Abstract
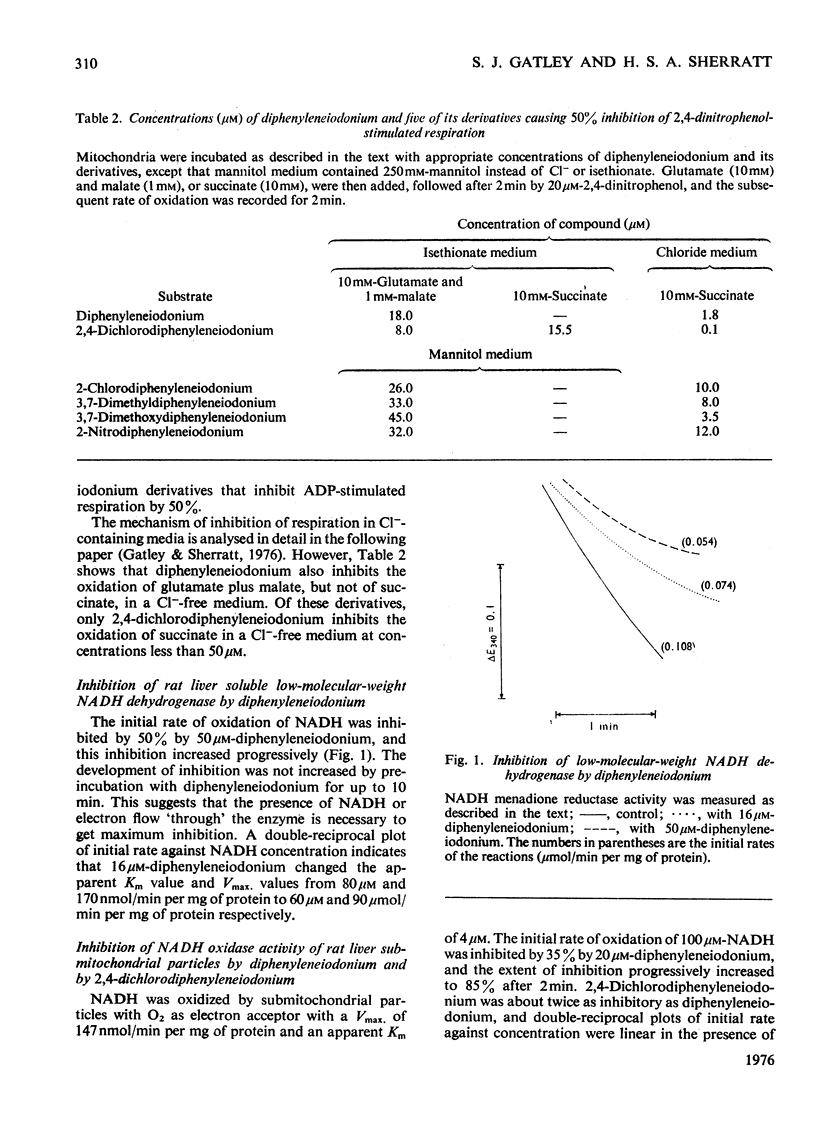
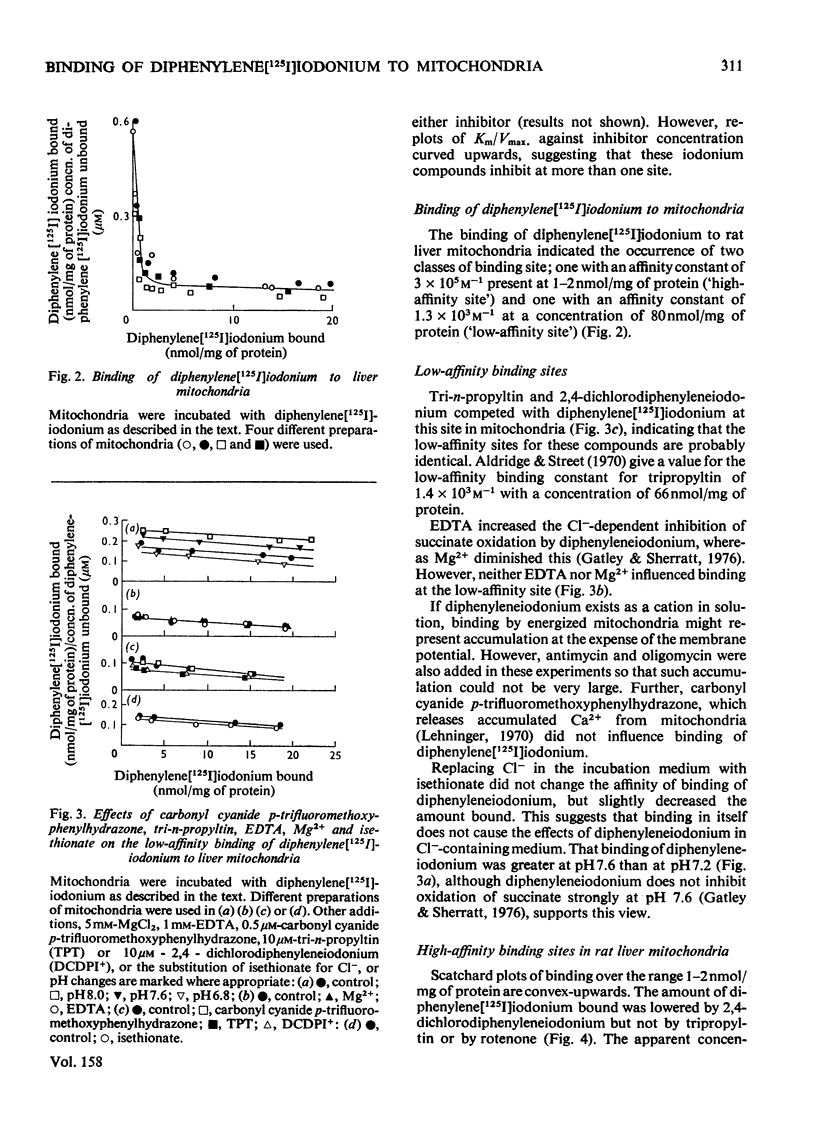
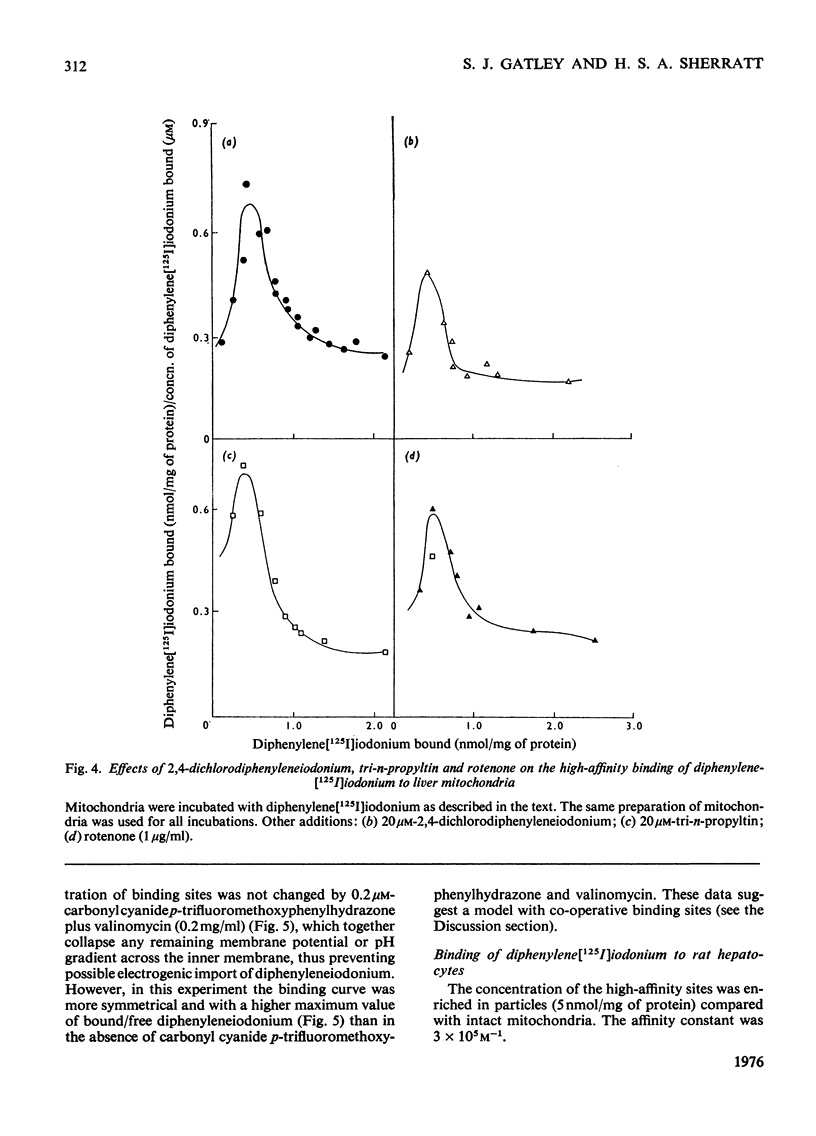
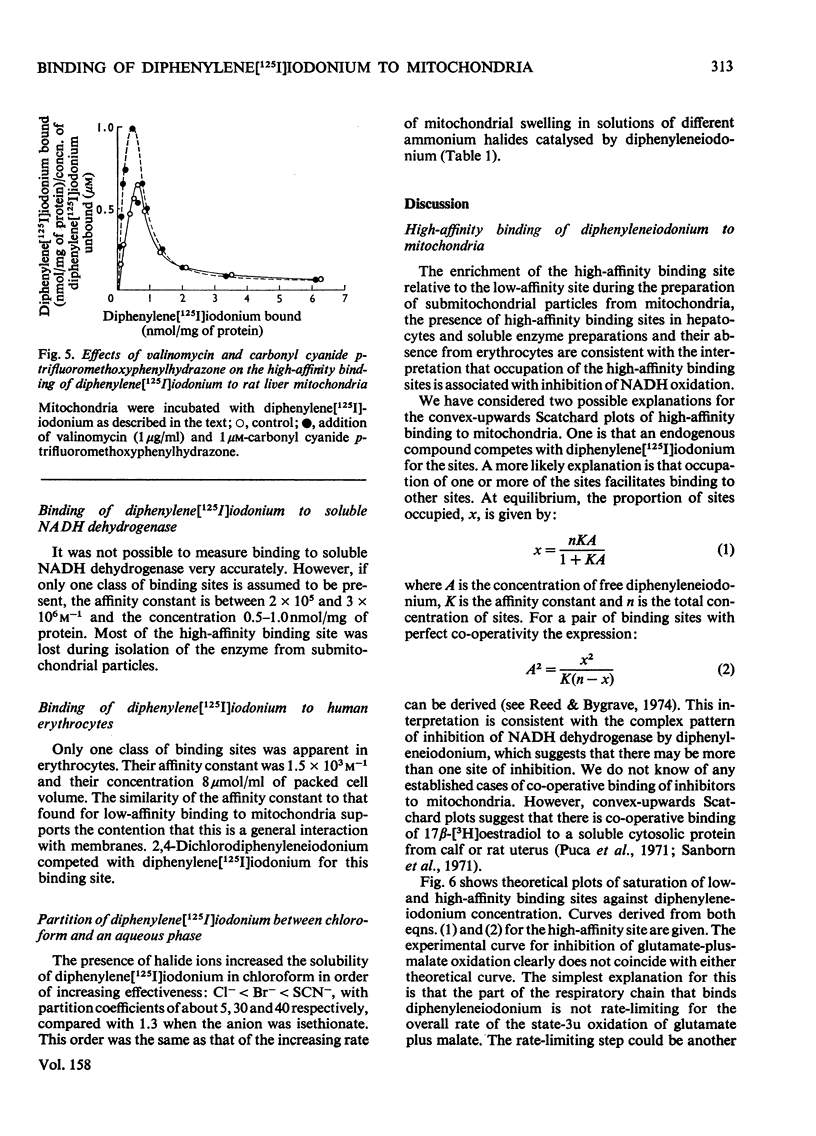
1. Several ring-substituted derivatives of diphenyleneiodonium catalyse the exchange of Cl- and OH- ions across the inner membrane of rat liver mitochondria. They also inhibit state 3 and state 3u oxidations of glutamate plus malate in the presence of Cl- more than in its absence. Most have activities similar to diphenyleneiodonium, although 2,4-dichlorodiphenyleneiodonium is up to 50 times more active. 2. Diphenyleneiodonium inhibits soluble rat liver NADH dehydrogenase and NADH oxidation by rat liver sub-mitochondrial particles directly; 2,4-dichlorodiphenyleneiodonium is only about twice as inhibitory. 3. Liver mitochondria contain two classes of binding sites for diphenylene[125I]iodonium, namely high-affinity sites with an affinity constant of 3 X 10(5) M-1 (1--2 nmol/mg of protein), and low-affinity sites with an affinity constant of 1.3 X 10(3) M-1 (80 nmol/mg of protein). Both sites occur in hepatocytes with a relative enrichment of the low-affinity site. Nadh dehydrogenase preparations only apparently contain high-affinity binding sites. Only low-affinity sites occur in erythrocytes. 4. 2,4-Dichlorodiphenyleneiodonium competes with diphenylene[125I]iodonium for both low- and high-affinity sites, whereas tri-n-propyltin only competes for the low-affinity sites. 