms of the marginal likelihood consistent with the observed data.9 EFS data were not coarsened to 1.5-month intervals for the purposes of Kaplan–Meier curve estimation or associated comparisons via logrank tests.
The primary analysis was based on a stratified grouped relative risk (Cox) model, stratified by the three randomization strata, thus allowing for separate baseline hazard functions for FL-high, FL-intermediate/low, and non-FL. The primary histology-stratified grouped Cox model was further parametrically adjusted for two covariates: enrolling site (6-df) and age at randomization (1-df, assuming linearity in the log-hazard). The primary analysis was based on a 1-df robust Wald test of treatment in the histology-stratified grouped Cox model, adjusted for site and age. Interactions of vitamin D supplementation with randomization strata, median age (≥62 years), sex, baseline vitamin D (≤20, 21–30, >30), and 11 pre-specified genetic variants were investigated in secondary analyses, where the 5 smallest sites were pooled for stability (3-df for site groups), and subgroup-specific hazard ratios were summarized in a forest plot including 90% confidence intervals (CI). Interactions were tested using robust Wald tests.
Secondary endpoints included response at week 13 (binary) and overall survival (OS) (time from randomization to death from any cause, right-censored by time of last follow-up). Planned exploratory analyses included the subset of patients with FL and potential associations of known vitamin D-related polymorphisms with outcomes. All statistical analyses were conducted using SAS v9.4 software.
Based on the RESORT trial,10 we anticipated the three-year EFS in the control arm would be 40%. We hypothesized that vitamin D supplementation would reduce the event rate by 45%: Hazard Ratio (HR) = 0.55, corresponding with an increase in three-year EFS from 40% to 60.4%. Assuming a 10% loss to follow-up, a 2:1 randomized trial with 210 treated patients, 140 supplemented and 70 controls, provides 81% power to detect HR = 0.55 using a two-sided α = 0.05 level test.
Ethics
The study was reviewed and approved at the coordinating site by the University of Rochester Research Subjects Review Board (RSRB00066593) and at each of the six participating academic medical centers by their institutional ethics review boards. Written consent was obtained from all patients before enrollment and the study was registered before enrolling patients (ClinicalTrials.gov NCT03078855).
Role of the funding source
The funders had no role in the study design, data collection, analysis, interpretation or writing of the manuscript.
Results
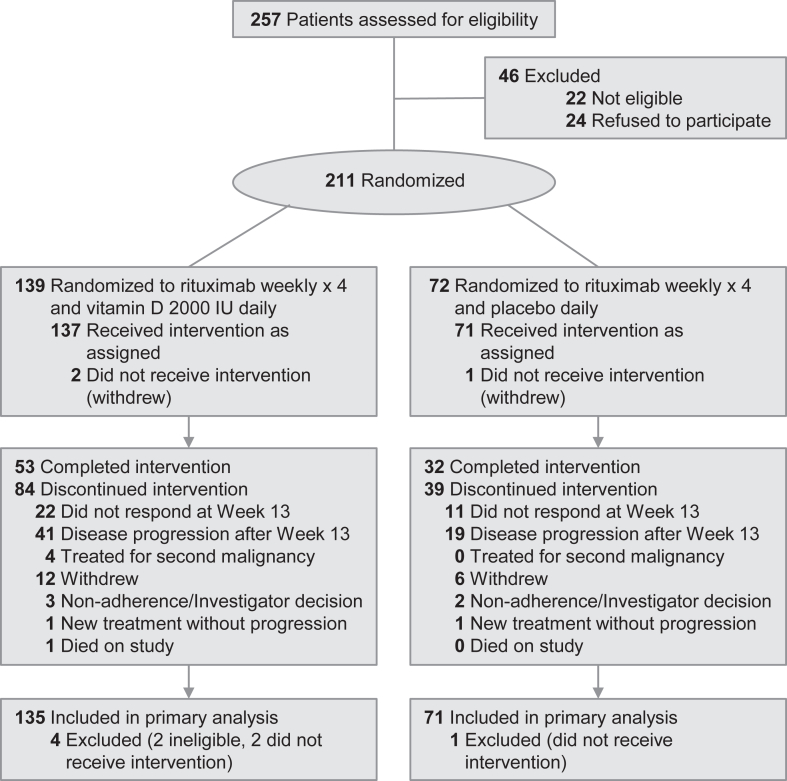
Between September 7, 2017 and March 8, 2022, 211 patients were enrolled and randomized. Two patients were later determined to be ineligible and three withdrew prior to receiving vitamin D/placebo, leaving 206 patients for the primary analysis cohort: 135 randomized to vitamin D and 71 randomized to placebo (Fig. 1).
