Abstract
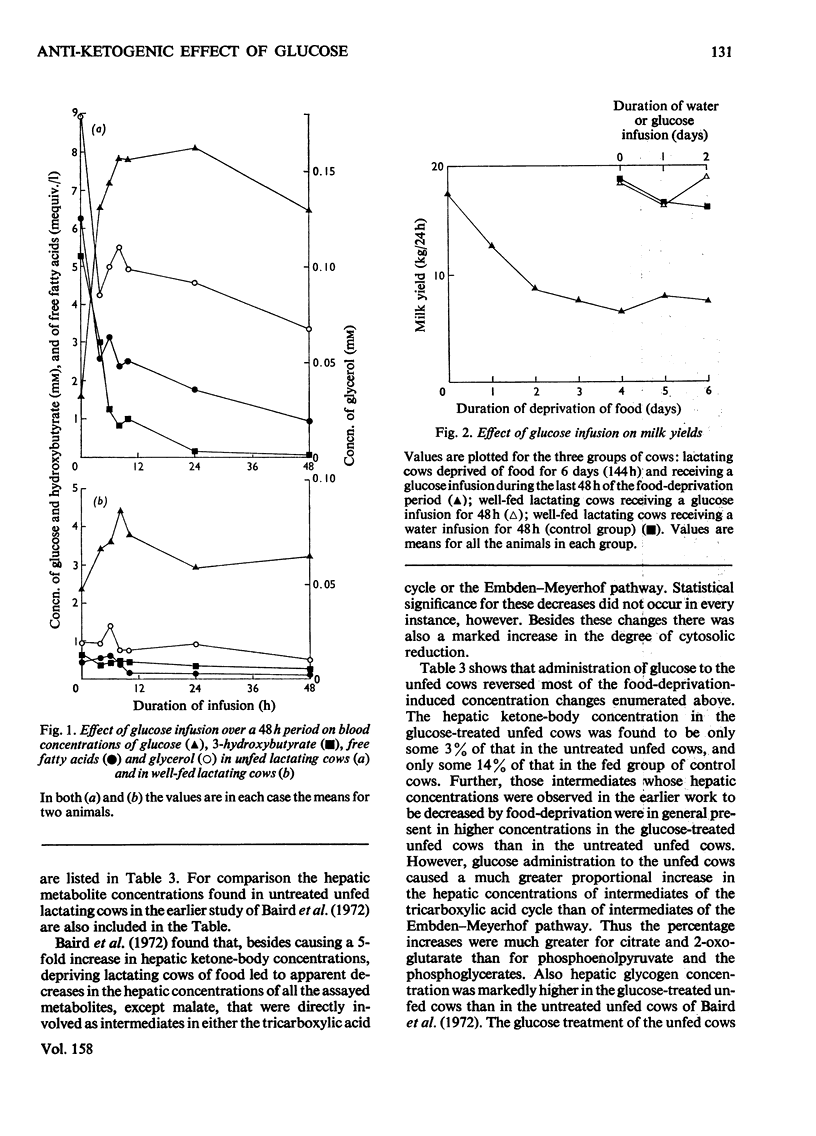
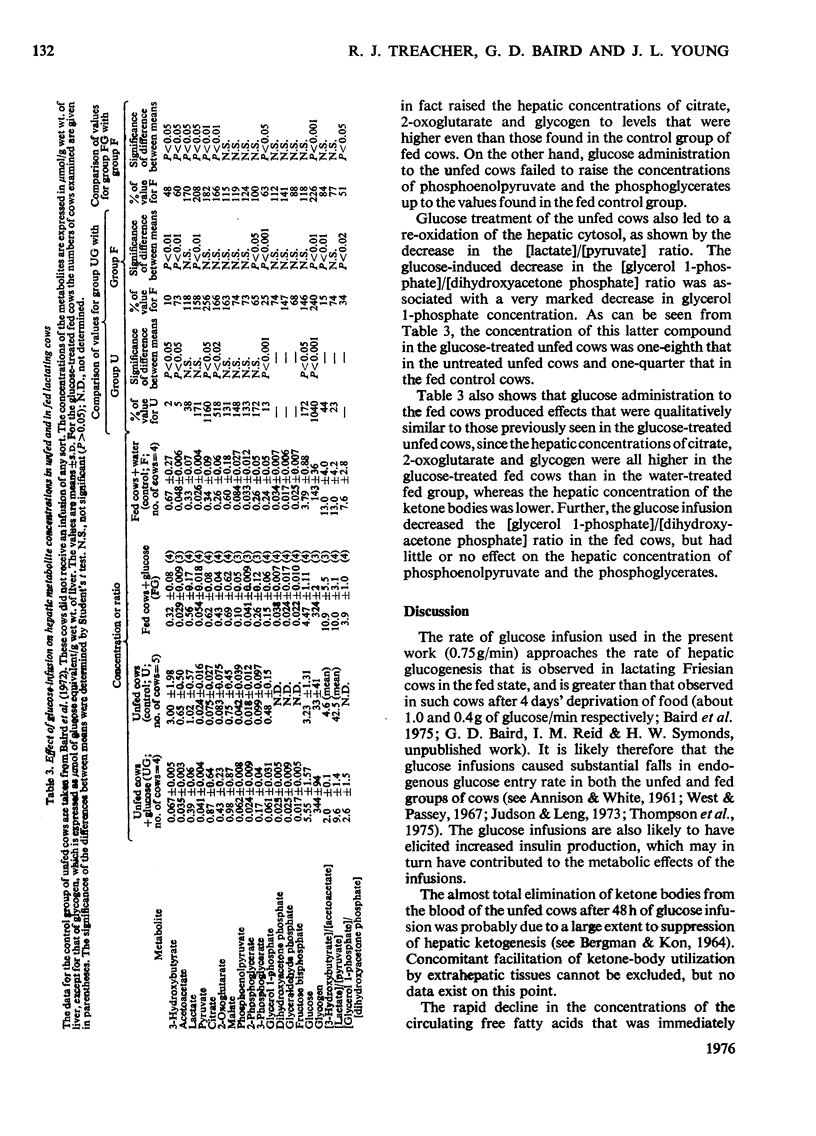
1. The aim of this study was to determine the effect of a constant infusion of glucose on the ketosis that is observed when dairy cows are deprived of food in early lactation. 2. Cows in early lactation were first deprived of food for 4 days (96h) to induce a 'fasting ketosis'. Glucose was then infused intravenously at a constant rate of 0.75 g/min for 48h while deprivation of food was maintained. At the end of this 48 h period, blood and liver ketone-body concentrations had decreased to values well below those found in healthy fed cows. 3. On the assumption that the anti-ketogenic effect of glucose was mainly due to suppression of hepatic ketogenesis, it was concluded that two anti-ketogenic mechanisms had been identified. These were (a) a decrease in the availability of free fatty acids for hepatic oxidation, and (b) anti-ketogenic changes within the liver itself. 4. These latter anti-ketogenic changes were twofold. The first was a major increase in the hepatic concentrations of citrate and 2-oxoglutarate. The second was an increase in the degree of oxidation of the hepatic cytosol. It was proposed that both these intrahepatic changes might indicate an augmentation of the quantity of oxaloacetate available for condensation with acetyl-CoA derived from fat oxidation. 5. Hepatic glycerol 1-phosphate concentration fell substantially after glucose infusion. 6. Glucose infusion into fed cows produced qualitatively similar effects to those observed in the unfed cows. However, blood and liver ketone-body concentrations were not decreased to the same extent in the fed cows as in the unfed cows.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., WHITE R. R. Glucose utilization in sheep. Biochem J. 1961 Jul;80:162–169. doi: 10.1042/bj0800162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMAN E. N., KON K. ACETOACETATE TURNOVER AND OXIDATION RATES IN OVINE PREGNANCY KETOSIS. Am J Physiol. 1964 Feb;206:449–452. doi: 10.1152/ajplegacy.1964.206.2.449. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J. Gluconeogenesis in the cow. The effects of a glucocorticoid on hepatic intermediary metabolism. Biochem J. 1970 Mar;116(5):865–874. doi: 10.1042/bj1160865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J., Hibbitt K. G. Effects of starvation on intermediary metabolism in the lactating cow. A comparison with metabolic changes occurring during bovine ketosis. Biochem J. 1972 Aug;128(5):1311–1318. doi: 10.1042/bj1281311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G. D., Heitzman R. J. Mode of action of a glucocorticoid on bovine intermediary metabolism. Possible role in controlling hepatic ketogenesis. Biochim Biophys Acta. 1971 Oct;252(1):184–198. doi: 10.1016/0304-4165(71)90107-3. [DOI] [PubMed] [Google Scholar]
- Baird G. D., Hibbitt K. G., Hunter G. D. Biochemical aspects of bovine ketosis. Biochem J. 1968 May;107(5):683–689. doi: 10.1042/bj1070683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman E. N. Hyperketonemia-ketogenesis and ketone body metabolism. J Dairy Sci. 1971 Jun;54(6):936–948. doi: 10.3168/jds.S0022-0302(71)85950-7. [DOI] [PubMed] [Google Scholar]
- Bieberdorf F. A., Chernick S. S., Scow R. O. Effect of insulin and acute diabetes on plasma FFA and ketone bodies in the fasting rat. J Clin Invest. 1970 Sep;49(9):1685–1693. doi: 10.1172/JCI106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Holloway P. A., Aberti K. G. The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem J. 1975 Jun;148(3):353–362. doi: 10.1042/bj1480353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRITZ I. B. Factors influencing the rates of long-chain fatty acid oxidation and synthesis in mammalian systems. Physiol Rev. 1961 Jan;41:52–129. doi: 10.1152/physrev.1961.41.1.52. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., REIM M. Steady state equilibria of some DPN-linked reactions and the oxidation/reduction state of the DPN/DPNH system in the cytoplasmatic compartment of liver cells in vivo. Biochem Biophys Res Commun. 1961 Mar 10;4:159–162. doi: 10.1016/0006-291x(61)90262-5. [DOI] [PubMed] [Google Scholar]
