Abstract
1. The regulation of glucose uptake and disposition in skeletal muscle was studied in the isolated perfused rat hindquarter. 2. Insulin and exercise, induced by sciatic-nerve stimulation, enhanced glucose uptake about tenfold in fed and starved rats, but were without effect in rats with diabetic ketoacidosis. 3. At rest, the oxidation of lactate (0.44 mumol/min per 30 g muscle in fed rats) was decreased by 75% in both starved and diabetic rats, whereas the release of alanine and lactate (0.41 and 1.35 mumol/min per 30 g respectively in the fed state) was increased. Glycolysis, defined as the sum of lactate+alanine release and lactate oxidation, was not decreased in either starvation or diabetes. 4. In all groups, exercise tripled O2 consumption (from approximately 8 to approximately 25 mumol/min per 30 g of muscle) and increased the release and oxidation of lactate five- to ten-fold. The differences in lactate release between fed, starved and diabetic rats observed at rest were no longer apparent; however, lactate oxidation was still several times greater in the fed group. 5. Perfusion of the hindquarter of a fed rat with palmitate, octanoate or acetoacetate did not alter glucose uptake or lactate release in either resting or exercising muslce; however, lactate oxidation was significantly inhibited by acetoacetate, which also increased the intracellular concentration of acetyl-CoA. 6. The data suggest that neither that neither glycolysis nor the capacity for glucose transport are inhbitied in the perfused hindquarter during starvation or perfusion with fatty acids or ketone bodies. On the other hand, lactate oxidation is inhibited, suggesting diminished activity of pyruvate dehydrogenase. 