Fig. 1.
Flow of patients through the ILyAD randomized clinical trial.
Demographics of study participants were balanced between the arms and are detailed in Table 1. The majority of patients, 137 (66%) had FL; smaller numbers had MZL or SLL. The median age at randomization was 62 years (IQR 54–70), 118 (57%) of patients were female, and 182 (89%) were white. The mean vitamin D serum level at baseline was 33 ng/mL (range 6–80 ng/mL, SD 11.4). Approximately half of patients were taking some form of vitamin D supplementation up to the protocol-defined limit of 1000 IU daily. Only 25 (13%) of patients had baseline vitamin D < 20 ng/mL. PTH levels were elevated in only six patients at baseline, with modest inverse correlation with vitamin D levels (Supplementary Figure S1).
Table 1.
Demographics table by arm.
| Patient characteristicsa | Vitamin D (N = 135) | Placebo (N = 71) |
|---|---|---|
| Age at randomization, years, Median (IQR) | 62 (54–70) | 64 (56–71) |
| Male | 57 (42) | 31 (44) |
| Race | ||
| White | 120 (89) | 62 (87) |
| African American | 10 (7) | 5 (7) |
| Other/Unknown race | 5 (4) | 4 (6) |
| Ethnicity | ||
| Hispanic | 10 (8) | 5 (7) |
| Non-Hispanic | 123 (92) | 63 (93) |
| Unknown | 2 | 3 |
| Body Mass Index, kg/m2, Median (IQR) | 29 (25–33) | 29 (25–33) |
| <25 (normal) | 31 (23) | 16 (23) |
| 25–29 (overweight) | 45 (33) | 27 (38) |
| ≥30 (obese) | 59 (44) | 28 (39) |
| Histology | ||
| Marginal Zone Lymphoma | 33 (24) | 14 (20) |
| Small Lymphocytic Lymphoma | 13 (10) | 9 (13) |
| Follicular Lymphoma | 89 (66) | 48 (68) |
| FLIPI Low/Intermediate (0–2) | 62 (70) | 32 (67) |
| FLIPI High (3–5) | 27 (30) | 16 (33) |
| FL Grade 1 | 35 (39) | 21 (44) |
| FL Grade 2 | 44 (49) | 24 (50) |
| FL Grade 3a | 10 (11) | 3 (6) |
| Ann Arbor Stage | ||
| Stage II | 20 (15) | 7 (10) |
| Stage III | 45 (33) | 27 (38) |
| Stage IV | 70 (52) | 37 (52) |
| Baseline Vitamin D, ng/mL, mean [SD]b | 33 [11] | 33 [12] |
| ≤20 (deficient) | 15 (12) | 10 (14) |
| >20–30 (insufficient) | 39 (30) | 18 (26) |
| >30 (sufficient) | 75 (58) | 42 (60) |
| Baseline PTH > ULN (65 pg/mL)c | 3 (2) | 3 (4) |
| Taking vitamin D supplement/multivitamind | 60 (44) | 30 (42) |
Frequency (column percent) is reported unless otherwise specified.
Baseline vitamin D not measured in 6 patients in the vitamin D arm and 1 in the placebo arm.
Baseline PTH not measured in 8 patients in the vitamin D arm and 2 in the placebo arm.
Total vitamin D supplementation ≤1000 IU daily permitted per protocol.
