Abstract
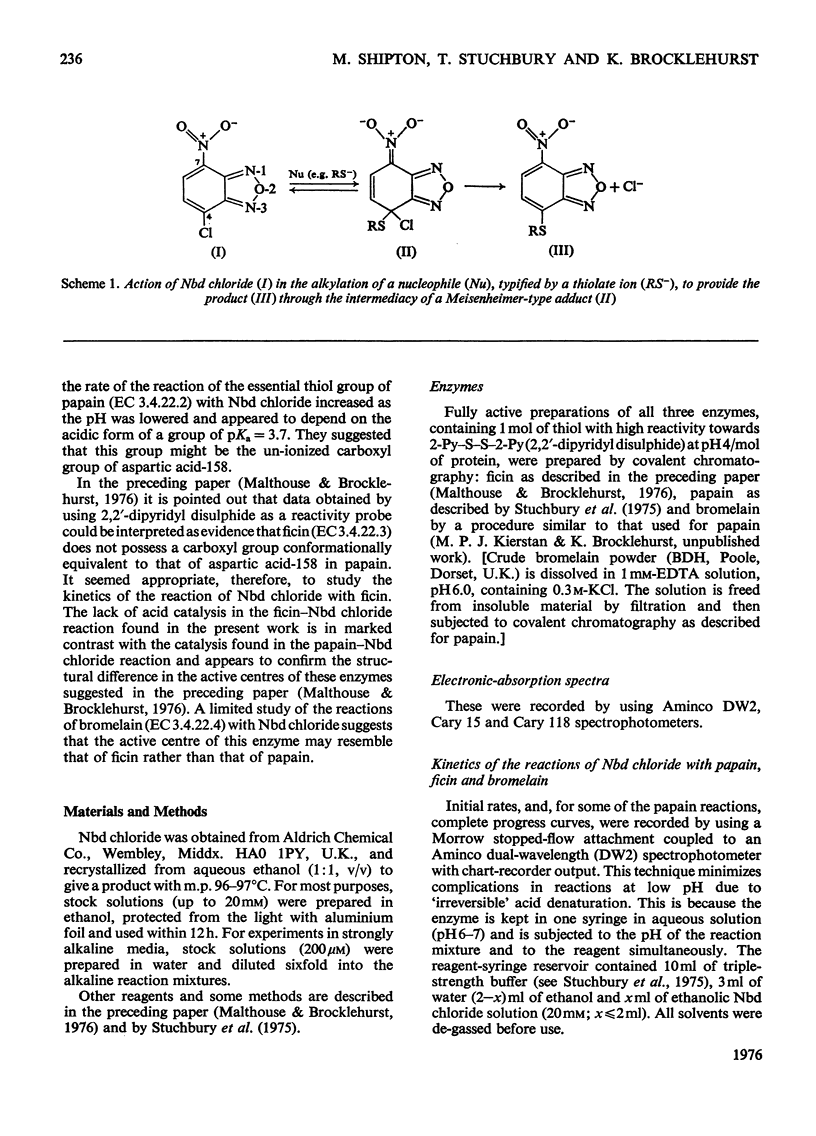
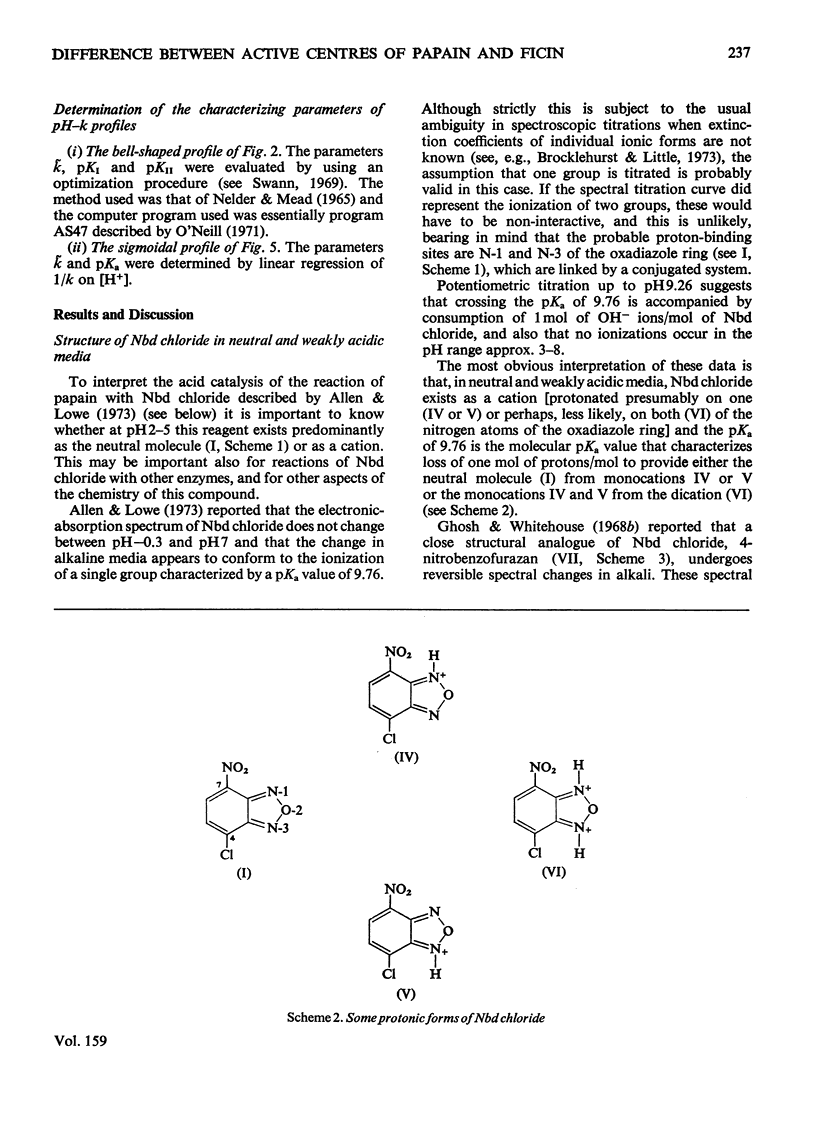
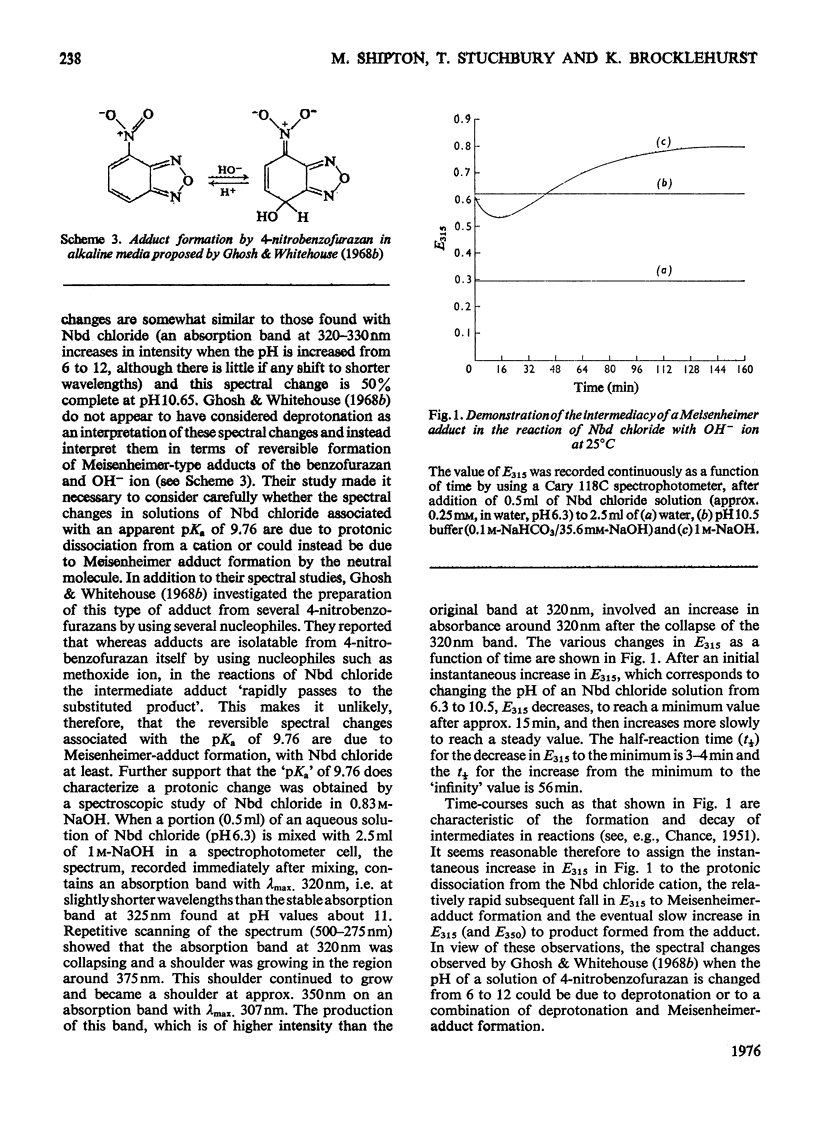
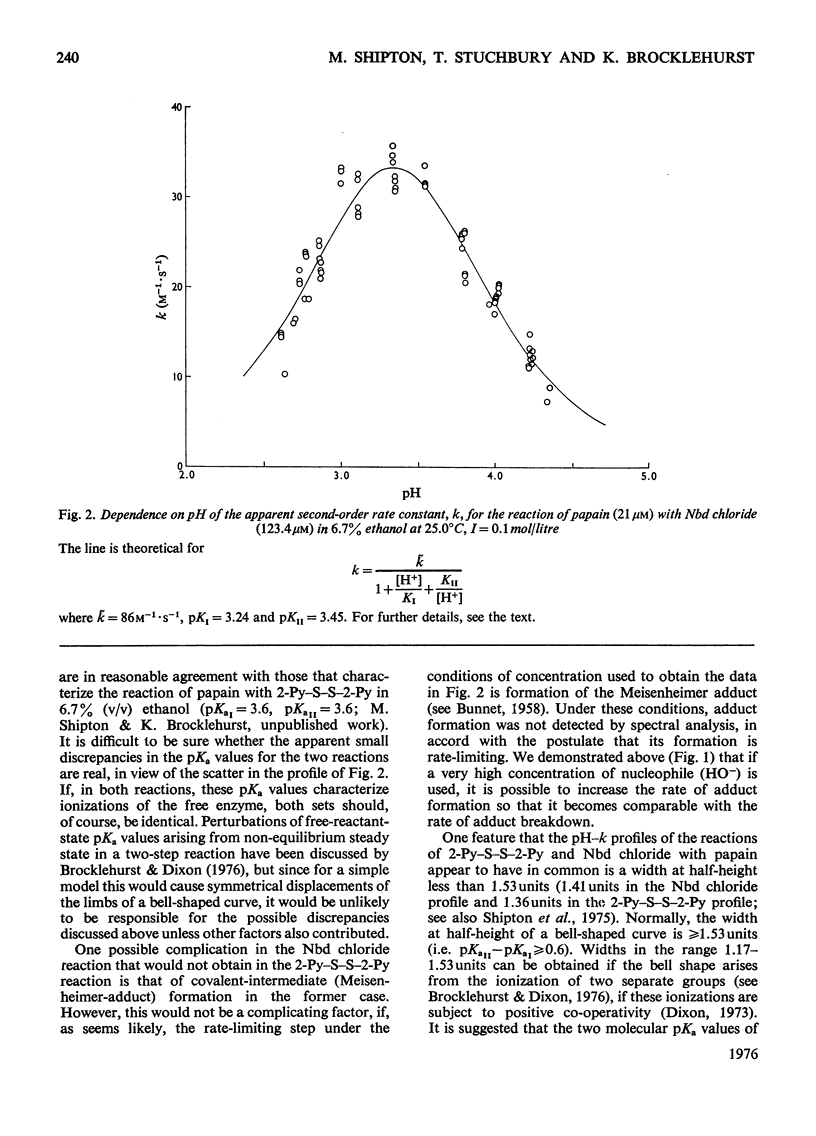
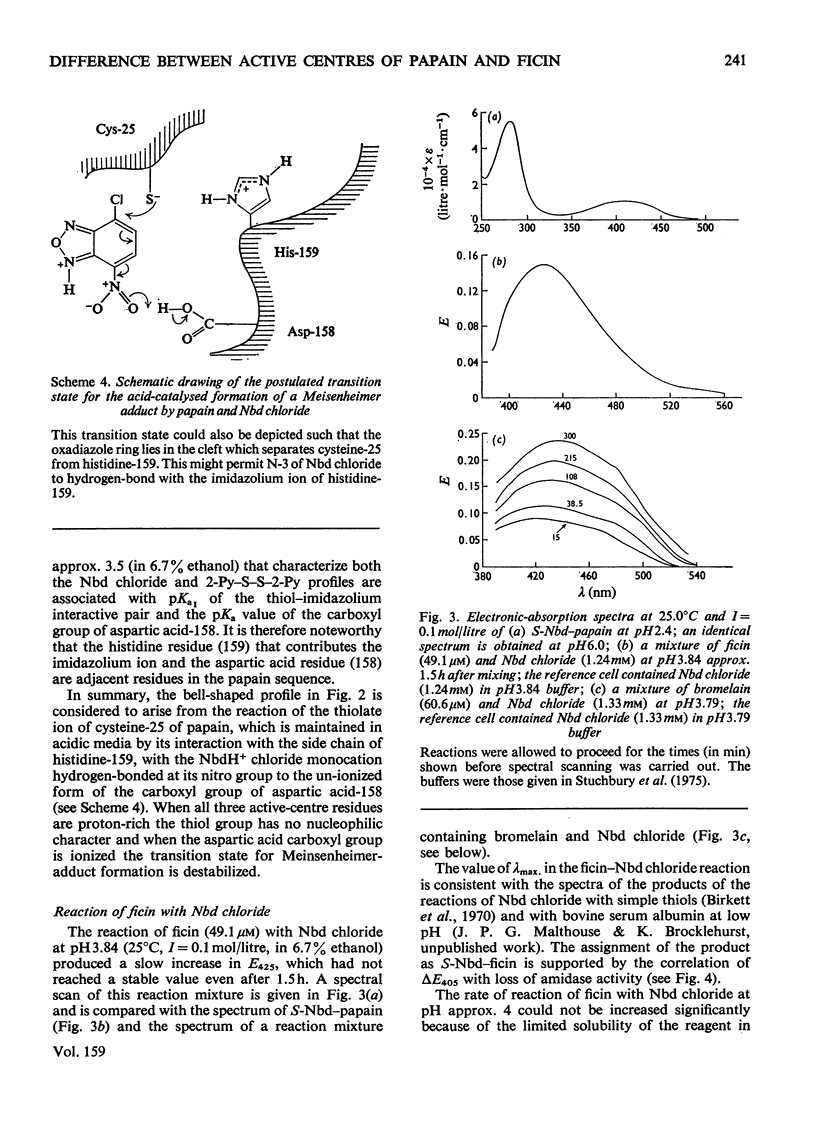
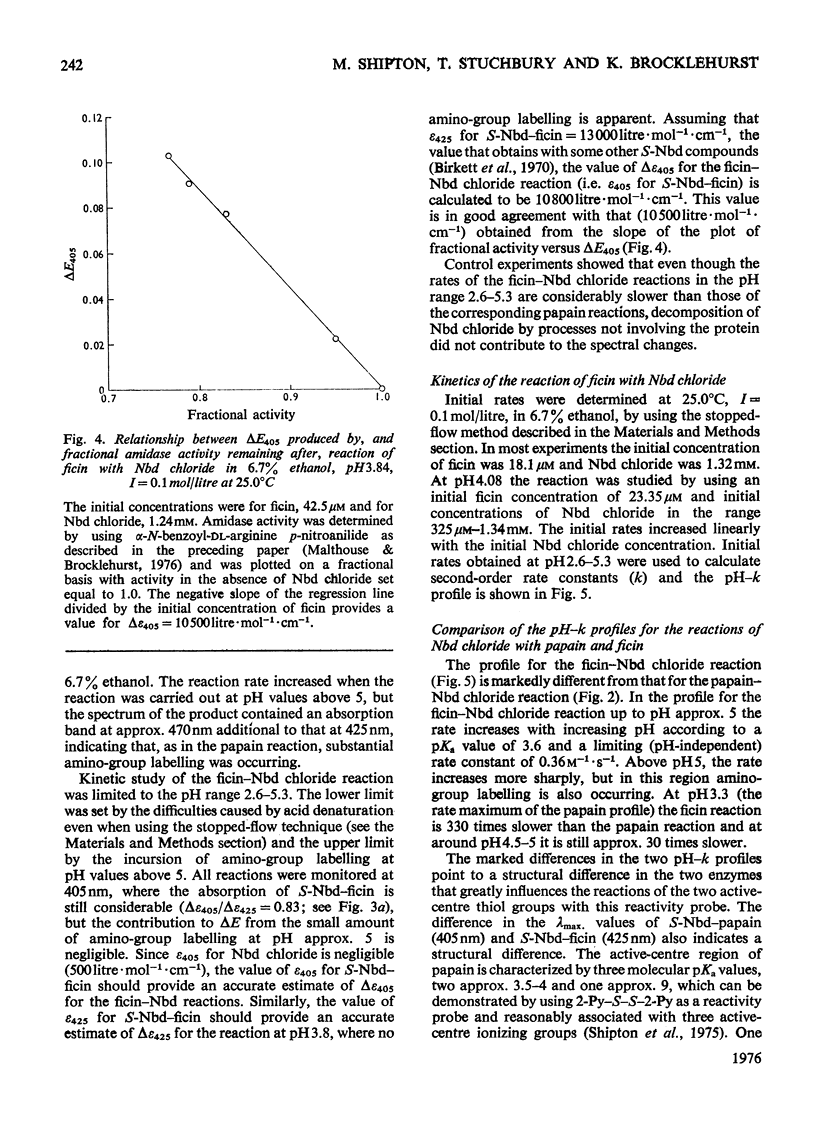
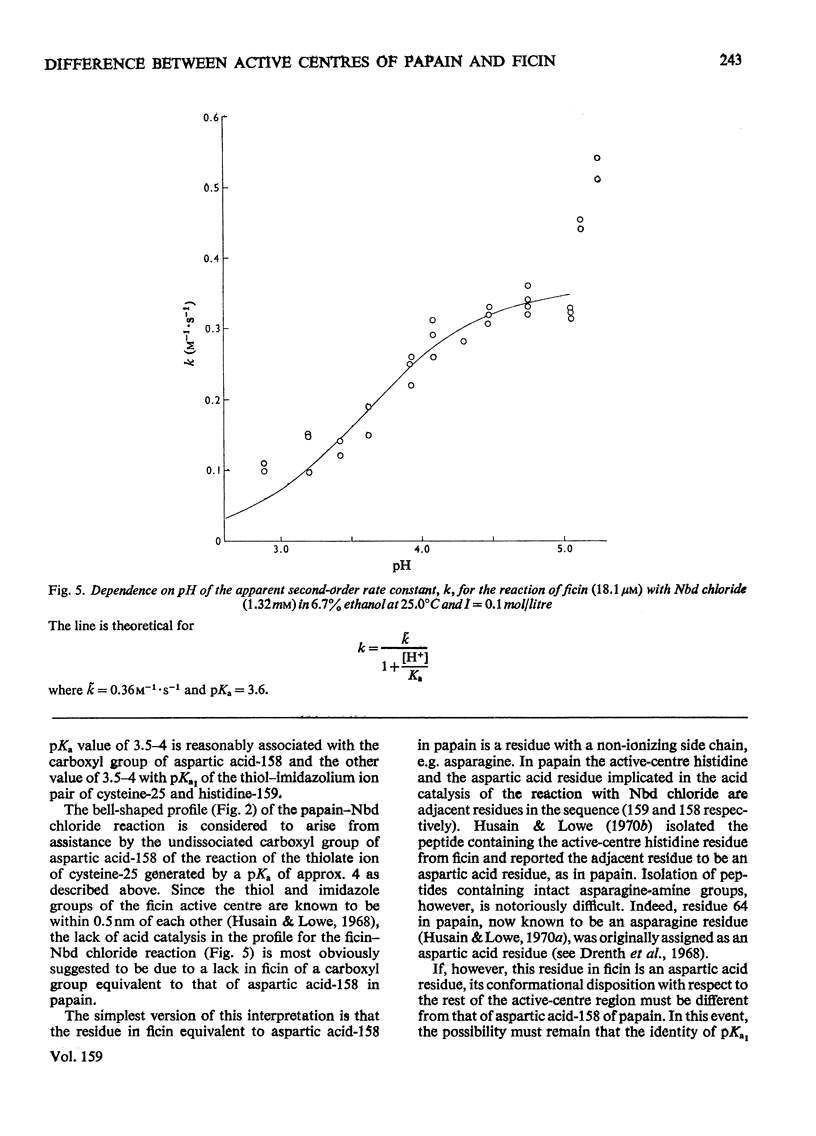
1. 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole (Nbd chloride) was used as a reactivity probe to characterize the active centres of papin (EC 3.4.22.2), ficin (EC 3.4.22.3) and bromelain (EC 3.4.22.4). 2. In the pH range 0-8 Nbd chloride probably exists mainly as a monocation, possibly with the proton located on N-1 of the oxadiazole ring. 3. Spectroscopic evidence is presented for the intermediacy of Meisenheimer-type adducts in the reaction of Nbd chloride with nucleophiles. 