Abstract
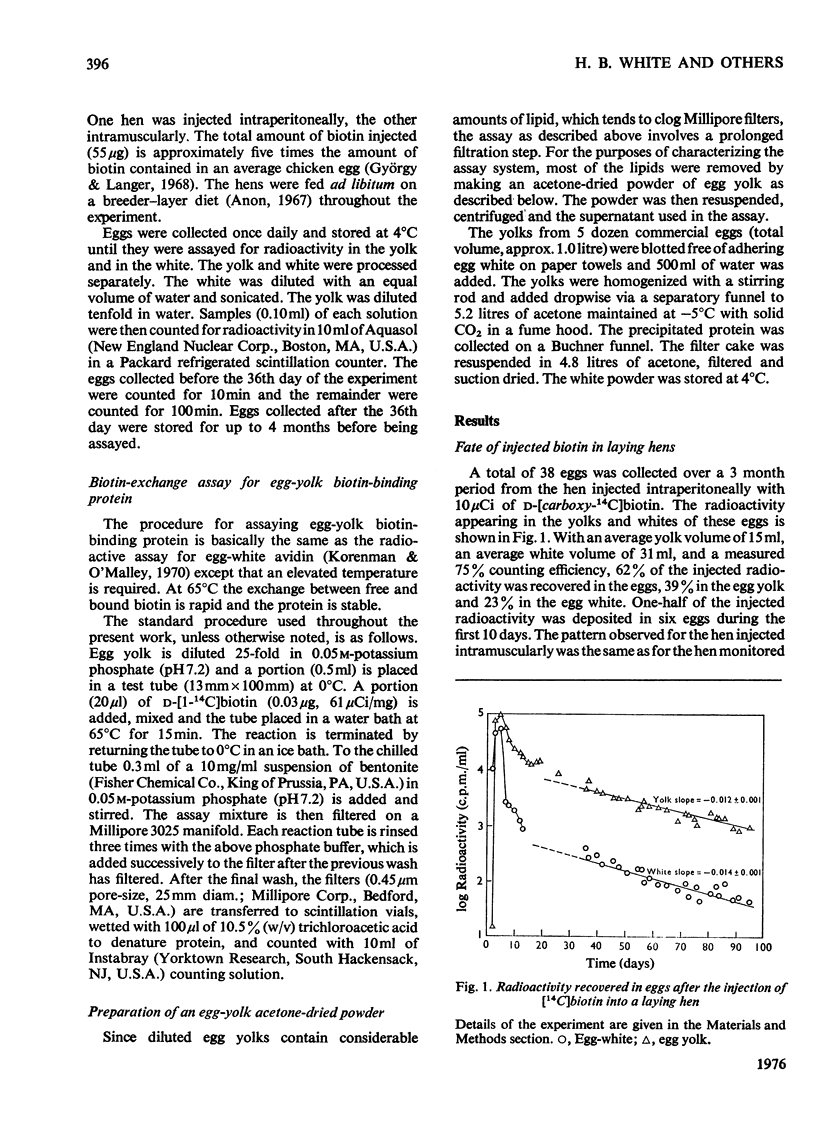
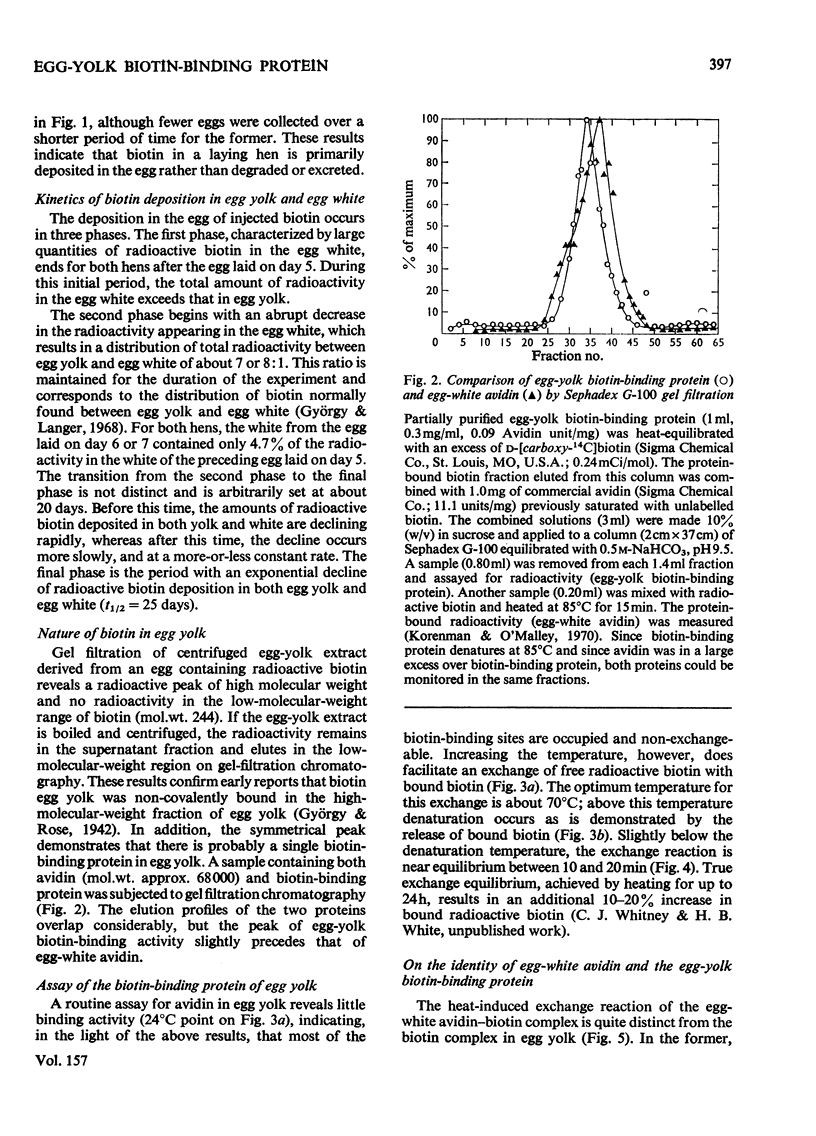
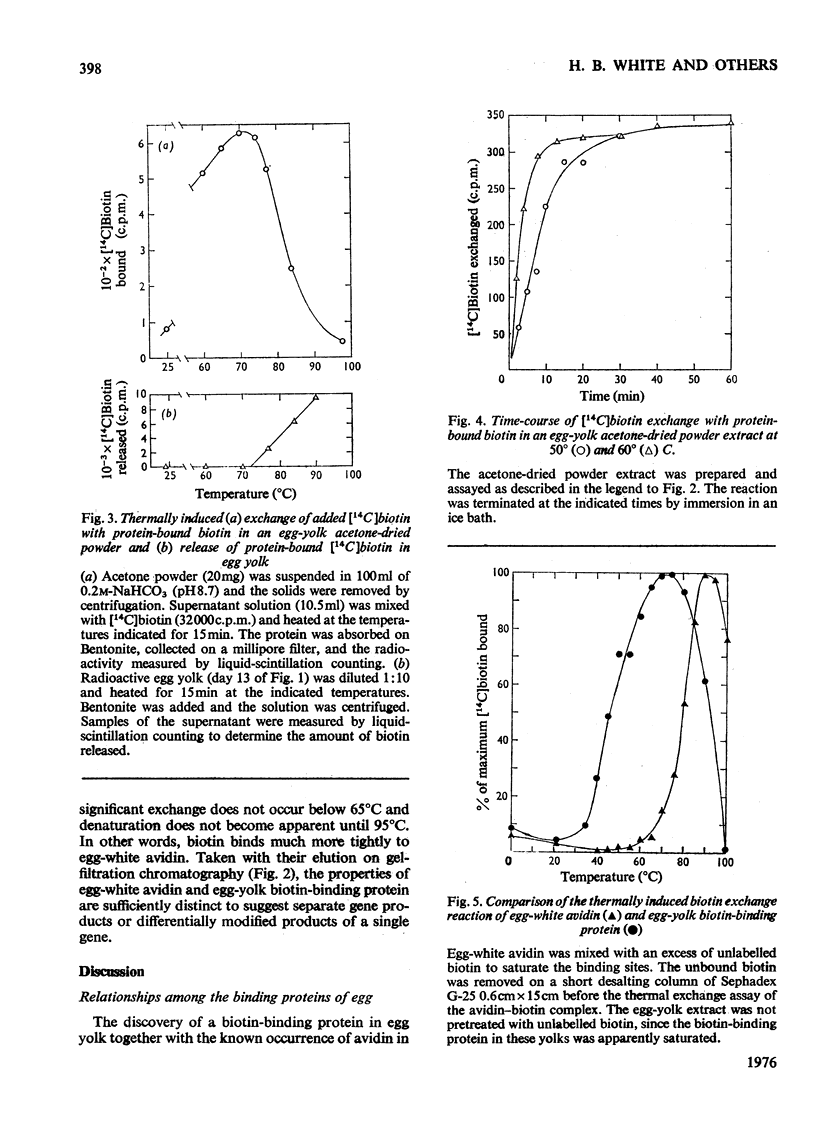
1. Biotin in chicken egg yolk is non-covalently bound to a specific protein that comprises 0.03% of the total yolk protein (0.8 mg/yolk). This biotin-binding protein is not detectable by the normal avidin assay owing to the biotin being tightly bound. Exchange of [14C]biotin for bound biotin at 65 degrees C is the basis of an assay for this protein. 2. Biotin-binding protein from egg yolk is distinguishable from egg-white avidin on Sephadex G-100 gel filtration, although the sizes of the two proteins appear quite similar. 3. Biotin-binding protein is denatured at a lower temperature and freely exchanges biotin at lower temperatures than does avidin. 4. The biotin-binding protein in egg yolk is postulated to be responsible for the deposition of biotin in egg yolk. D-[carboxyl-14C]Biotin injected into laying hens rapidly appears in the egg bound to yolk biotin-binding protein and avidin. Over 60% of the radioactivity is eventually deposited in eggs. The kinetics of biotin deposition in the egg suggests a 25 day half-life for an intracellular biotinyl-coenzyme pool in the laying hen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Muto Y., Hosoya N. Vitamin A transport in chicken plasma: isolation and characterization of retinol-binding protein (RBP), prealbumin (PA), and RBP--PA complex. J Lipid Res. 1975 May;16(3):200–210. [PubMed] [Google Scholar]