- Heitzman R. J., Herriman I. D., Mallinson C. B. Some effects of glucocorticoids on the subcellular distribution of the activities of citrate synthase and phosphoenolpyruvate carboxykinase in livers of rats and cows. FEBS Lett. 1972 Jan 15;20(1):19–21. doi: 10.1016/0014-5793(72)80006-1. [DOI] [PubMed] [Google Scholar]
- Judson G. J., Leng R. A. Studies on the control of gluconeogenesis in sheep: effect of glucose infusion. Br J Nutr. 1973 Mar;29(2):159–174. doi: 10.1079/bjn19730092. [DOI] [PubMed] [Google Scholar]
- Katz M. L., Bergman E. N. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969 Apr;216(4):953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- Kronfeld D. S. Ketosis in pregnant sheep and lactating cows. A review. Aust Vet J. 1972 Dec;48(12):680–687. doi: 10.1111/j.1751-0813.1972.tb09250.x. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of ketogenesis and clinical aspects of the ketotic state. Metabolism. 1972 May;21(5):471–489. doi: 10.1016/0026-0495(72)90059-5. [DOI] [PubMed] [Google Scholar]
- Moreno F. J., Sánchez-Urrutia L., Medina J. M., Sánchez-Medina F., Mayor F. Stimulation of phosphoenolpyruvate carboxykinase (guanosine triphosphate) activity by low concentrations of circulating glucose in perfused rat liver. Biochem J. 1975 Jul;150(1):51–58. doi: 10.1042/bj1500051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrago E., Young J. W., Lardy H. A. Carbohydrate supply as a regulator of rat liver phosphoenolpyruvate carboxykinase activity. Science. 1967 Dec 22;158(3808):1572–1573. doi: 10.1126/science.158.3808.1572. [DOI] [PubMed] [Google Scholar]
- Thompson J. R., Weiser G., Seto K., Black A. L. Effect of glucose load on synthesis of plasma glucose in lactating cows. J Dairy Sci. 1975 Mar;58(3):362–370. doi: 10.3168/jds.S0022-0302(75)84573-5. [DOI] [PubMed] [Google Scholar]
- Treacher R. J. An apparatus for the intravenous infusion of fluids into unrestrained cattle during long periods. Vet Rec. 1973 Dec 8;93(23):596–597. doi: 10.1136/vr.93.23.596. [DOI] [PubMed] [Google Scholar]
- West C. E., Passey R. F. Effect of glucose load and of insulin on the metabolism of glucose and of palmitate in sheep. Biochem J. 1967 Jan;102(1):58–64. doi: 10.1042/bj1020058. [DOI] [PMC free article] [PubMed] [Google Scholar]


