Abstract
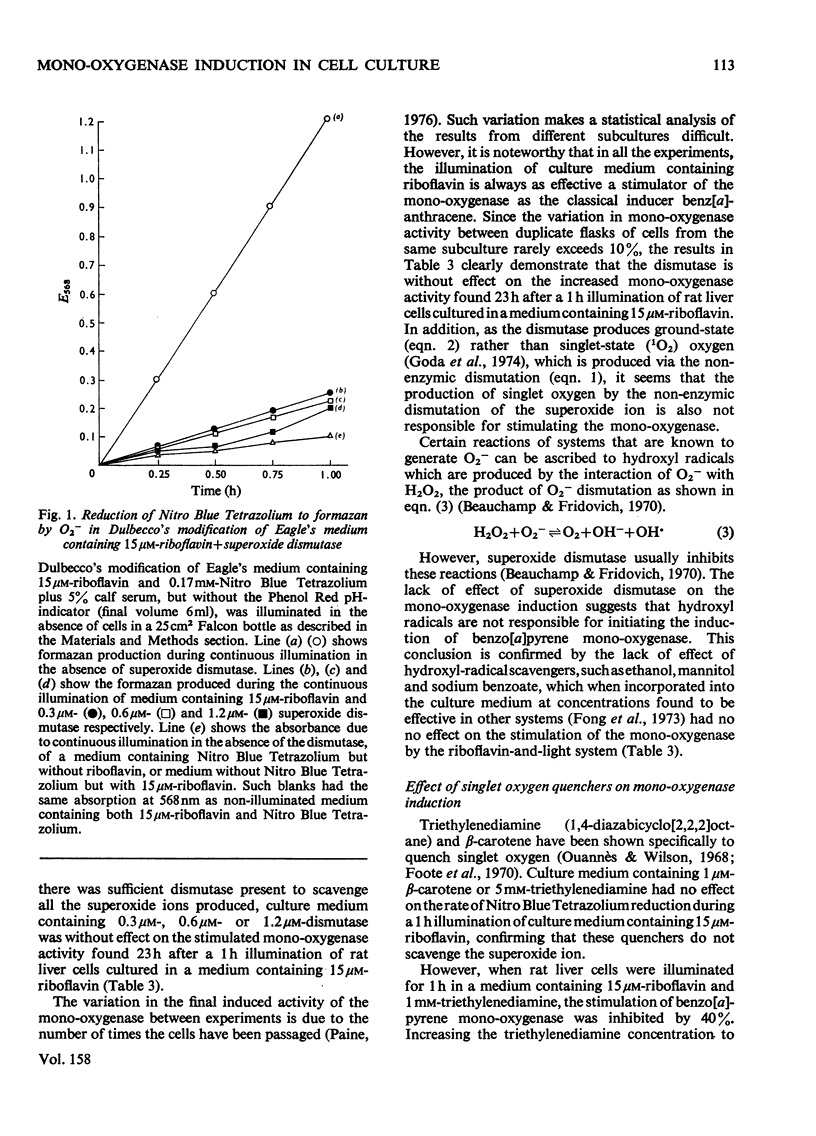
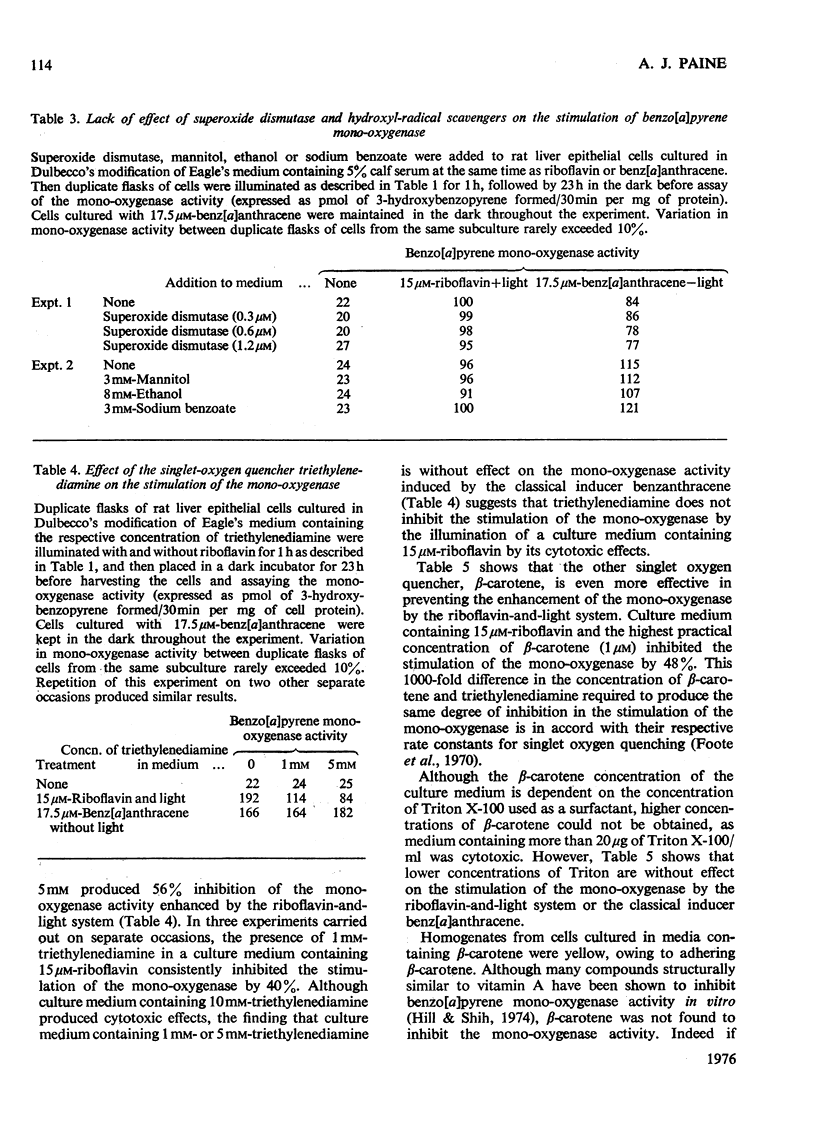
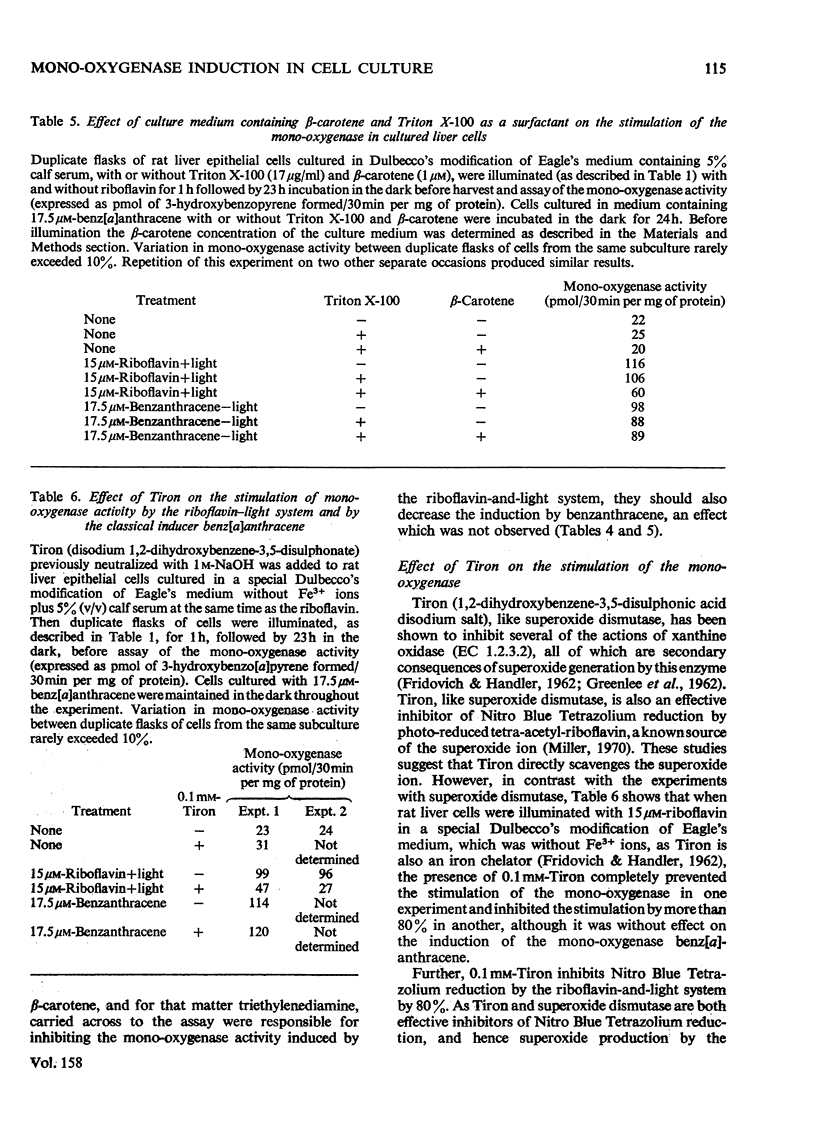
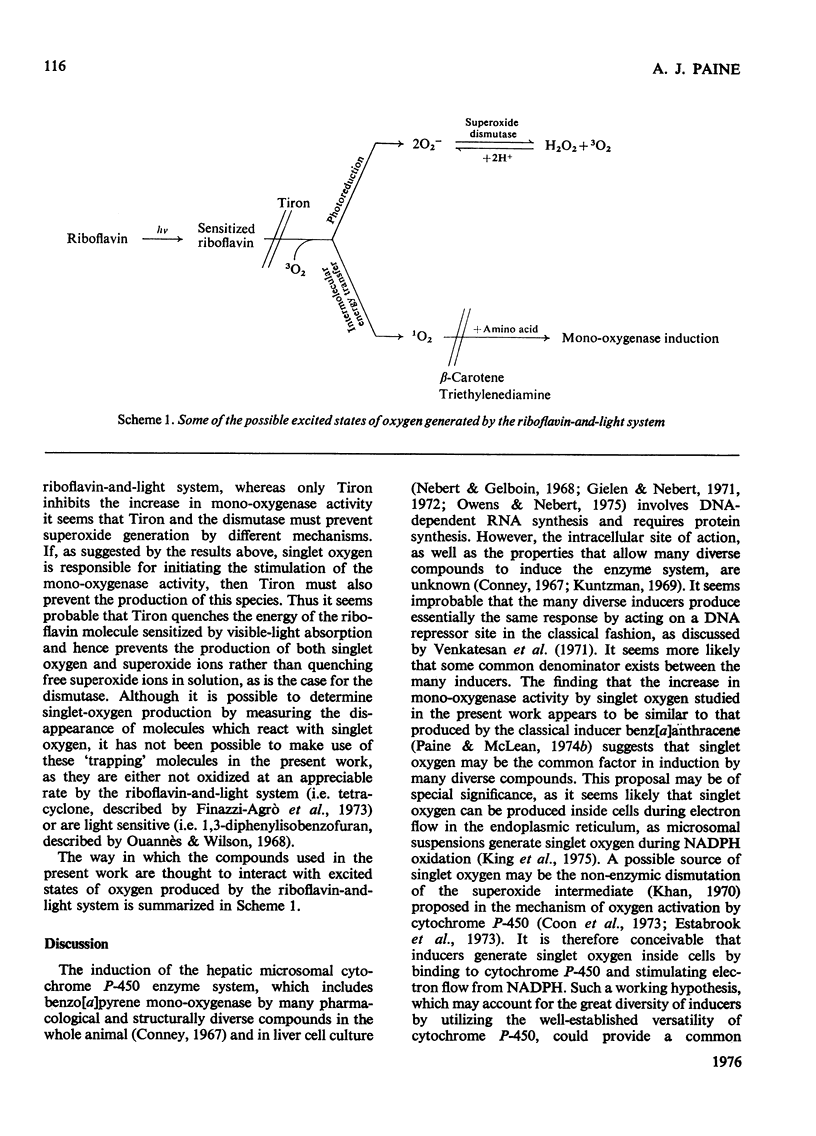
1. The photochemical generation of excited states of oxygen in liver cell culture by the mild ilumination of culture medium containing riboflavin, results in stimulation of benzo[a]pyrene 3-mono-oxygenase, a cytochrome P-450-linked mono-oxygenase. 2. The same large increase in mono-oxygenase activity was found when medium containing riboflavin was illuminated in the absence of cells and then stored in the dark for 24h before contact with the cells. From this it may be inferred that stimulation is due to the formation of a stable inducer in the culture medium. Further experiments indicate that the stable inducer is due to the photo-oxidation of an amino acid. 3. Evidence that singlet oxygen is responsible for initiating the stimulation of the mono-oxygenase is based on the use of molecules that scavenge particular active oxygen species. Of all the scavengers tested, only those that scavenge single oxygen inhibited the stimulation. 4. A hypothesis is developed to relate the stimulation of the mono-oxygenase by singlet oxygen in cultured cells to the regulation of the cytochrome P-450 enzyme system in vivo. It is suggested that single oxygen generation within cells may be a common factor linking the many structurally diverse inducers of the enzyme system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Roerig D. L., Pederson T. C. Evidence for superoxide generation by NADPH-cytochrome c reductase of rat liver microsomes. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1133–1137. doi: 10.1016/0006-291x(72)90952-7. [DOI] [PubMed] [Google Scholar]
- Ballou D., Palmer G., Massey V. Direct demonstration of superoxide anion production during the oxidation of reduced flavin and of its catalytic decomposition by erythrocuprein. Biochem Biophys Res Commun. 1969 Sep 10;36(6):898–904. doi: 10.1016/0006-291x(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Coon M. J., Strobel H. W., Boyer R. F. On the mechanism of hydroxylation reactions catalyzed by cytochrome P-450. Drug Metab Dispos. 1973 Jan-Feb;1(1):92–97. [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Debey P., Balny C. Production of superoxide ions in rat liver microsomes. Biochimie. 1973;55(3):329–332. doi: 10.1016/s0300-9084(73)80133-6. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Matsubara T., Mason J. I., Werringloer J., Baron J. Studies on the molecular function of cytochrome P-450 during drug metabolism. Drug Metab Dispos. 1973 Jan-Feb;1(1):98–110. [PubMed] [Google Scholar]
- FRIDOVICH I., HANDLER P. Xanthine oxidase. V. Differential inhibition of the reduction of various electron acceptors. J Biol Chem. 1962 Mar;237:916–921. [PubMed] [Google Scholar]
- Finazzi Agró F., De Sole P., Rotilio G., Mondoví B. Influence of catalase and superoxide dismutase on side oxidations involving singlet oxygen. Ital J Biochem. 1973 Sep-Dec;22(5):217–231. [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- GREENLEE L., FRIDOVICH I., HANDLER P. Chemiluminescence induced by operation of iron-flavoproteins. Biochemistry. 1962 Sep;1:779–783. doi: 10.1021/bi00911a008. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V., Wiebel F. J., Kinoshita N. Microsomal aryl hydrocarbon hydroxylases: on their role in polycyclic hydrocarbon carcinogenesis and toxicity and the mechanism of enzyme induction. Biochem Soc Symp. 1972;34:103–133. [PubMed] [Google Scholar]
- Gielen J. E., Nebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. 3. Effects of various sera, hormones, biogenic amines, and other endogenous compounds on the enzyme activity. J Biol Chem. 1972 Dec 10;247(23):7591–7602. [PubMed] [Google Scholar]
- Gielen J. E., Nebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. I. Stimulation of enzyme activity in nonhepatic cells and in hepatic cells by phenobarbital, polycyclic hydrocarbons, and 2,2-bis(p-chlorophenyl)-1,1,1-trichloroethane. J Biol Chem. 1971 Sep 10;246(17):5189–5198. [PubMed] [Google Scholar]
- Goda K., Kimura T., Thayer A. L., Kees K., Schaap A. P. Singlet molecular oxygen in biological systems: non-quenching of singlet oxygen-mediated chemiluminescence by superoxide dismutase. Biochem Biophys Res Commun. 1974 Jun 4;58(3):660–666. doi: 10.1016/s0006-291x(74)80469-9. [DOI] [PubMed] [Google Scholar]
- Hartz J. W., Deutsch H. F. Preparation and physicochemical properties of human erythrocuprein. J Biol Chem. 1969 Sep 10;244(17):4565–4572. [PubMed] [Google Scholar]