4. The pH-dependence of the second-order rate constants (k) of the reactions of the three enzymes with Nbd chloride was determined at 25 degrees C, I = 0.1 mol/litre in 6.7% (v/v) ethanol in the pH range 2.5-5, where, at least for papain and ficin, the reactions occur specifically with their active-centre thiol groups. The pH-k profile for the papain reaction is bell-shaped (pKaI = 3.24, pKaII = 3.44 and k = 86M(-1)-s(-1), whereas that for ficin is sigmoidal (pKa = 3.6, k = 0.36M(-1)-s(-1), the rate increasing with increasing pH. The profile for the bromelain reaction appears to resemble that for the ficin reaction, but is complicated by amino-group labelling. 5. The bell-shaped profile of the papain reaction is considered to arise from the reaction of the thiolate ion of cysteine-25, maintained in acidic media by interaction with the side chain of histidine-159, with the Nbd chloride monocation hydrogen-bonded at its nitro group to the un-ionized form of the carboxyl group of aspartic acid-158. The lack of acid catalysis in the corresponding reactions of ficin and probably of bromelain suggests that these enzymes may lack carboxyl groups conformationally equivalent to that of aspartic acid-158 of papain. The possible consequences of this for the catalytic sites of these enzymes is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Lowe G. Investigation of the active site of papain with fluorescent probes. Biochem J. 1973 Aug;133(4):679–686. doi: 10.1042/bj1330679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett D. J., Dwek R. A., Radda G. K., Richards R. E., Salmon A. G. Probes for the conformational transitions of phosphorylase b. Effect of ligands studied by proton relaxation enhancement, fluorescence and chemical reactivities. Eur J Biochem. 