- Arinze J. C., Mistry S. P. Hepatic acetyl CoA carboxylase, propionyl CoA carboxylase and pyruvate carboxylase activities during embryonic development and growth in chickens. Proc Soc Exp Biol Med. 1970 Nov;135(2):553–556. [PubMed] [Google Scholar]
- Board R. G., Fuller R. Non-specific antimicrobial defences of the avian egg, embryo and neonate. Biol Rev Camb Philos Soc. 1974 Feb;49(1):15–49. doi: 10.1111/j.1469-185x.1974.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Brewer L. E., Edwards H. M., Jr Studies on the biotin requirement of broiler breeders. Poult Sci. 1972 Mar;51(2):619–624. doi: 10.3382/ps.0510619. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Roth T. F. Changes in specific sequestration of protein during transport into the developing oocyte of the chicken. Biochim Biophys Acta. 1973 Apr 16;298(4):951–955. doi: 10.1016/0005-2736(73)90398-2. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. RAW HEN EGG WHITE AND THE ROLE OF IRON IN GROWTH INHIBITION OF SHIGELLA DYSENTERIAE, STAPHYLOCOCCUS AUREUS, ESCHERICHIA COLI AND SACCHAROMYCES CEREVISIAE. Science. 1944 Jul 7;100(2584):14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- Sonneborn D. W., Hansen H. J. Vitamin B12 binders of chicken serum and chicken proventriculus are immunologically similar. Science. 1970 May 1;168(3931):591–592. doi: 10.1126/science.168.3931.591. [DOI] [PubMed] [Google Scholar]