7. Differences in the regulation of glucose metabolism in heart and skeletal muscle and the role of the glucose/fatty acid cycle in each tissue are discussed.
Full text
PDF


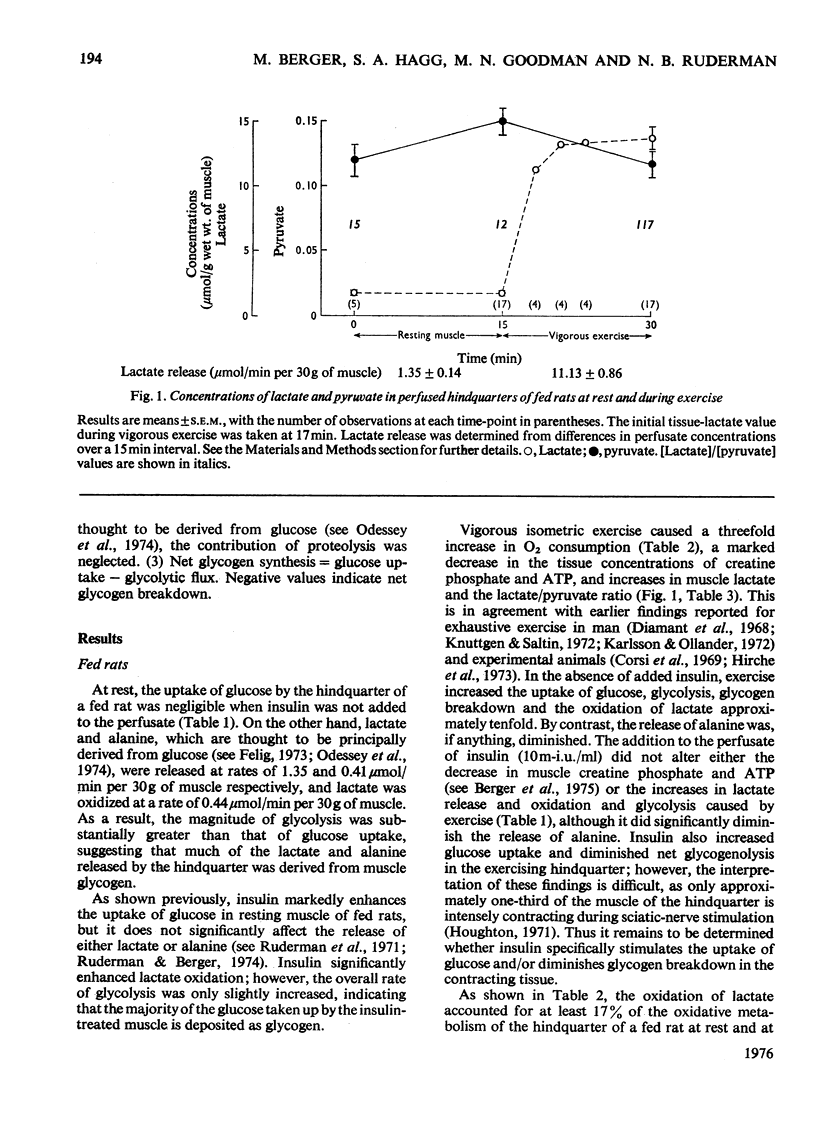
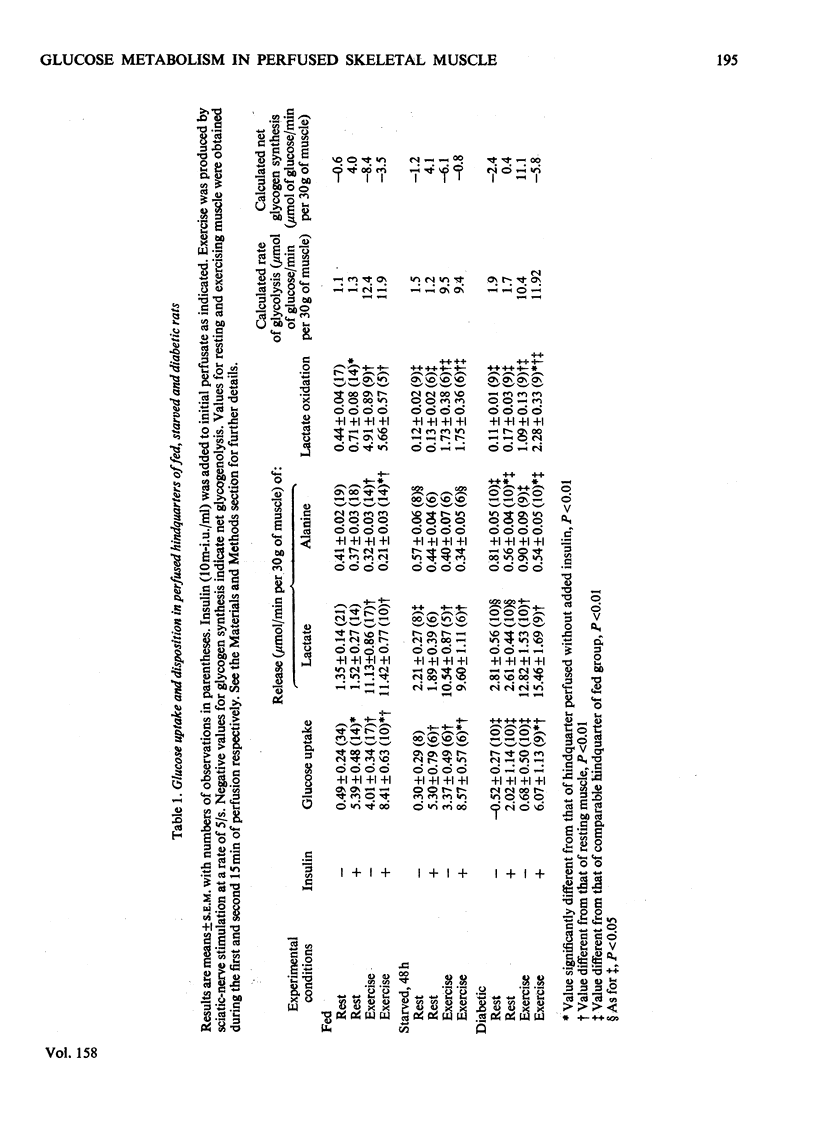
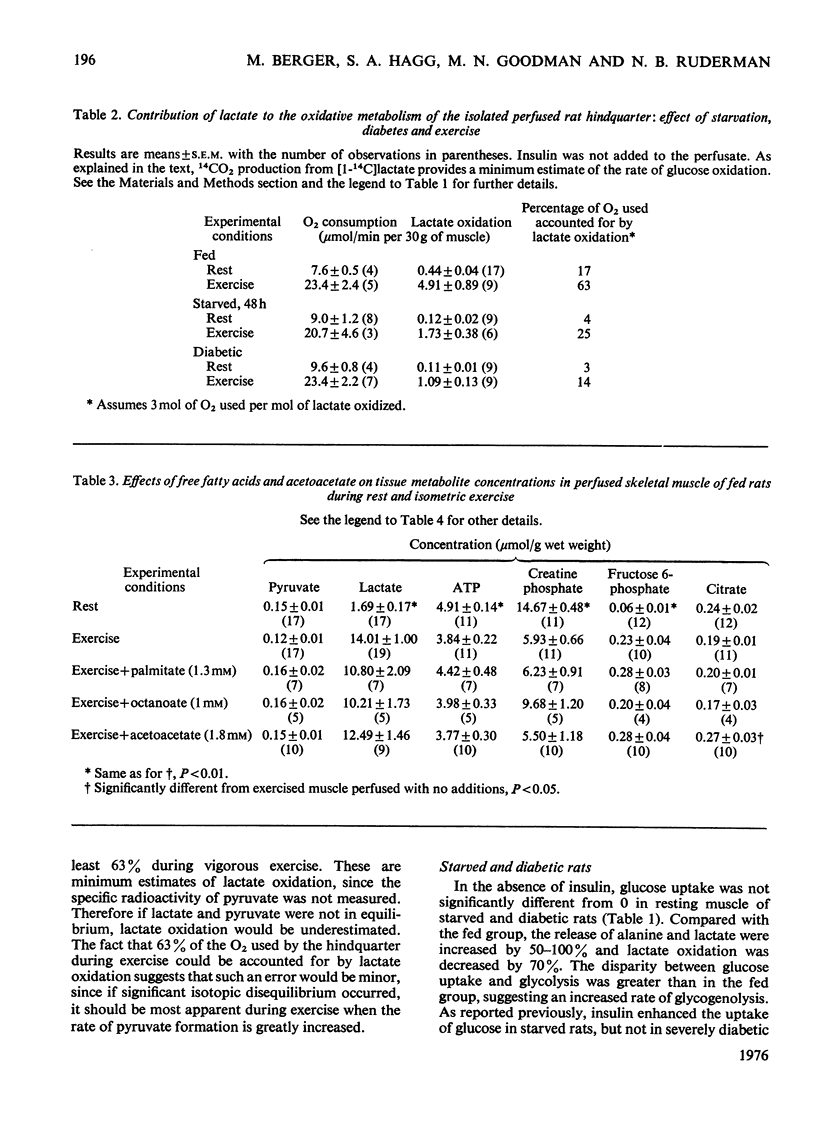




Selected References
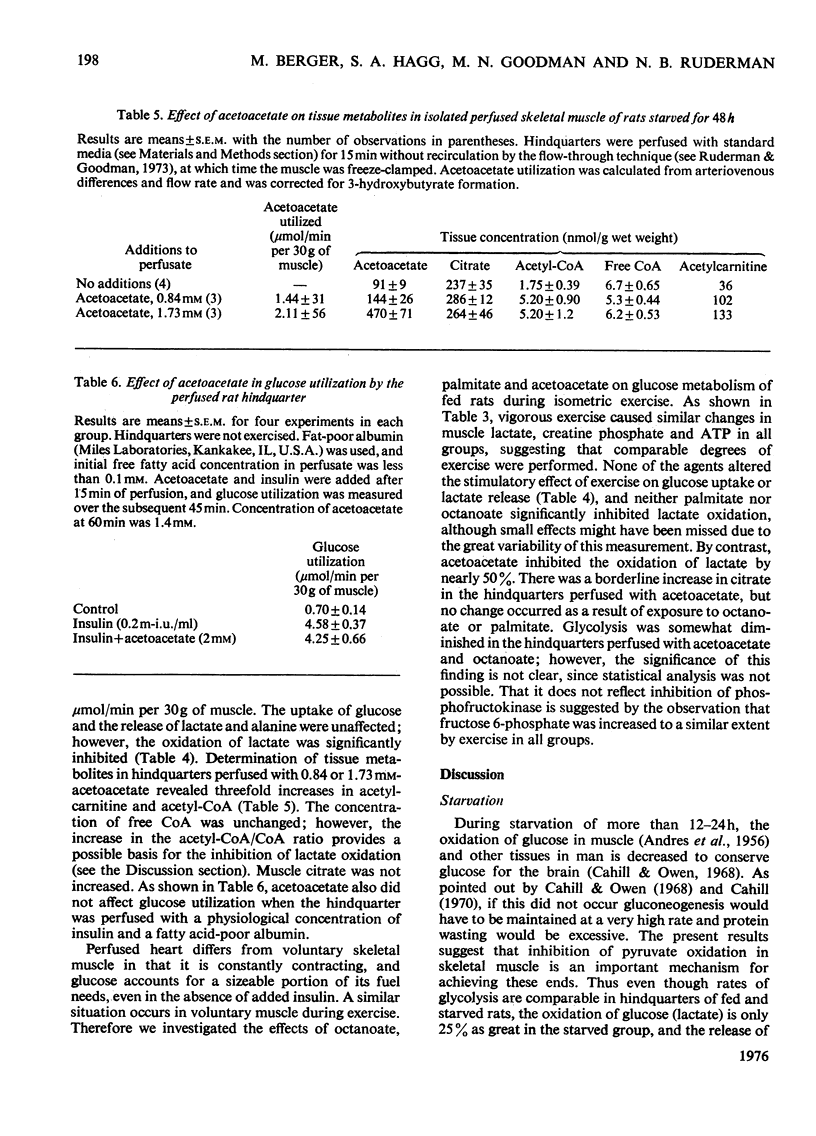
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrouny G. A. Differential patterns of glycogen metabolism in cardiac and skeletal muscles. Am J Physiol. 1969 Sep;217(3):686–693. doi: 10.1152/ajplegacy.1969.217.3.686. [DOI] [PubMed] [Google Scholar]
- Beatty C. H., Bocek R. M. Interrelation of carbohydrate and palmitate metabolism in skeletal muscle. Am J Physiol. 1971 Jun;220(6):1928–1934. doi: 10.1152/ajplegacy.1971.220.6.1928. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Interaction of insulin and exercise on glucose uptake. Biochem J. 1975 Jan;146(1):231–238. doi: 10.1042/bj1460231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J., Hultman E. A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest. 1967;19(3):218–228. doi: 10.3109/00365516709090629. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Corsi A., Midrio M., Granata A. L. In situ utilization of glycogen and blood glucose by skeletal muscle during tetanus. Am J Physiol. 1969 Jun;216(6):1534–1541. doi: 10.1152/ajplegacy.1969.216.6.1534. [DOI] [PubMed] [Google Scholar]