5. The high-affinity sites are apparently associated with NADH dehydrogenase and the low-affinity sites probably represent electrostatic binding of diphenylene[125I]iodonium to phospholipids. The high-affinity site does not appear to be associated with a rate-limiting stage of NADH oxidation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge W. N., Street B. W. Oxidative phosphorylation. The relation between the specific binding of trimethylytin and triethyltin to mitochondria and their effects on various mitochondrial functions. Biochem J. 1971 Aug;124(1):221–234. doi: 10.1042/bj1240221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. The specific binding of trimethyltin and triethyltin to rat liver mitochondria. Biochem J. 1970 Jun;118(1):171–179. doi: 10.1042/bj1180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMONA T., KEARNEY E. B. STUDIES ON THE RESPIRATORY CHAIN-LINKED REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE DEHYDROGENASE. VI. FURTHER PURIFICATION AND PROPERTIES OF THE ENZYME FROM BEEF HEART. J Biol Chem. 1964 Jul;239:2328–2334. [PubMed] [Google Scholar]
- Gatley S. J., Al-Bassam S. S., Taylor J. R., Sherratt H. S. Inhibition by diphenyleneiodonium and by some of its substituted derivatives of gluconeogenesis in isolated rat hepatocytes. Biochem Soc Trans. 1975;3(2):333–335. doi: 10.1042/bst0030333. [DOI] [PubMed] [Google Scholar]
