Abstract
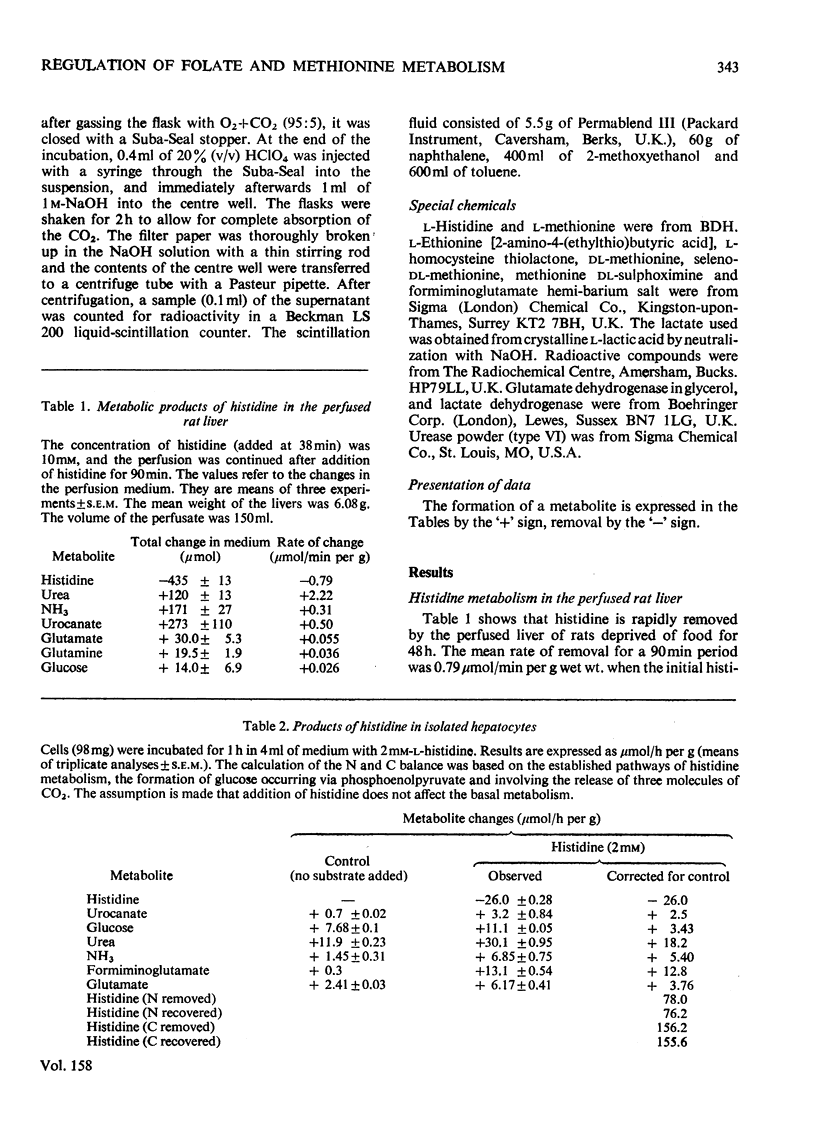
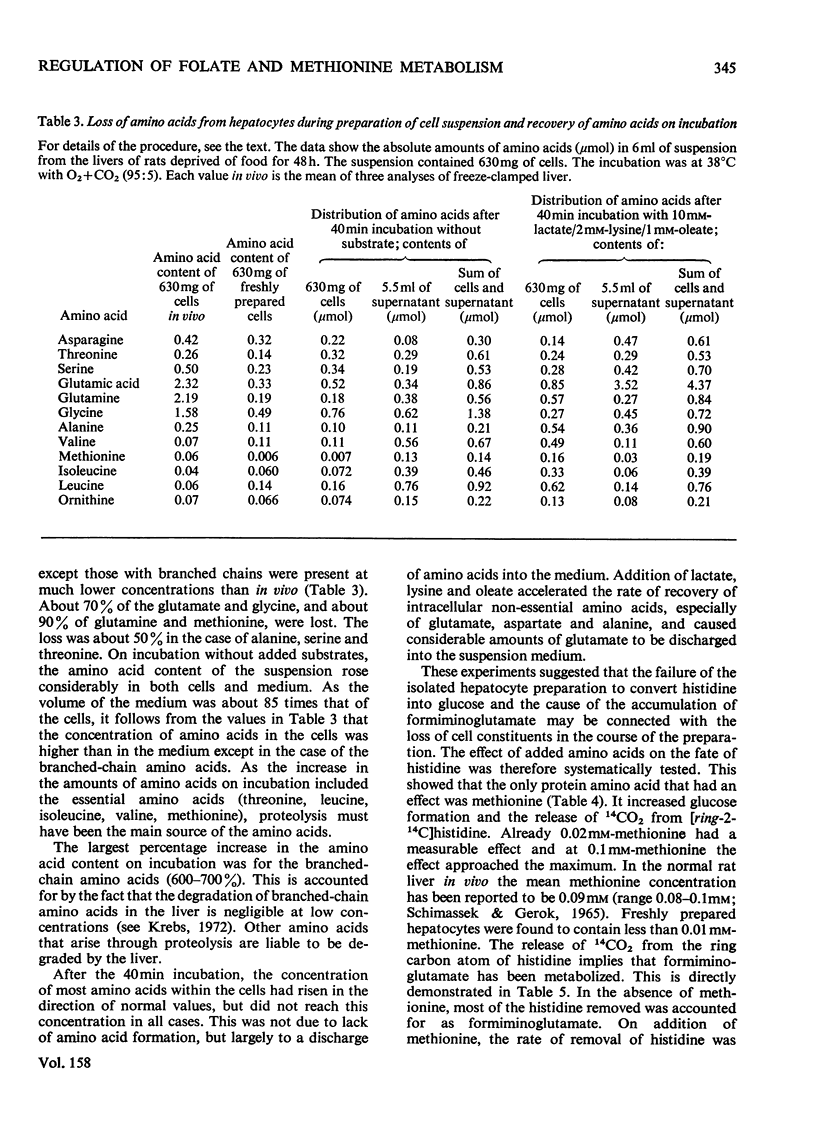
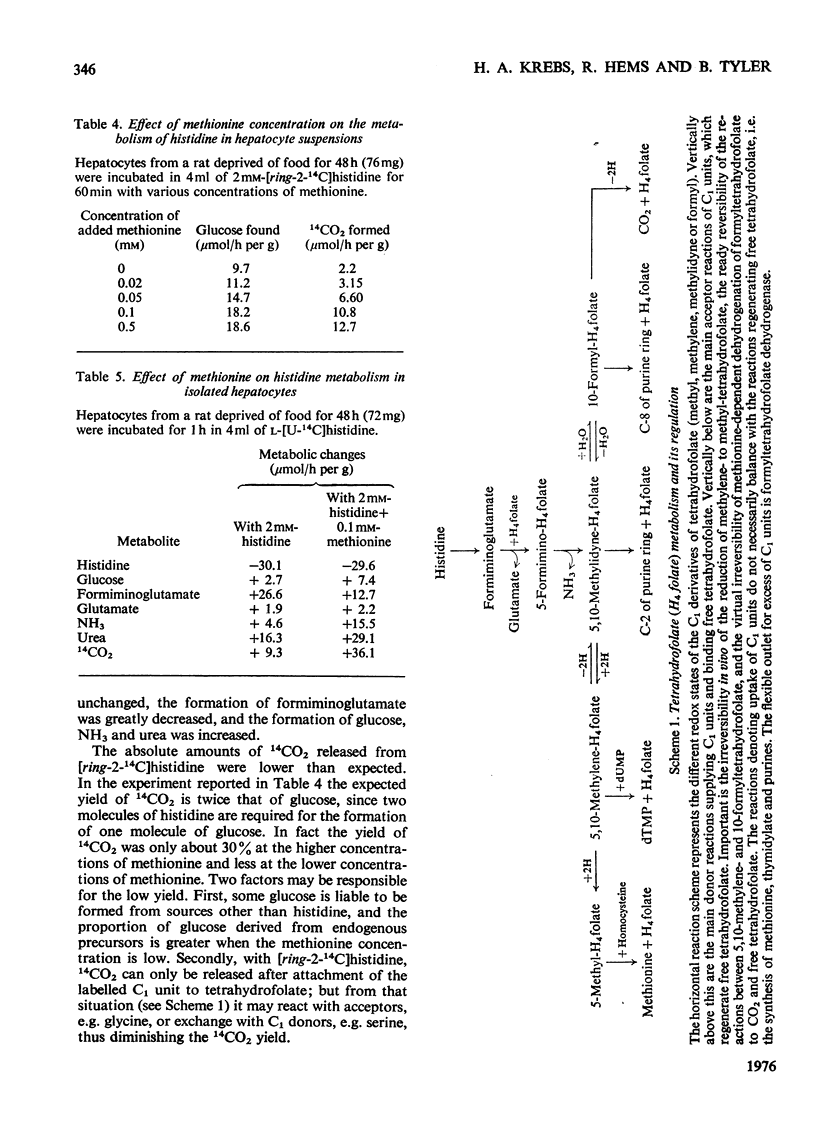
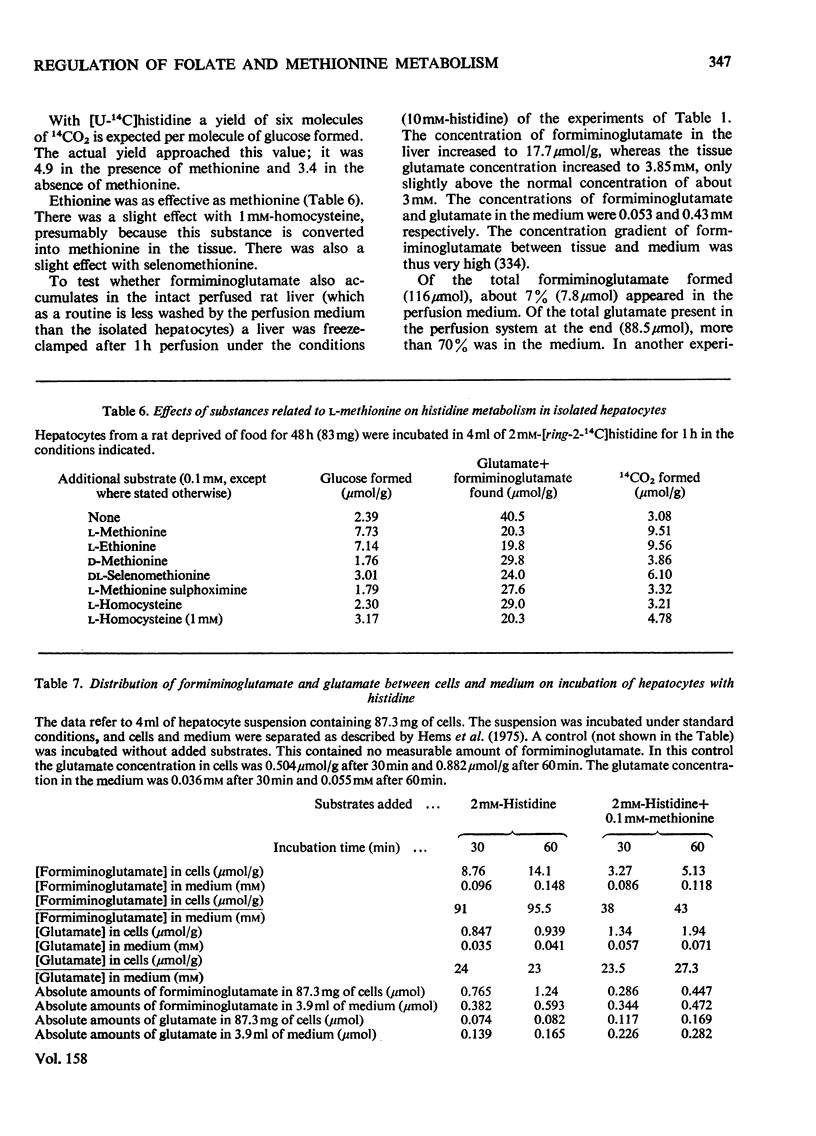
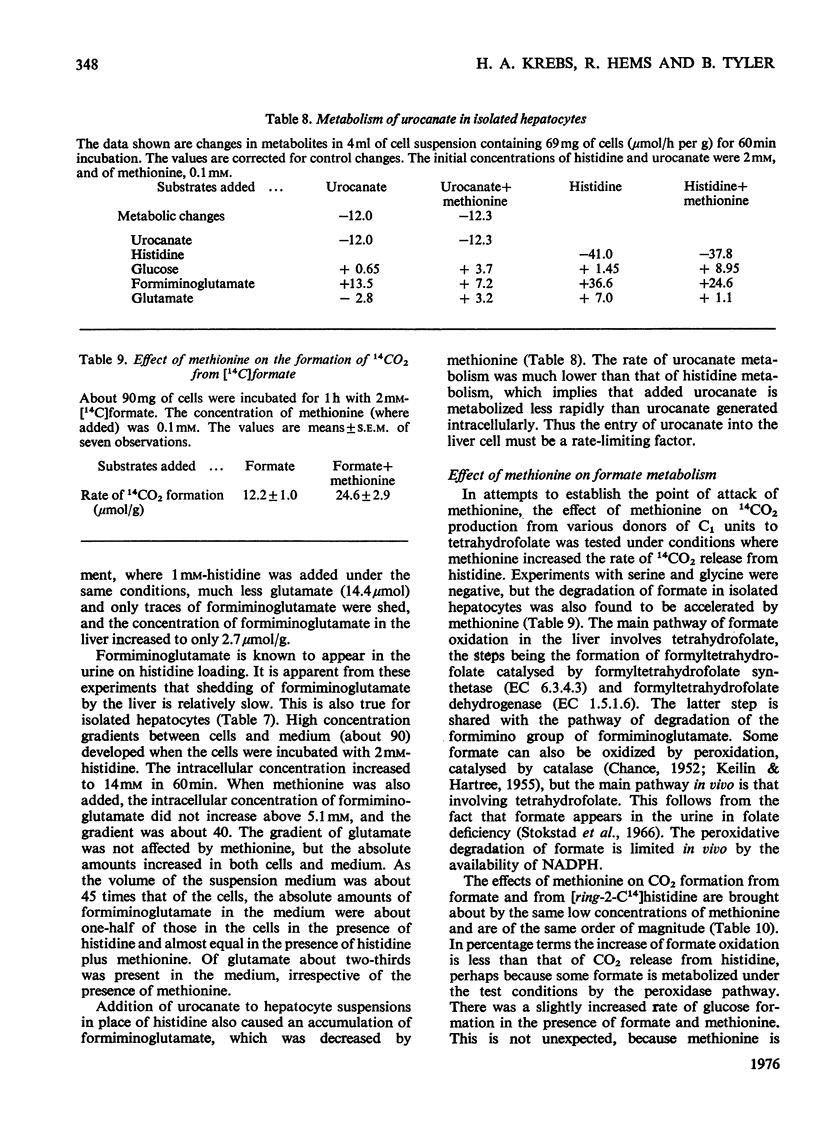
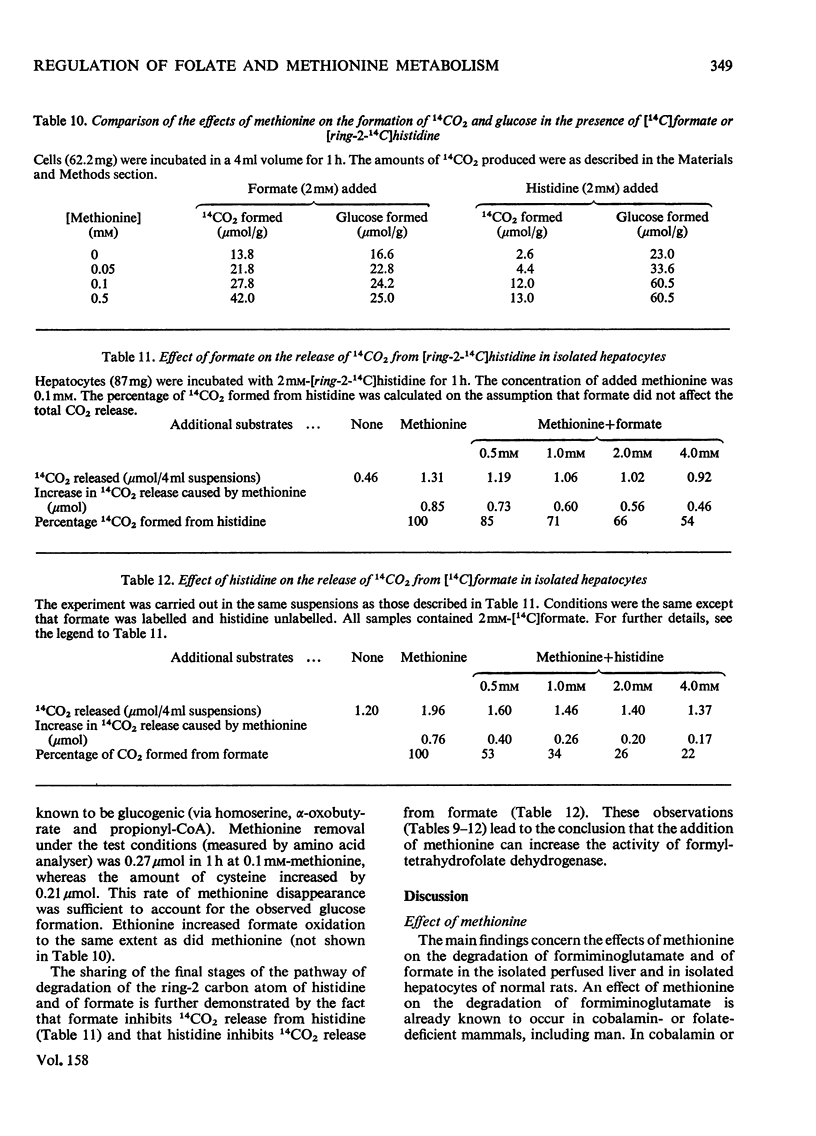
1. The isolated perfused rat liver and suspensions of isolated rat hepatocytes fail to form glucose from histidine, in contrast with the liver in vivo. Both rat liver preparations readily metabolize histidine. The main end product is N-formiminoglutamate. In this respect the liver preparations behave like the liver of cobalamin- or folate-deficient mammals. 