- Hill D. L., Shih T. W. Vitamin A compounds and analogs as inhibitors of mixed-function oxidases that metabolize carcinogenic polycyclic hydrocarbons and other compounds. Cancer Res. 1974 Mar;34(3):564–570. [PubMed] [Google Scholar]
- Khan A. U. Singlet molecular oxygen from superoxide anion and sensitized fluorescence of organic molecules. Science. 1970 Apr 24;168(3930):476–477. doi: 10.1126/science.168.3930.476. [DOI] [PubMed] [Google Scholar]
- King M. M., Lai E. K., McCay P. B. Singlet oxygen production associated with enzyme-catalyzed lipid peroxidation in liver microsomes. J Biol Chem. 1975 Aug 25;250(16):6496–6502. [PubMed] [Google Scholar]
- Kuntzman R. Drugs and enzyme induction. Annu Rev Pharmacol. 1969;9:21–36. doi: 10.1146/annurev.pa.09.040169.000321. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marshall W. J., McLean A. E. A requirement for dietary lipids for induction of cytochrome P-450 by phenobarbitone in rat liver microsomal fraction. Biochem J. 1971 May;122(4):569–573. doi: 10.1042/bj1220569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Miller R. W. Reactions of superoxide anion, catechols, and cytochrome c. Can J Biochem. 1970 Aug;48(8):935–939. doi: 10.1139/o70-145. [DOI] [PubMed] [Google Scholar]
