Abstract
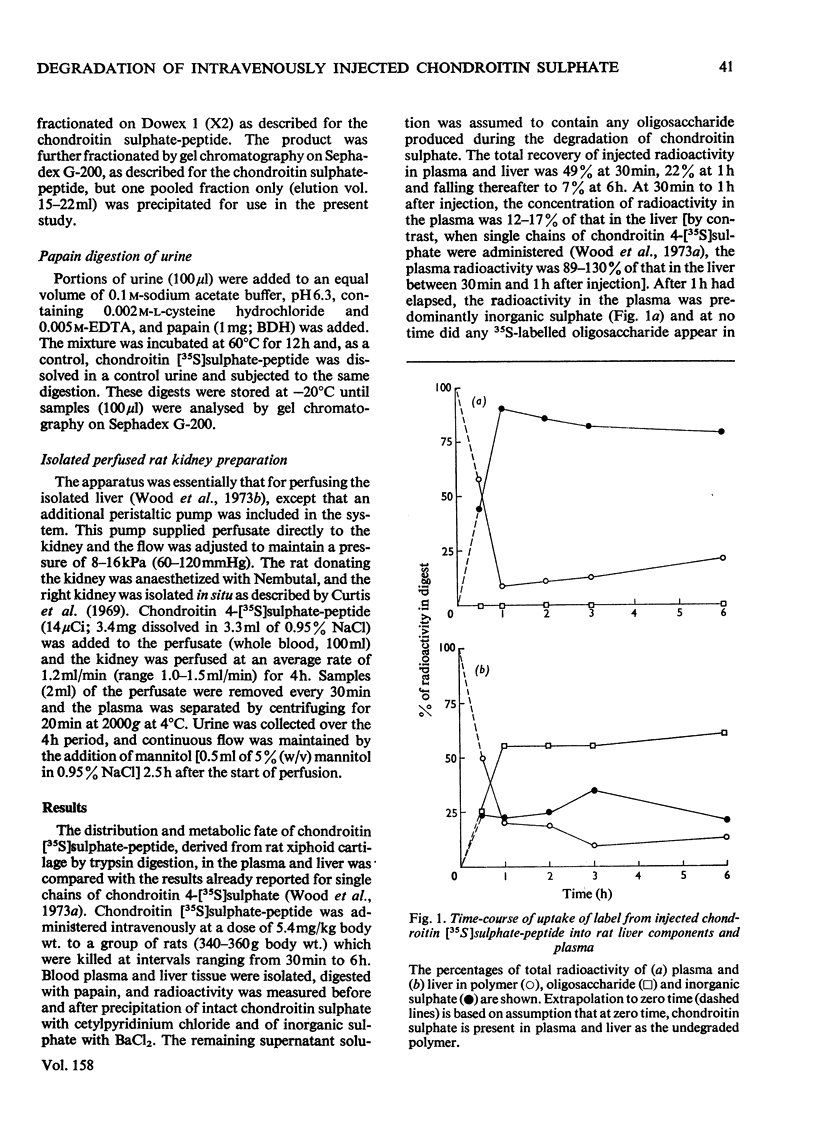
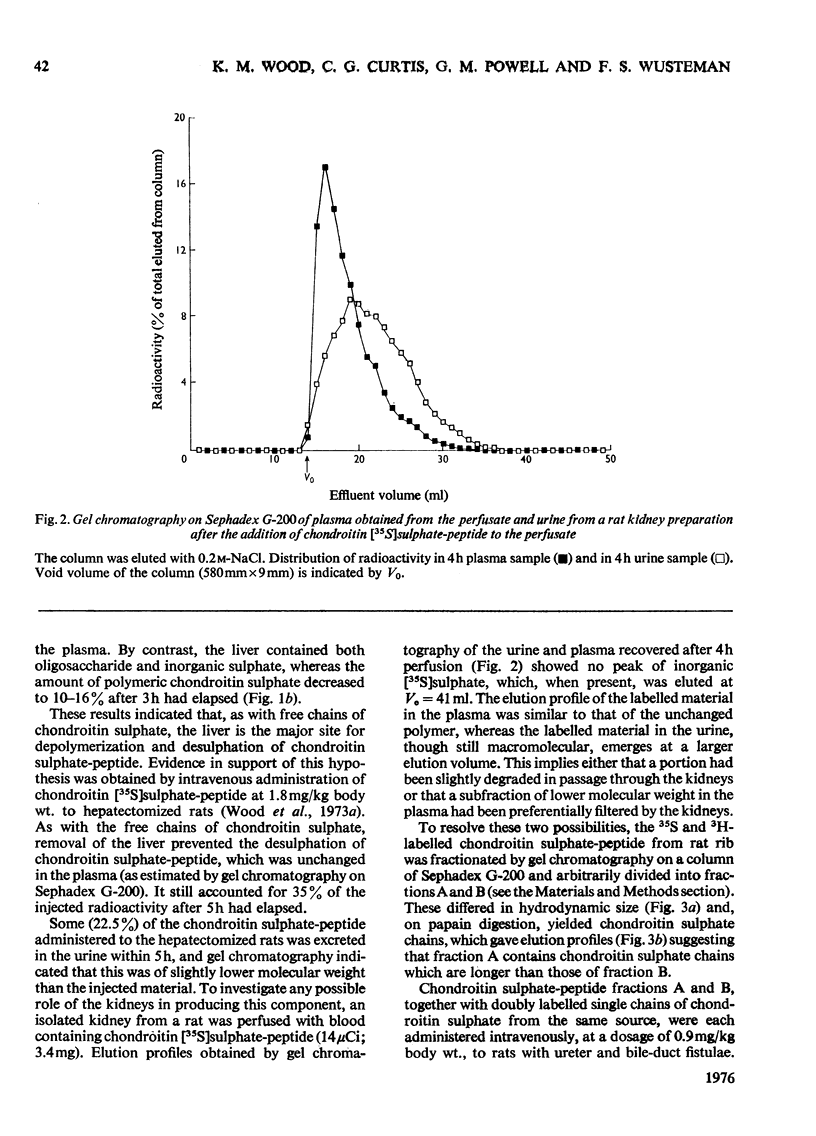
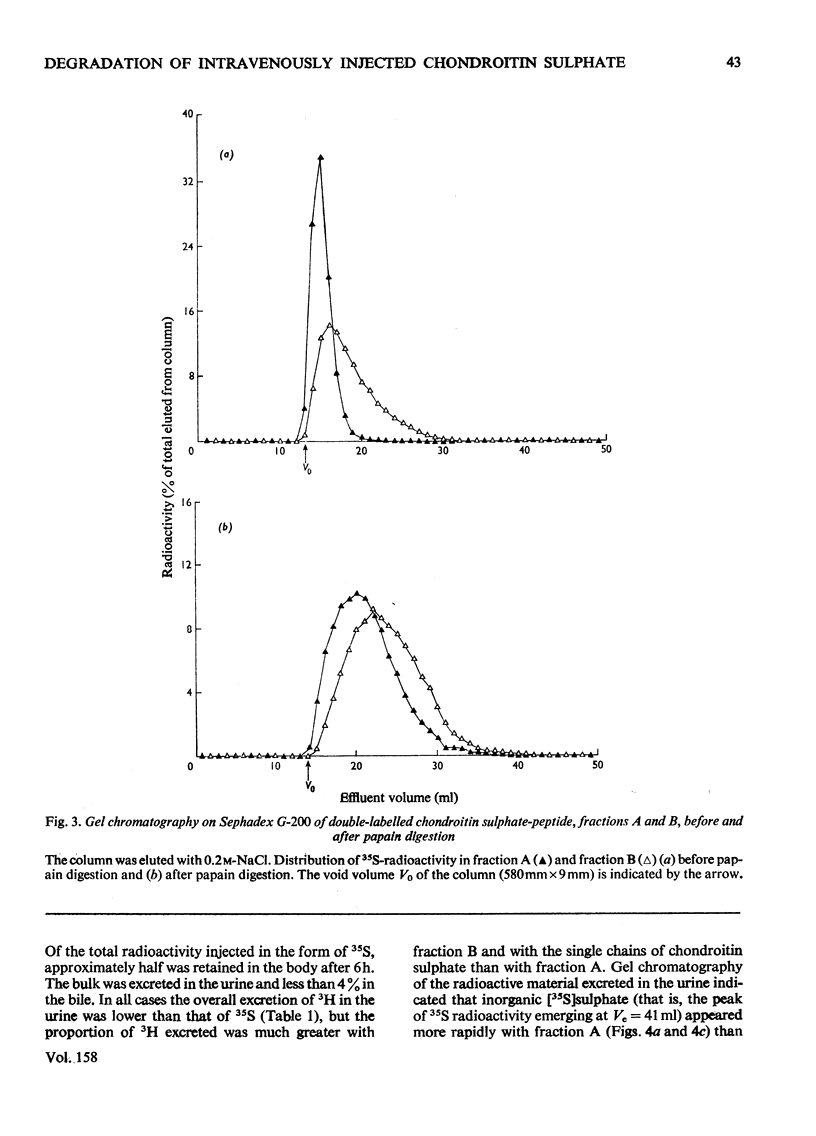
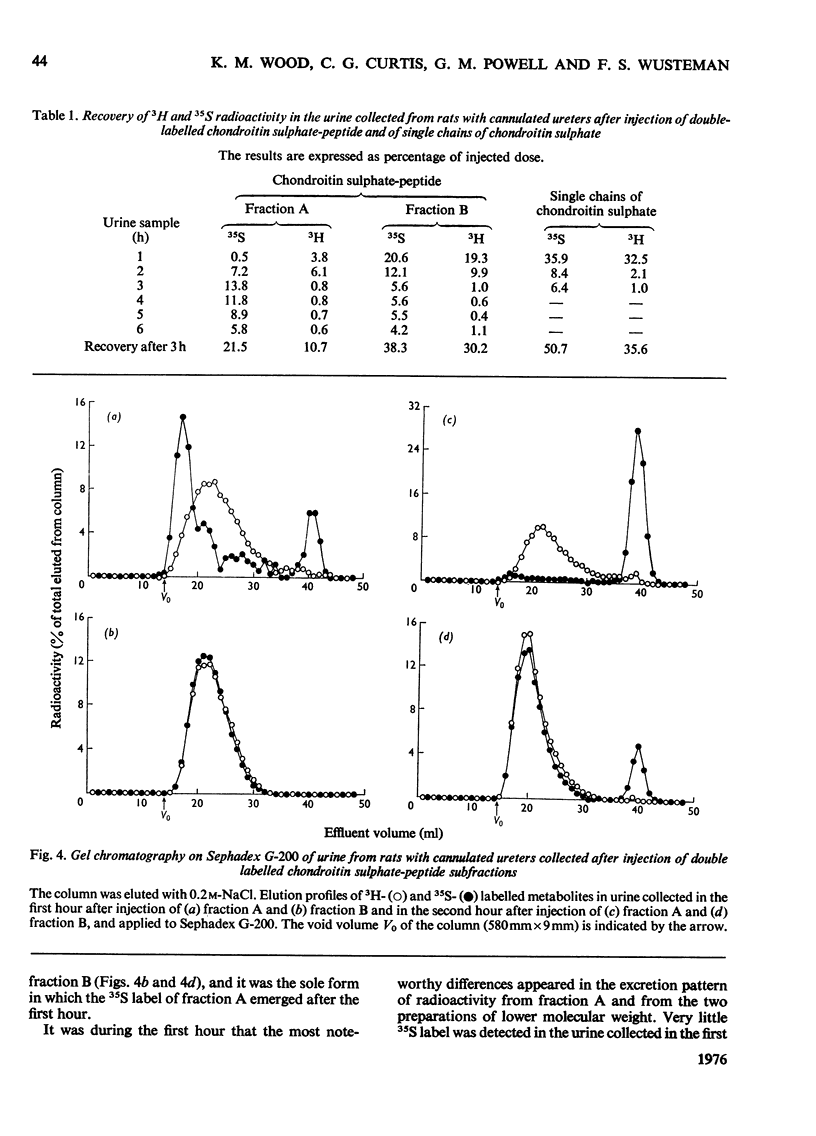
The degradation of intravenously administered chondroitin sulphate-peptide, obtained by trypsin digestion of rat cartilage preparations labelled in vitro with 35S (and, in some cases, with 3H), was studied in rats. As with free chains of chondroitin sulphate, the major site of accumulation and degradation in the body was the liver, although peptide-linked chains were taken up more rapidly than free chains. In the first 2h after intravenous injection of a chondroitin sulphate-peptide fraction, labelled macromolecular components were excreted in the urine. These were shown to be chondroitin sulphate-peptide of the same degree of sulphation but of smaller average size than the injected material. A similar observation was made when free chains of chondroitin sulphate from the same source were administered intravenously. An isolated perfused rat kidney