- DAWSON D. M., ROMANUL F. C. ENZYMES IN MUSCLES. II. HISTOCHEMICAL AND QUANTITATIVE STUDIES. Arch Neurol. 1964 Oct;11:369–378. doi: 10.1001/archneur.1964.00460220031004. [DOI] [PubMed] [Google Scholar]
- Diamant B., Karlsson J., Saltin B. Muscle tissue lactate after maximal exercise in man. Acta Physiol Scand. 1968 Mar;72(3):383–384. doi: 10.1111/j.1748-1716.1968.tb03861.x. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Green M. R., Landau B. R. Contribution of the pentose cycle to glucose metabolism in muscle. Arch Biochem Biophys. 1965 Sep;111(3):569–575. doi: 10.1016/0003-9861(65)90236-5. [DOI] [PubMed] [Google Scholar]
- Hagg S. A., Taylor S. I., Ruberman N. B. Glucose metabolism in perfused skeletal muscle. Pyruvate dehydrogenase activity in starvation, diabetes and exercise. Biochem J. 1976 Aug 15;158(2):203–210. doi: 10.1042/bj1580203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEANRENAUD B. Dynamic aspects of adipose tissue metabolism: a review. Metabolism. 1961 Jul;10:535–581. [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Rannels D. E., Munger B. L., Morgan H. E. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc. 1974 Apr;33(4):1098–1104. [PubMed] [Google Scholar]
- Karlsson J., Ollander B. Muscle metabolites with exhaustive static exercise of different duration. Acta Physiol Scand. 1972 Nov;86(3):309–314. doi: 10.1111/j.1748-1716.1972.tb05337.x. [DOI] [PubMed] [Google Scholar]
- Knuttgen H. G., Saltin B. Muscle metabolites and oxygen uptake in short-term submaximal exercise in man. J Appl Physiol. 1972 May;32(5):690–694. doi: 10.1152/jappl.1972.32.5.690. [DOI] [PubMed] [Google Scholar]