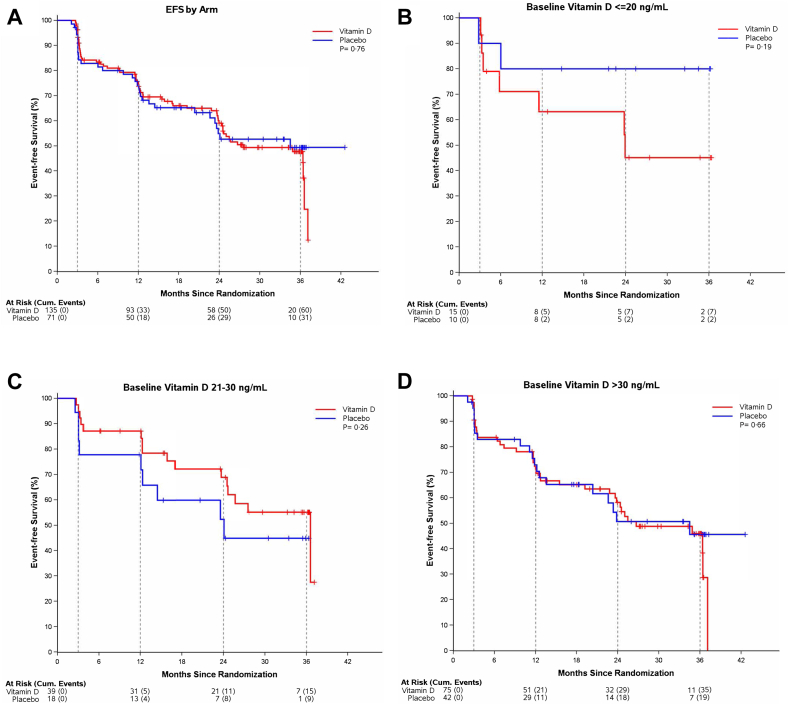
At week 13 the mean vitamin D level increased to 41.6 ng/mL (SD 10.1) in the vitamin D arm vs. remaining stable (31.3 ng/mL, SD 11.2) in the placebo arm. These differences persisted at one- and three-year timepoints (Supplementary Figure S2). Among the 206 eligible and treated patients (median follow-up of 19.6 months, IQR 9.3–33.5, with follow-up defined as time to event or censoring, whichever occurs first), the median EFS was 34.5 months (95% CI, 24–42 months). Without adjusting for other patient characteristics, there was insufficient evidence of a difference in EFS between the two arms (P = 0.76): three-year EFS in the vitamin D arm was 47.7% (95% CI 39.0–58.4) compared to 49.5% (95% CI, 37.6–65.0) in the placebo arm (Fig. 2A).
Fig. 2.
Event-Free Survival by Arm. Event-free survival among eligible, treated patients (A) randomized to vitamin D (n = 135) or placebo (n = 71) (B) in patients with baseline vitamin D less than or equal to 20 ng/mL, (C) in patients with baseline vitamin D 21–30 ng/mL and (D) baseline vitamin D greater than 30 ng/mL. The dotted vertical lines represent protocol-directed imaging assessments at Week 13 and Months 12, 24 and 36. Reported P values are for the unstratified logrank test.
Adjusting for site and age at randomization, and stratifying by the three randomization strata, the estimated HR for vitamin D supplementation from the primary analysis was 1.28 (95% CI, 0.83–1.98, P = 0.26), with insufficient evidence of benefit (HR < 1) or harm (HR > 1). Excluding non-follicular patients, there remained insufficient evidence of an effect of vitamin D supplementation in the subgroup of FL patients (n = 137, HR = 1.49, P = 0.21). There was also insufficient evidence of an effect of vitamin D supplementation within clinically relevant baseline vitamin D level subgroups (Fig. 2B, C, 2D) or within FL and non-FL histologies (Supplementary Figures S3 and S4), as evaluated by unadjusted logrank tests. Additional subgroup analyses of patients with FL and low/intermediate, high, or intermediate/high FLIPI scores, patients with stage 3 or 4 disease, and patients with baseline vitamin D equal to or less than 40 ng/mL also showed no effect of vitamin D supplementation on EFS (Supplementary Figures S5–S9).
At week 13, 172 patients (84%) responded to treatment using Lugano criteria, including a complete response in 110 patients and a partial response in 62 patients. There was no difference in response between the vitamin D and placebo arms (both 84%). As expected, response varied by subtype, detailed in Supplementary Table S1.
Observed toxicity was as expected with rituximab treatment. Adverse events of all grades occurring in more than 5% of patients are shown in Table 2. Adverse events of interest potentially associated with vitamin D supplementation were rare and detailed in Table 3. Hypercalcemia was observed in two patients, both in the placebo arm. Incidental findings of renal calculi upon imaging were found in three patients assigned to the vitamin D arm. One additional patient on the vitamin D arm experienced a symptomatic renal calculus and was taken off study drug. Three participants assigned to vitamin D experienced bone fractures; two in the setting of trauma. Adverse events grades 3 through 5 which occurred in more than one patient are shown in Table 4. There was one death on study due to complications from COVID infection. Six additional participants (three vitamin D; three placebo) subsequently died after completion of study participation. Among the 206 eligible and treated patients (median follow-up of 35.1 months, IQR 22.9–45.1, with follow-up defined as time to death or censoring, whichever occurs first), the crude OS rate was 96.6% (95% CI, 93.1–98.6%). There was no significant difference between the vitamin D and placebo arms (P = 0.47, Supplementary Figure S10).
Table 2.