- Stratil A. Transferrin and albumin loci in chickens, Gallus gallus L. Comp Biochem Physiol. 1968 Jan;24(1):113–121. doi: 10.1016/0010-406x(68)90962-6. [DOI] [PubMed] [Google Scholar]
- Teraoka H., Numa S. Content, synthesis and degradation of acetyl-coenzyme A carboxylase in the liver of growing chicks. Eur J Biochem. 1975 May 6;53(2):465–470. doi: 10.1111/j.1432-1033.1975.tb04087.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. A comparison of conalbumin and transferrin in the domestic fowl. Biochem J. 1962 May;83:355–364. doi: 10.1042/bj0830355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J. Serum proteins and the livetins of hen's-egg yolk. Biochem J. 1962 May;83:346–355. doi: 10.1042/bj0830346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter W. P., Buss E. G., Clagett C. O., Boucher R. V. The nature of the biochemical lesion in avian renal riboflavinuria. I. Effect of genotype on renal riboflavin metabolism. Comp Biochem Physiol. 1967 Sep;22(3):889–896. doi: 10.1016/0010-406x(67)90779-7. [DOI] [PubMed] [Google Scholar]
- Winter W. P., Buss E. G., Clagett C. O., Boucher R. V. The nature of the biochemical lesion in avian renal riboflavinuria. II. The inherited change of a riboflavin-binding protein from blood and eggs. Comp Biochem Physiol. 1967 Sep;22(3):897–906. doi: 10.1016/0010-406x(67)90780-3. [DOI] [PubMed] [Google Scholar]