- Montesano R., Saint Vincent L., Tomatis L. Malignant transformation in vitro of rat liver cells by dimethylnitrosamine and N-methyl-N'-nitro-N-nitrosoguanidine. Br J Cancer. 1973 Sep;28(3):215–220. doi: 10.1038/bjc.1973.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. II. Cellular responses during enzyme induction. J Biol Chem. 1968 Dec 10;243(23):6250–6261. [PubMed] [Google Scholar]
- Owens I. S., Nebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver-derived cell cultures. Stimulation of "cytochrome P1-450-associated" enzyme activity by many inducing compounds. Mol Pharmacol. 1975 Jan;11(1):94–104. [PubMed] [Google Scholar]
- Paine A. J. Effect of amino acids and inducers on the activity of the microsomal mono-oxygenase system in rat liver cell culture. Chem Biol Interact. 1976 Jun;13(3-4):307–315. doi: 10.1016/0009-2797(76)90083-1. [DOI] [PubMed] [Google Scholar]
- Paine A. J., McLean A. E. Induction of aryl hydrocarbon hydroxylase by a light-driven superoxide generating system in liver cell culture. Biochem Biophys Res Commun. 1974 May 20;58(2):482–486. doi: 10.1016/0006-291x(74)90390-8. [DOI] [PubMed] [Google Scholar]
- Paine A. J., McLean A. E. Role of adrenochrome in aryl hydrocarbon hydroxylase induction by epinephrine in rat liver cell culture. Biochem Pharmacol. 1974 Jul 1;23(13):1910–1913. doi: 10.1016/0006-2952(74)90201-9. [DOI] [PubMed] [Google Scholar]
- Venkatesan N., Arcos J. C., Argus M. F. Induction and repression of microsomal drug-metabolizing enzymes by polycyclic hydrocarbons and phenobarbital: theoretical models. J Theor Biol. 1971 Dec;33(3):517–537. doi: 10.1016/0022-5193(71)90094-4. [DOI] [PubMed] [Google Scholar]