2. Additions of L-methionine in physiological concentrations (or of ethionine [2-amino-4-(ethylthio)butyric acid]) promotes the degradation of formiminoglutamate, as is already known to be the case in cobalamin of folate deficiency. Added methionine also promotes glucose formation from histidine. 3. Addition of methionine accelerates the oxidation of formate to bicarbonate by hepatocytes. 4. A feature common to cobalamin-deficient liver and the isolated liver preparations is taken to be a low tissue methionine concentration, to be expected in cobalamin deficiency through a decreased synthesis of methionine and caused in liver preparations by a washing out of amino acids during the handling of the tissue. 5. The available evidence is in accordance with the assumption that methionine does not directly increase the catalytic capacity of formyltetrahydrofolate dehydrogenase; rather, that an increased methionine concentration raises the concentration of S-adenosylmethionine, thus leading to the inhibition of methylenetetrahydrofolate reductase activity [Kutzbach & Stokstad (1967) Biochim. Biophys. Acta 139, 217-220; Kutzbach & Stokstad (1971) Methods Enzymol. 18B, 793-798], that this inhibition causes an increase in the concentration of methylenetetrahydrofolate and the C1 tetrahydrofolate derivatives in equilibrium with methylenetetrahydrofolate, including 10-formyltetrahydrofolate; that the increased concentration of the latter accelerates the formyltetrahydrofolate dehydrogenase reaction, because the normal concentration of the substrate is far below the Km value of the enzyme for the substrate. 6. The findings are relevant to the understanding of the regulation of both folate and methionine metabolism. When the methionine concentration is low, C1 units are preserved by the decreased activity of formyltetrahydrofolate dehydrogenase and are utilized for the synthesis of methionine, purines and pyrimidines. On the other hand when the concentration of methionine, and hence adenosylmethionine, is high and there is a surplus of C1 units as a result of excess of dietary supply, formyltetrahydrofolate dehydrogenase disposes of the excess. When ample dietary supply causes an excess of methionine, which has to be disposed of by degradation, the increased activity of formyltetrahydrofolate dehydrogenase decreases the supply of methyltetrahydrofolate. Thus homocysteine, instead of being remethylated, enters the pathway of degradation via cystathionine. 7. The findings throw light on the biochemical abnormalities associated with cobalamin deficiency (megaloblastic anaemia), especially on the 'methylfolate-trap hypothesis'. This is discussed. 8...