1971 Jun 29;20(4):494–508. doi: 10.1111/j.1432-1033.1971.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Birkett D. J., Price N. C., Radda G. K., Salmon A. G. The reactivity of SH groups with a fluorogenic reagent. FEBS Lett. 1970 Feb 25;6(4):346–348. doi: 10.1016/0014-5793(70)80095-3. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Crook E. M., Kierstan M. The mutability of stem bromelain: evidence for perturbation by structural transitions of the parameters that characterize the reaction of the essential thiol group of bromelain with 2,2'-dipyridyl disulphide. Biochem J. 1972 Jul;128(4):979–982. doi: 10.1042/bj1280979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. PH-dependence of the steady-state rate of a two-step enzymic reaction. Biochem J. 1976 Apr 1;155(1):61–70. doi: 10.1042/bj1550061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Enzyme-substrate compounds. Adv Enzymol Relat Subj Biochem. 1951;12:153–190. doi: 10.1002/9780470122570.ch3. [DOI] [PubMed] [Google Scholar]
- Clark P. E., Lowe G. The pKa value of the active site histidine in photo-oxidised papain. FEBS Lett. 1976 Jan 1;61(1):25–27. doi: 10.1016/0014-5793(76)80162-7. [DOI] [PubMed] [Google Scholar]
- Dixon H. B. Shapes of curves of pH-dependence of reactions. Biochem J. 1973 Jan;131(1):149–154. doi: 10.1042/bj1310149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Swen H. M., Wolthers B. G. Structure of papain. Nature. 1968 Jun 8;218(5145):929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- Ghosh P. B., Whitehouse M. W. 