Incidence of adverse events occurring in more than 5% of patients.
| Adverse eventa | Grade | Vitamin D N = 137 (%) | Placebo N = 72 (%) |
|---|---|---|---|
| Fatigue | 1 | 38 (28) | 17 (24) |
| 2 | 3 (2) | 2 (3) | |
| Infusion related reaction | 1 | 10 (7) | 5 (7) |
| 2 | 40 (29) | 17 (24) | |
| 3 | 0 (0) | 1 (1) | |
| Injection site reaction | 1 | 12 (9) | 6 (8) |
| 2 | 3 (2) | 3 (4) | |
| Abdominal pain | 1 | 10 (7) | 3 (4) |
| 2 | 1 (1) | 0 (0) | |
| Constipation | 1 | 8 (6) | 4 (6) |
| 2 | 2 (1) | 0 (0) | |
| Diarrhea | 1 | 8 (6) | 2 (3) |
| 2 | 0 (0) | 2 (3) | |
| Nausea | 1 | 8 (6) | 5 (7) |
| 2 | 2 (1) | 2 (3) | |
| Infection—COVID-19 | 1 | 5 (4) | 4 (6) |
| 2 | 3 (2) | 4 (6) | |
| 3 | 3 (2) | 1 (1) | |
| 5 | 1 (1) | 0 (0) | |
| Upper respiratory infection | 1 | 3 (2) | 2 (3) |
| 2 | 8 (6) | 6 (8) | |
| Arthralgia | 1 | 10 (7) | 4 (6) |
| 2 | 2 (1) | 2 (3) | |
| Back pain | 1 | 12 (9) | 3 (4) |
| 2 | 3 (2) | 3 (4) | |
| Pain in extremity | 1 | 8 (6) | 4 (6) |
| Headache | 1 | 16 (12) | 4 (6) |
| 2 | 2 (1) | 1 (1) | |
| 3 | 1 (1) | 0 (0) | |
| Cough | 1 | 10 (7) | 4 (6) |
| 2 | 1 (1) | 0 (0) | |
| Dyspnea | 1 | 5 (4) | 4 (6) |
| 2 | 2 (1) | 0 (0) | |
| Pruritus | 1 | 3 (2) | 4 (6) |
| 2 | 4 (3) | 0 (0) | |
| Hypertension | 1 | 6 (4) | 1 (1) |
| 2 | 7 (5) | 4 (6) | |
| 3 | 3 (2) | 0 (0) |
Patients appear only once in each row.
Table 3.
Incidence of adverse events of interest to vitamin D supplementation.
| Adverse event | Grade | Vitamin D arm | Placebo arm |
|---|---|---|---|
| Hypercalcemia | 1 | 0 | 2 |
| Renal calculi | 1 | 2 | 0 |
| 2 | 1 | 0 | |
| 3 | 1 | 0 | |
| Bone fractures | 2 | 1 | 0 |
| 3 | 2 | 0 |
Table 4.
Incidence of grade 3-5 adverse events occurring more than once.
| Adverse eventa | Vitamin DN = 137 | Placebo N = 72 |
|---|---|---|
| Anemia | 1 | 1 |
| COVID19 infectionb | 4 | 1 |
| Sepsis | 1 | 1 |
| Bone fracture | 2 | 0 |
| Syncope | 4 | 0 |
| Hypertension | 3 | 1 |
| Hypotension | 1 | 1 |
| Thromboembolism | 2 | 0 |
Patients appear only once in each row based on the worst grade experienced in each category.
One patient in the vitamin D arm experienced grade 5 COVID infection while on study.
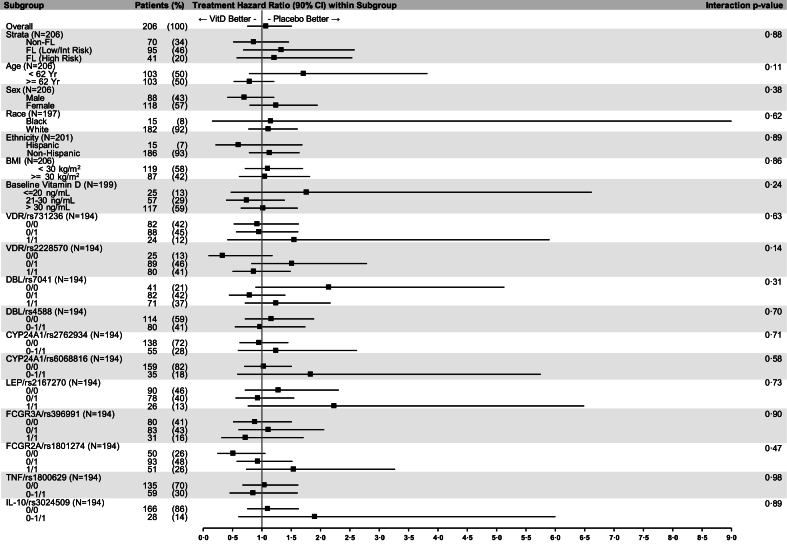
Polymorphisms in the vitamin D pathway11 and biomarkers of rituximab response12 were evaluated by whole exome sequencing of germline DNA in 194 patients. There was no association of benefit from vitamin D supplementation with polymorphisms of vitamin D receptor, vitamin D binding protein, CYP24A1 or biomarkers potentially associated with rituximab response, detailed in Fig. 3.