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN D. D., SILVA O. L., GARDINER R. C., SILVERMAN M. Metabolism of formiminoglutamic acid by vitamin B12 and folic acid-deficient rats fed excess methionine. J Biol Chem. 1960 Jul;235:2058–2062. [PubMed] [Google Scholar]
- BUCHANAN J. M., ELFORD H. L., LOUGHLIN R. E., MCDOUGALL B. M., ROSENTHAL S. THE ROLE OF VITAMIN B12 IN METHYL TRANSFER TO HOMOCYSTEINE. Ann N Y Acad Sci. 1964 Apr 24;112:756–773. doi: 10.1111/j.1749-6632.1964.tb45053.x. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehring K. U., Batra K. K., Stokstad E. L. The effect of methionine on folic acid and histidine metabolism in perfused rat liver. Biochim Biophys Acta. 1972 Oct 25;279(3):498–512. doi: 10.1016/0304-4165(72)90172-9. [DOI] [PubMed] [Google Scholar]
- CHANCE B. Effect of pH upon the reaction kinetics of the enzyme-substrate compounds of catalase. J Biol Chem. 1952 Feb;194(2):471–481. [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Krebs H. A. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem J. 1974 Aug;142(2):327–337. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. D., Kyle W. E., Martin J. L., Pick A. M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem Biophys Res Commun. 1975 Sep 2;66(1):81–87. doi: 10.1016/s0006-291x(75)80297-x. [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D. Methionine metabolism in mammals: the biochemical basis for homocystinuria. Metabolism. 1974 Apr;23(4):387–398. doi: 10.1016/0026-0495(74)90057-2. [DOI] [PubMed] [Google Scholar]
- Gawthorne J. M., Smith R. M. Folic acid metabolism in vitamin B12-deficient sheep. Effects of injected methionine on methotrexate transport and the activity of enzymes associated with folate metabolism in liver. Biochem J. 1974 Jul;142(1):119–126. doi: 10.1042/bj1420119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT V., SULLIVAN L. W. Formiminoglutamicaciduria in humans with megaloblastic anemia: diminution by methionine or glycine. Proc Soc Exp Biol Med. 1963 Feb;112:304–305. doi: 10.3181/00379727-112-28023. [DOI] [PubMed] [Google Scholar]
- HERBERT V., ZALUSKY R. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J Clin Invest. 1962 Jun;41:1263–1276. doi: 10.1172/JCI104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffbrand A. V. Letter: The methylfolate trap. Lancet. 1975 Jun 21;1(7921):1380–1381. doi: 10.1016/s0140-6736(75)92290-4. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Karlsson I. M. Ion-exchange chromatography of physiological sulphur amino acids on a highly crosslinked resin. J Chromatogr. 1972 Oct 5;72(1):93–103. doi: 10.1016/0021-9673(72)80011-6. [DOI] [PubMed] [Google Scholar]
- KEILIN D., HARTREE E. F. Catalase, peroxidase and metmyoglobin as catalysts of coupled peroxidatic reactions. Biochem J. 1955 Jun;60(2):310–325. doi: 10.1042/bj0600310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISLIUK R. L. THE ROLE OF ADENOSYLMETHIONINE AND 5-METHYLTETRAHYDROFOLATE IN THE REGULATION OF THE METABOLISM OF SINGLE CARBON UNITS. Medicine (Baltimore) 1964 Nov;43:711–713. doi: 10.1097/00005792-196411000-00014. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Freedland R. A., Hems R., Stubbs M. Inhibition of hepatic gluconeogenesis by ethanol. Biochem J. 1969 Mar;112(1):117–124. doi: 10.1042/bj1120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A. Some aspects of the regulation of fuel supply in omnivorous animals. Adv Enzyme Regul. 1972;10:397–420. doi: 10.1016/0065-2571(72)90025-8. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Galloway E., Stokstad E. L. Influence of vitamin B12 and methionine on levels of folic acid compounds and folate enzymes in rat liver. Proc Soc Exp Biol Med. 1967 Mar;124(3):801–805. doi: 10.3181/00379727-124-31857. [DOI] [PubMed] [Google Scholar]