- Neely J. R., Bowman R. H., Morgan H. E. Effects of ventricular pressure development and palmitate on glucose transport. Am J Physiol. 1969 Apr;216(4):804–811. doi: 10.1152/ajplegacy.1969.216.4.804. [DOI] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A., Denton R. M., Pogson C. I. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Reimer F., Löffler G., Wieland O. H. Untersuchungen zum muskulären Kohlenhydrat- und Fettstoffwechsel am Modell des hämoglobin-frei perfundierten Rattenhinterbeins. Verh Dtsch Ges Inn Med. 1974;80:1231–1233. [PubMed] [Google Scholar]
- Ruderman N. B., Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974 Sep 10;249(17):5500–5506. [PubMed] [Google Scholar]
- Ruderman N. B., Goodman M. N. Regulation of ketone body metabolism in skeletal muscle. Am J Physiol. 1973 Jun;224(6):1391–1397. doi: 10.1152/ajplegacy.1973.224.6.1391. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Ross P. S., Berger M., Goodman M. N. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem J. 1974 Jan;138(1):1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- SACKTOR B., WORMSER-SHAVIT E., WHITE J. I. DIPHOSPHOPYRIDINE NUCLEOTIDE-LINKED CYTOPLASMIC METABOLITES IN RAT LEG MUSCLE IN SITU DURING CONTRACTION AND RECOVERY. J Biol Chem. 1965 Jun;240:2678–2681. [PubMed] [Google Scholar]
- Thompson M. P., Williamson D. H. Metabolic interactions of glucose, acetoacetate and adrenaline in rat submaxillary gland in vitro. Biochem J. 1975 Mar;146(3):635–644. doi: 10.1042/bj1460635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]
- Williamson D. H. Tissue-specific direction of blood metabolites. Symp Soc Exp Biol. 1973;27:283–298. [PubMed] [Google Scholar]