Fig. 3.
Forest Plot of Subgroup Hazard Ratios. Plot of vitamin D intervention hazard ratios within patient characteristics at baseline. Genetic polymorphisms in genes associated with the vitamin D pathway and response to rituximab are represented by 0/0 (no minor alleles), 0/1 (one minor allele), 1/1 (two minor alleles), 0–1/1 (one or two minor alleles). Each histology-stratified grouped Cox model included indicators for intervention, baseline characteristic, their interaction, indicators for the two highest enrolling sites, and continuous age. Significance of interactions and confidence intervals were calculated in SAS based on robust Wald tests.
Discussion
We hypothesized that vitamin D supplementation could represent an inexpensive, nontoxic strategy to improve rituximab efficacy in patients with low tumor burden indolent lymphoma. In our rigorous, double-blind, placebo-controlled study, we did not demonstrate a benefit of vitamin D supplementation when added to rituximab for either response or EFS. The observed event rate in our trial, including higher responses in FL compared with other indolent histologies, was in keeping with other recently published trials.13,14 Planned subgroup analyses of patients with FL and patients with advanced stage and low baseline vitamin D levels still failed to demonstrate any benefit of this intervention.
Obtained mostly through sun exposure but also available from food sources and dietary supplements, vitamin D in humans is metabolized in the liver and kidney to its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D). A provocative study in mice has shown that disrupted vitamin D signaling alters the intestinal microbiome, affecting immunity to cancer.15 Clinical evidence supports an association of improved survival in patients with higher vitamin D levels at diagnosis in several malignancies, and retrospective analyses have demonstrated improved survival with higher levels of vitamin D among patients with newly diagnosed diffuse large B-cell lymphoma16 and longer treatment free survival in patients with chronic lymphocytic leukemia.17 Recent studies have also demonstrated that vitamin D insufficiency is associated with lower response rates and inferior OS in patients with aggressive B-cell lymphomas treated with chimeric antigen receptor T-cell therapy.18 Pretreatment vitamin D deficiency is also significantly associated with impaired progression-free and OS in Hodgkin lymphoma.19
Unlike the case in solid tumors, expression of the vitamin D receptor is absent in indolent lymphomas,20 and gene expression studies have shown vitamin D deficiency does not induce changes in the transcriptional program of non-Hodgkin lymphomas.21 Therefore, any role of supplementation in the setting of indolent lymphoma would be an indirect effect on the immune microenvironment. Antibody-dependent cell-mediated cytotoxicity (ADCC) is the major mechanism of rituximab action in patients with indolent lymphoma. Supplementation with vitamin D has been shown to improve rituximab-mediated cellular cytotoxicity in blood samples from patients with vitamin D insufficiency.22 Vitamin D also modulates the activity of CD4+T-cells, which promote follicle center cell proliferation, and are critical to maintenance of the malignant microenvironment of FL.20 A clinical trial of vitamin D supplementation for patients with indolent lymphoma was conducted in the pre-rituximab era using one microgram daily of oral alfacalcidol, a vitamin D analogue, and demonstrated a single-agent overall response rate of 24%.23 Despite this rationale, we did not demonstrate any impact of vitamin D supplementation.