failed to de-sulphate circulating chondroitin sulphate-peptide, but a component of lower average molecular weight was excreted in the urine. When a chondroitin sulphate-peptide fraction of relatively larger hydrodynamic volume was administered, very little chondroitin sulphate appeared in the urine in the first 2h. It was concluded that, depending on size and/or peptide content, the chondroitin sulphate-peptide released from connective tissues into the circulation would probably be subjected to one of two alternative fates. The smaller fragments are more likely to be excreted in the urine, whereas the larger ones are taken up by the liver and there degraded to inorganic sulphate and undefined carbohydrate components.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadò R., Ingmar B., Lindahl U., Wasteson A. Depolymerisation and desulphation of chondroitin sulphate by enzymes from embryonic chick cartilage. FEBS Lett. 1974 Feb 1;39(1):49–52. doi: 10.1016/0014-5793(74)80014-1. [DOI] [PubMed] [Google Scholar]
- Curtis C. G., Powell G. M., Dodgson K. S. The site of metabolism of potassium L-tyrosine O- 35 S-sulphate in the rat. Biochem Pharmacol. 1969 Oct;18(10):2551–2558. doi: 10.1016/0006-2952(69)90369-4. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse H., Tekolf W., von Figura K., Buddecke E. Metabolism of sulfated glycosaminoglycans in cultivated bovine arterial cells. II. Quantitative studies on the uptake of 35SO4-labeled proteoglycans. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):943–952. doi: 10.1515/bchm2.1975.356.s1.943. [DOI] [PubMed] [Google Scholar]
- Liau Y. H., Horowitz M. I. Desulfation and depolymerization of chondroitin 4-sulfate and its degradation products by rat stomach, liver and small intestine. Proc Soc Exp Biol Med. 1974 Sep;146(4):1037–1043. doi: 10.3181/00379727-146-38242. [DOI] [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Revell P. A., Muir H. The excretion and degradation of chondroitin 4-sulphate administered to guinea pigs as free chondroitin sulphate and as proteoglycan. Biochem J. 1972 Nov;130(2):597–606. doi: 10.1042/bj1300597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Wasteson A., Lindahl U., Hallén A. Mode of degradation of the chondroitin sulphate proteoglycan in rat costal cartilage. Biochem J. 1972 Dec;130(3):729–738. doi: 10.1042/bj1300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973 Mar 10;248(5):1634–1642. [PubMed] [Google Scholar]
- Wood K. M., Wusteman F. S., Curtis C. G. The degradation of intravenously injected chondroitin 4-sulphate in the rat. Biochem J. 1973 Aug;134(4):1009–1013. doi: 10.1042/bj1341009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K. M., Wusteman F. S., Curtis C. G. The metabolic fate of chondroitin (35S)sulphate proteoglycan in the rat. Biochem Soc Trans. 1975;3(4):500–502. doi: 10.1042/bst0030500. [DOI] [PubMed] [Google Scholar]
- Wusteman F. S., Davidson E. A. The linkage region in the polypeptide of pig costal cartilage proteoglycan. Connect Tissue Res. 1975;3(2):123–133. doi: 10.3109/03008207509152170. [DOI] [PubMed] [Google Scholar]


