Abstract
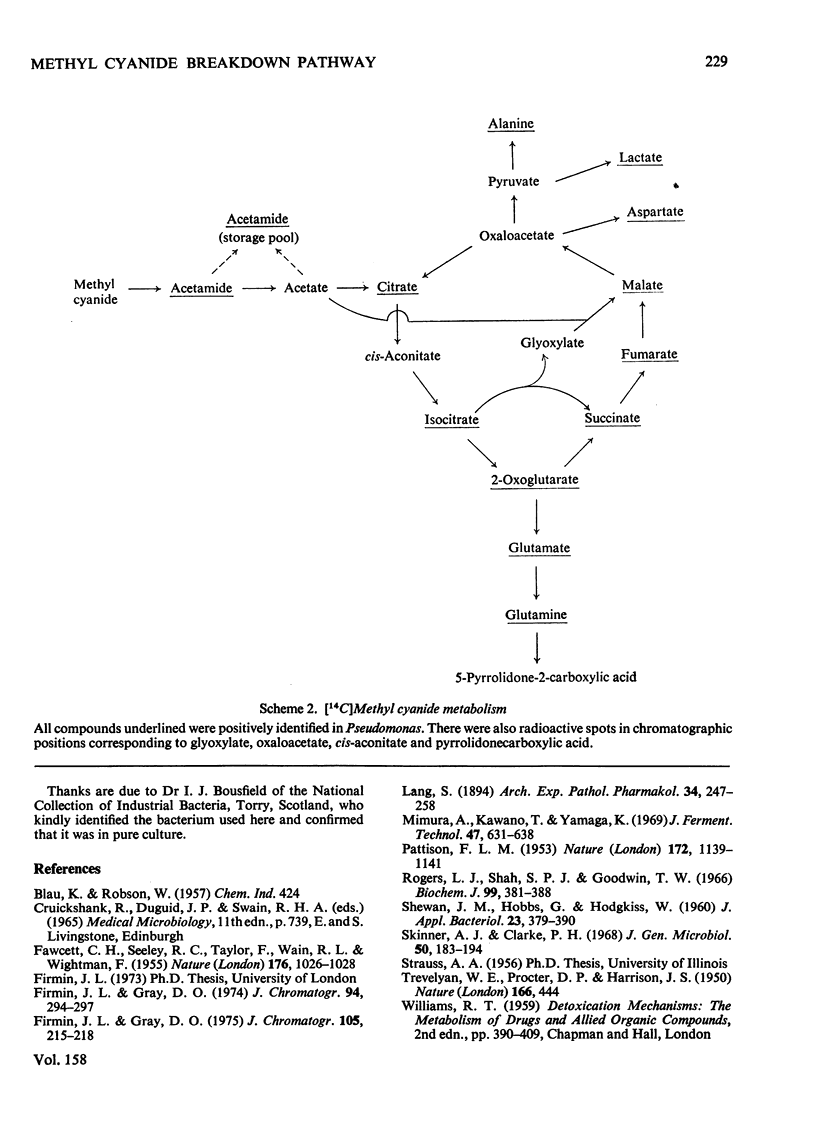
[2-14C]Methyl cyanide (acetonitrile) is metabolized to citrate, succinate, fumarate, malate, glutamate, pyrrolidonecarboxylic acid and aspartate. Non-radioactive acetamide and acetate compete with 14C from methyl cyanide, and [2-14C]acetate and [2-14C]methyl cyanide are metabolized at similar rates, giving identical products. This evidence, combined with the inhibitory effect of fluoroacetate and arsenite on methyl cyanide metabolism, indicates that the pathway is: methyl cyanide leads to acetamide leads to acetate leads to tricarboxylic acid-cycle intermediates. The pathway was investigated in a species of Pseudomonas (group III; N.C.I.B. 10477), but comparison of labelling patterns suggests that it also exists in several higher plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Firmin J. L., Gray D. O. Paper chromatography of acetamide and its application to metabolic studies. J Chromatogr. 1975 Feb 19;105(1):215–218. doi: 10.1016/s0021-9673(01)81112-2. [DOI] [PubMed] [Google Scholar]
- PATTISON F. L. Toxic fluorine compounds. Nature. 1953 Dec 19;172(4390):1138–1141. doi: 10.1038/1721139a0. [DOI] [PubMed] [Google Scholar]
- Rogers L. J., Shah S. P., Goodwin T. W. Intracellular localization of mevalonate-activating enzymes in plant cells. Biochem J. 1966 May;99(2):381–388. doi: 10.1042/bj0990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner A. J., Clarke P. H. Acetate and acetamide mutants of Pseudomonas aeruginosa 8602. J Gen Microbiol. 1968 Feb;50(2):183–194. doi: 10.1099/00221287-50-2-183. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]