In our study, the chosen dose of 2000 IU of vitamin D3 is a standard daily dose that is readily available over the counter in soft gels, capsules, tablets, and liquids. This dose adequately supplements serum 25(OH)D levels while maintaining a substantial margin of safety below the upper level of intake (4000 IU) at which point the potential risk for harm begins to increase. Two thousand IU daily has been shown to effectively raise 25(OH)D levels over a prolonged period of administration without evidence of toxicity in previous human studies,24 and this strategy and dose of vitamin D has been utilized in other NIH-sponsored prospective clinical trials in cancer populations.25
Robust prospective trials like ours evaluating the role of vitamin D supplementation in cancer are limited in number, and generally have been negative. A recent single arm study in acute myeloid leukemia combined vitamin D with chemotherapy and did not demonstrate any benefit in OS.26 A randomized double blind trial comparing vitamin D supplementation (1200 IU daily) vs. placebo after surgery for lung cancer failed to demonstrate any significant difference in relapse free survival or OS; in this study a subgroup of patients with early stage adenocarcinoma and low baseline vitamin D appeared to derive some benefit.27 A study evaluating vitamin D (2000 IU daily) or placebo after surgery in patients with gastrointestinal cancer similarly did not result in significant improvement in relapse-free survival at five years.25 Another study in patients with colorectal cancer evaluating the addition of high dose or standard dose vitamin D in combination with chemotherapy and bevacizumab resulted in no significant difference in progression-free survival.28
Retrospective cohort and observational studies in cancer have used varying definitions of vitamin D insufficiency in correlation with outcome, precluding the ability to determine an optimal level of vitamin D for treatment response as appropriate thresholds for serum vitamin D vary significantly between different patient subgroups and desired outcomes. In our study, we enrolled patients regardless of baseline vitamin D level, which was blinded to treating physicians. We chose to not limit study participation based on baseline serum 25(OH)D levels for several reasons, including: 1) The highest 25(OH)D level in our previous study of patients with follicular lymphoma was 63 ng/mL,29 minimizing concern for potential toxicity with our chosen dose of 2000 IU daily; 2) we excluded patients with other clinical manifestations of low 25(OH)D levels, namely osteoporosis requiring prescription treatment; 3) 25(OH)D levels vary substantially by season, race and BMI; 4) our intention in designing this study was to be as inclusive as possible to maximize generalizability across a broad population of patients with indolent lymphoma. Our data demonstrate that patients randomized to vitamin D had higher levels by week 13, confirming compliance and similar to observations from another study that utilized the 2000 IU daily schedule.30 In evaluating previously published ranges of defined vitamin D insufficiency, the outcome of rituximab therapy in our trial was not impacted by vitamin D supplementation even for patients with the lowest vitamin D levels at study entry.
Response to oral vitamin D supplementation may be dependent on certain VDR genotypes,31 for example, approximately 80% of the variation in vitamin D binding protein concentration is explained by genetic variation in the vitamin D-binding protein.32 In our study, using whole exome sequencing, we did not demonstrate any subgroup who appeared to benefit from vitamin D supplementation. Only 15% of patients in this study were Hispanic or Black, which limits the ability to draw conclusions about these populations.
Strengths of our study include the placebo-controlled design, uniform treatment with rituximab, and a representative US patient population treated at seven cancer centers with varying latitude (Rochester, NY 43.1566°N to Miami, FL 25.7617°N). While these latitudes are representative of the US population it should be noted that countries with different latitudes may have different distributions of vitamin D levels which may impact the generalization of our results. Notably, relatively few patients in our study were vitamin D deficient as only 13% of patients had baseline vitamin D less than 20 ng/mL. This is in contrast to earlier studies and international studies showing much higher levels of deficiency in patients diagnosed with lymphoma,29 and likely is a result from increased screening for vitamin D deficiency and supplementation in the US, despite calls to avoid over-screening and over-prescribing of supplemental vitamin D for the healthy population.33 This small number of patients with vitamin D deficiency precludes a definitive statement as to benefit to supplementation in this group. Our study used a target hazard ratio of 0.55 resulting in our modest sample size. This hazard ratio is similar to the effect observed when adding lenalidomide to rituximab in patients with relapsed follicular lymphoma.34 A larger study may have had the ability to detect a small benefit to vitamin D supplementation, however, given the rarity of indolent lymphoma, such a study would not be feasible given limitations of eligible patients and funding restrictions. Our study was conducted during the COVID pandemic which may have influenced patients’ willingness to participate in this study.
In conclusion, our study demonstrates the importance of prospective, double-blind, placebo-controlled studies to definitively address the role of vitamin D supplementation as therapy for cancer. Although there was no toxicity signal, based upon our findings, there is no role for routine vitamin D supplementation to improve outcomes of rituximab therapy for indolent lymphoma, as tested in this study, in the absence of other indication or further data. The previously demonstrated association of low vitamin D with outcome in patients with indolent lymphoma treated with rituximab appears to represent a surrogate for illness and comorbidity rather than a modifiable predictive risk factor. Our results add to a limited literature showing no benefit of vitamin D supplementation in cancer therapy and have broad implications for ongoing and planned supplementation studies in other malignancies.