- Gatley S. J., Sherratt H. S. The effects of diphenyleneiodonium and of 2,4-dichlorodiphenyleneiodonium on mitochondrial reactions. Mechanism of the inhibition of oxygen uptake as a consequence of the catalysis of the chloride/hydroxyl-ion exchange. Biochem J. 1976 Aug 15;158(2):317–326. doi: 10.1042/bj1580317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. C., Clark M. G., Bloxham D. P., Lardy H. A. Mechanism of action of the hypoglycemic agent diphenyleneiodonium. J Biol Chem. 1973 Sep 10;248(17):6050–6056. [PubMed] [Google Scholar]
- Holland P. C., Sherratt H. S. Biochemical effects of the hypoglycaemic compound diphenyleneiodonnium. Catalysis of anion-hydroxyl ion exchange across the inner membrane of rat liver mitochondria and effects on oxygen uptake. Biochem J. 1972 Aug;129(1):39–54. doi: 10.1042/bj1290039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan D. J., Singer T. P., Casida J. E. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. 13. Binding sites of rotenone, piericidin A, and amytal in the respiratory chain. J Biol Chem. 1968 Feb 25;243(4):834–843. [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharo R. L., Sordahl L. A., Vyas S. R., Sanadi D. R. Studies on dihydronicotinamide adenine dinucleotide ubiquinone reductase. I. Assay of ubiquinone reductase activity in submitochondrial particles and extracts. J Biol Chem. 1966 Oct 25;241(20):4771–4780. [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen-binding proteins of calf uterus. Partial purification and preliminary characterization of two cytoplasmic proteins. Biochemistry. 1971 Sep 28;10(20):3769–3780. doi: 10.1021/bi00796a020. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. A re-evaluation of energy-independent calcium-ion binding by rat liver mitochondria. Biochem J. 1974 Sep;142(3):555–566. doi: 10.1042/bj1420555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn B. M., Rao B. R., Korenman S. G. Interaction of 17 -estradiol and its specific uterine receptor. Evidence for complex kinetic and equilibrium behavior. Biochemistry. 1971 Dec 21;10(26):4955–4962. doi: 10.1021/bi00802a019. [DOI] [PubMed] [Google Scholar]
- Schäfer G., Bojanowski D. Interaction of biguanides with mitochondrial and synthetic membranes. Eur J Biochem. 1972 May 23;27(2):364–375. doi: 10.1111/j.1432-1033.1972.tb01846.x. [DOI] [PubMed] [Google Scholar]
- Schäfer G. Interaction of biguanides with mitochondrial and synthetic membranes. The role of phospholipids as natural binding sites. Eur J Biochem. 1974 Jun 1;45(1):57–66. doi: 10.1111/j.1432-1033.1974.tb03529.x. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Carbohydrate metabolism. Biochem J. 1968 Dec;110(3):521–527. doi: 10.1042/bj1100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P., Gutman M. The DPNH dehydrogenase of the mitochondrial respiratory chain. Adv Enzymol Relat Areas Mol Biol. 1971;34:79–153. doi: 10.1002/9780470122792.ch3. [DOI] [PubMed] [Google Scholar]
- Williams J. N., Jr A comparative study of cytochrome ratios in mitochondria from organs of the rat, chicken, and guinea pig. Biochim Biophys Acta. 1968 Aug 20;162(2):175–181. doi: 10.1016/0005-2728(68)90100-x. [DOI] [PubMed] [Google Scholar]