- Kutzbach C., Stokstad E. L. Feedback inhibition of methylene-tetrahydrofolate reductase in rat liver by S-adenosylmethionine. Biochim Biophys Acta. 1967 May 16;139(1):217–220. doi: 10.1016/0005-2744(67)90140-4. [DOI] [PubMed] [Google Scholar]
- Lavoie A., Tripp E., Hoffbrand A. V. The effect of vitamin B12 deficiency on methylfolate metabolism and pteroylpolyglutamate synthesis in human cells. Clin Sci Mol Med. 1974 Dec;47(6):617–630. doi: 10.1042/cs0470617. [DOI] [PubMed] [Google Scholar]
- Lombardini J. B., Talalay P. Formation, functions and regulatory importance of S-adenosyl-L-methionine. Adv Enzyme Regul. 1970;9:349–384. doi: 10.1016/s0065-2571(71)80054-7. [DOI] [PubMed] [Google Scholar]
- Nixon P. F., Bertino J. R. Interrelationships of vitamin B12 and folate in man. Am J Med. 1970 May;48(5):555–561. doi: 10.1016/0002-9343(70)90004-5. [DOI] [PubMed] [Google Scholar]
- SHAPIRO S. K., LOHMAR P., HERTENSTEIN M. Utilization of S-adenosylmethionine for the biosynthesis of methionine. Arch Biochem Biophys. 1963 Jan;100:74–79. doi: 10.1016/0003-9861(63)90036-5. [DOI] [PubMed] [Google Scholar]
- SILVERMAN M., PITNEY A. J. Dietary methionine and the excretion of formiminoglutamic acid by the rat. J Biol Chem. 1958 Nov;233(5):1179–1182. [PubMed] [Google Scholar]
- Schirch L., Ropp M. Serine transhydroxymethylase. Affinity of tetrahydrofolate compounds for the enzyme and enzyme-glycine complex. Biochemistry. 1967 Jan;6(1):253–257. doi: 10.1021/bi00853a039. [DOI] [PubMed] [Google Scholar]
- Shin Y. S., Beuhring K. U., Stokstad E. L. The relationships between vitamin B12 and folic acid and the effect of methionine on folate metabolism. Mol Cell Biochem. 1975 Nov 14;9(2):97–108. doi: 10.1007/BF01732201. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Osborne-White W. S., Gawthorne J. M. Folic acid metabolism in vitamin B12-deficient sheep. Effects of injected methionine on liver constituents associated with folate metabolism. Biochem J. 1974 Jul;142(1):105–117. doi: 10.1042/bj1420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin H. B., Winchell H. S., Kusubov N. Lack of effect of L-methionine ingestion on 14CO2 excretion from L-histidine (imidazole-2-14C) in folic acid and vitamin B12 deficient humans. Blood. 1970 Jan;35(1):86–89. [PubMed] [Google Scholar]
- Stokstad E. L., Webb R. E., Shah E. Effect of vitamin B-12 and folic acid on the metabolism of formiminoglutamate, formate, and propionate in the rat. J Nutr. 1966 Feb;88(2):225–232. doi: 10.1093/jn/88.2.225. [DOI] [PubMed] [Google Scholar]
- Thenen S. W., Stokstad E. L. Effect of methionine on specific folate coenzyme pools in vitamin B 12 deficient and supplemented rats. J Nutr. 1973 Mar;103(3):363–370. doi: 10.1093/jn/103.3.363. [DOI] [PubMed] [Google Scholar]
- Tisman G., Wu S. J., Safire G. E., Rodriguez E. Letter: The methylfolate-trap hypothesis. Lancet. 1975 May 24;1(7917):1184–1184. doi: 10.1016/s0140-6736(75)93154-2. [DOI] [PubMed] [Google Scholar]
- Vitale J. J., Hegsted D. M. Effects of dietary methionine and vitamin B12 deficiency on folate metabolism. Br J Haematol. 1969 Nov;17(5):467–475. doi: 10.1111/j.1365-2141.1969.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Pegg A. E., Lockwood D. H. Mechanisms and regulation of polyamine and putrescine biosynthesis in male genital glands and other tissues of mammals. Adv Enzyme Regul. 1969;7:291–323. doi: 10.1016/0065-2571(69)90024-7. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Spray G. H. Observations on folate metabolism in rats on a vitamin B 12-deficient diet. Br J Haematol. 1970 Sep;19(3):353–360. doi: 10.1111/j.1365-2141.1970.tb01632.x. [DOI] [PubMed] [Google Scholar]