Contributors
JWF, DRP were responsible for study conceptualization and design. JWF, BSK, ISL, JBC, PMR, CC, BLA, GS, PMB, JPL, and LJN identified and enrolled participants and interpreted the data. JWF, MTB, MS, AB, and DRP verified, analyzed and interpreted the data. FV and JMA assisted with pathology samples and polymorphism data. JGS performed imaging response assessments. JWF, MTB, MS, DRP wrote the manuscript. JWF, DP and MS had full access to all the data in the study and verified the underlying data. JWF takes responsibility for the integrity of the data and the accuracy of the data analyses. All authors read and approved the final version of the manuscript.
Data sharing statement
Whole exome sequencing data is available under controlled access at dbGaP accession phs003503. v1. p1. Deidentified non-genomic study data will be provided upon reasonable request. The trial protocol is available at clinicaltrials. gov/NCT03078855.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
We appreciate all the patients who participated in this trial, and study staff at each site who enabled this trial. Kate Hirnak provided analysis support for polymorphism data. Germline DNA sequencing was conducted at the Wilmot Cancer Institute, Genomics Shared Resource. We thank Donna Neuberg ScD, Arnold Freedman MD, and Benjamin Leder MD for their service on the Data and Safety Monitoring Committee for this study. This study was funded by the National Cancer Institute (R01CA214890 to JWF), and by the Lymphoma Epidemiology of Outcomes Consortium (U01CA195568). REDCap support was provided by the University of Rochester Clinical and Translational Science Institute grant (UL1TR002001).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102959.
Appendix A. Supplementary data
References
- 1.Ghielmini M., Vitolo U., Kimby E., et al. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL) Ann Oncol. 2013;24(3):561–576. doi: 10.1093/annonc/mds517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly J.L., Salles G., Goldman B., et al. Low serum vitamin D levels are associated with inferior survival in follicular lymphoma: a prospective evaluation in SWOG and LYSA studies. J Clin Oncol. 2015;33(13):1482–1490. doi: 10.1200/JCO.2014.57.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solal-Celigny P., Roy P., Colombat P., et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 4.Tracy S.I., Maurer M.J., Witzig T.E., et al. Vitamin D insufficiency is associated with an increased risk of early clinical failure in follicular lymphoma. Blood Cancer J. 2017;7(8) doi: 10.1038/bcj.2017.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seraphin G., Rieger S., Hewison M., Capobianco E., Lisse T.S. The impact of vitamin D on cancer: a mini review. J Steroid Biochem Mol Biol. 2023;231 doi: 10.1016/j.jsbmb.2023.106308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheson B.D., Fisher R.I., Barrington S.F., et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brice P., Bastion Y., Lepage E., et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 1997;15(3):1110–1117. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P., Grillo-López A.J., Link B.K., et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 9.Kalbfleisch J., Prentice R. Marginal likelihoods based on Cox's regression and life model. Biometrika. 1973;60:267–278. [Google Scholar]
- 10.Kahl B.S., Hong F., Williams M.E., et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol. 2014;32(28):3096–3102. doi: 10.1200/JCO.2014.56.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abouzid M., Glowka F., Kagan L., Karazniewicz-Lada M. Vitamin D metabolism gene polymorphisms and their associated disorders: a literature review. Curr Drug Metab. 2022;23(8):630–651. doi: 10.2174/1389200223666220627104139. [DOI] [PubMed] [Google Scholar]
- 12.Liu D., Tian Y., Sun D., Sun H., Jin Y., Dong M. The FCGR3A polymorphism predicts the response to rituximab-based therapy in patients with non-Hodgkin lymphoma: a meta-analysis. Ann Hematol. 2016;95(9):1483–1490. doi: 10.1007/s00277-016-2723-x. [DOI] [PubMed] [Google Scholar]
- 13.Kahl B.S., Jegede O.A., Peterson C., et al. Long-term follow-up of the RESORT study (E4402): a randomized phase III comparison of two different rituximab dosing strategies for low-tumor burden follicular lymphoma. J Clin Oncol. 2024 doi: 10.1200/JCO.23.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartron G., Bachy E., Tilly H., et al. Randomized phase III trial evaluating subcutaneous rituximab for the first-line treatment of low-tumor burden follicular lymphoma: results of a LYSA study. J Clin Oncol. 2023;41(19):3523–3533. doi: 10.1200/JCO.22.02327. [DOI] [PubMed] [Google Scholar]
- 15.Giampazolias E., Pereira da Costa M., Lam K.C., et al. Vitamin D regulates microbiome-dependent cancer immunity. Science. 2024;384(6694):428–437. doi: 10.1126/science.adh7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake M.T., Maurer M.J., Link B.K., et al. Vitamin D deficiency is associated with inferior event-free and overall survival in diffuse large B-cell lymphoma. J Clin Oncol. 2010;28(27):4191–4198. [Google Scholar]
- 17.Tadmor T., Melamed G., Alapi H., Gazit S., Patalon T., Rokach L. Vitamin D supplement for patients with early-stage chronic lymphocytic leukemia is associated with a longer time to first treatment. Blood Adv. 2024;8(14):3840–3846. doi: 10.1182/bloodadvances.2023011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nath K., Tomas A.A., Flynn J., et al. Vitamin D insufficiency and clinical outcomes with chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Transplant Cell Ther. 2022;28(11):751.e1–751.e7. doi: 10.1016/j.jtct.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin J.Q., Yin H., Wu J.Z., et al. 25-Hydroxy vitamin D deficiency predicts inferior prognosis in Hodgkin lymphoma. Leuk Res. 2021;105 doi: 10.1016/j.leukres.2021.106580. [DOI] [PubMed] [Google Scholar]
- 20.Hickish T., Cunningham D., Colston K., et al. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68(4):668–672. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donati B., Ferrari A., Ruffini A., et al. Gene expression profile unveils diverse biological effect of serum vitamin D in Hodgkin's and diffuse large B-cell lymphoma. Hematol Oncol. 2021;39(2):205–214. doi: 10.1002/hon.2827. [DOI] [PubMed] [Google Scholar]
- 22.Bittenbring J.T., Neumann F., Altmann B., et al. Vitamin d deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol. 2014;32(29):3242–3248. doi: 10.1200/JCO.2013.53.4537. [DOI] [PubMed] [Google Scholar]
- 23.Raina V., Cunningham D., Gilchrist N., Soukop M. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63(3):463–465. doi: 10.1038/bjc.1991.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith S.M., Gardner K.K., Locke J., Zwart S.R. Vitamin D supplementation during Antarctic winter. Am J Clin Nutr. 2009;89(4):1092–1098. doi: 10.3945/ajcn.2008.27189. [DOI] [PubMed] [Google Scholar]
- 25.Urashima M., Ohdaira H., Akutsu T., et al. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: the AMATERASU randomized clinical trial. JAMA. 2019;321(14):1361–1369. doi: 10.1001/jama.2019.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouchel P.L., Berard E., Tavitian S., et al. Vitamin C and D supplementation in acute myeloid leukemia. Blood Adv. 2023;7(22):6886–6897. doi: 10.1182/bloodadvances.2023010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiba T., Morikawa T., Odaka M., et al. Vitamin D supplementation and survival of patients with non-small cell lung cancer: a randomized, double-blind, placebo-controlled trial. Clin Cancer Res. 2018;24(17):4089–4097. doi: 10.1158/1078-0432.CCR-18-0483. [DOI] [PubMed] [Google Scholar]
- 28.Ng K., Nimeiri H.S., McCleary N.J., et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or Metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. 2019;321(14):1370–1379. doi: 10.1001/jama.2019.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hutchinson K., Jahangiri S., Calvi L.M., Barr P., Friedberg J.W., Kelly J.L. Impact of dietary supplements, obesity and treatment initiation on serum vitamin D levels in patients with lymphoma. Leuk Lymphoma. 2015;56(2):508–511. doi: 10.3109/10428194.2014.919633. [DOI] [PubMed] [Google Scholar]
- 30.Yao P., Lu L., Hu Y., et al. A dose-response study of vitamin D3 supplementation in healthy Chinese: a 5-arm randomized, placebo-controlled trial. Eur J Nutr. 2016;55(1):383–392. doi: 10.1007/s00394-015-0859-4. [DOI] [PubMed] [Google Scholar]
- 31.Elnenaei M.O., Chandra R., Mangion T., Moniz C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation. Br J Nutr. 2011;105(1):71–79. doi: 10.1017/S0007114510003065. [DOI] [PubMed] [Google Scholar]
- 32.Powe C.E., Evans M.K., Wenger J., et al. Vitamin D–binding protein and vitamin D status of Black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manson J.E., Brannon P.M., Rosen C.J., Taylor C.L. Vitamin D deficiency–is there really a pandemic? N Engl J Med. 2016;375(19):1817–1820. doi: 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 34.Leonard J.P., Trneny M., Izutsu K., et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37(14):1188–1199. doi: 10.1200/JCO.19.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.