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole: a new fluorigenic reagent for amino acids and other amines. Biochem J. 1968 Jun;108(1):155–156. doi: 10.1042/bj1080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. B., Whitehouse M. W. Potential antileukemic and immunosuppressive drugs. Preparation and in vitro pharmacological acitivity of some benzo-2,1,3-oxadiazoles (benzofurazans) and their N-oxides (benzofuroxans). J Med Chem. 1968 Mar;11(2):305–311. doi: 10.1021/jm00308a027. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. A reinvestigation of residues 64-68 and 175 in papain. Evidence that residues 64 and 175 are asparagine. Biochem J. 1970 Feb;116(4):689–692. doi: 10.1042/bj1160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Evidence for histidine in the active site of papain. Biochem J. 1968 Aug;108(5):855–859. doi: 10.1042/bj1080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The amino acid sequence around the active-site cysteine and histidine residues of stem bromelain. Biochem J. 1970 Apr;117(2):341–346. doi: 10.1042/bj1170341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The amino acid sequence around the active-site cysteine and histidine residues, and the buried cysteine residue in ficin. Biochem J. 1970 Apr;117(2):333–340. doi: 10.1042/bj1170333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler H. G., Schneider F. Chemical modification of one carboxyl-group of papain abolishes the catalytic activity of the enzyme. FEBS Lett. 1974 Sep 1;45(1):79–81. doi: 10.1016/0014-5793(74)80815-x. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Preparation of fully active ficin from Ficus glabrata by covalent chromatography and characterization of its active centre by using 2,2'-depyridyl disulphide as a reactivity probe. Biochem J. 1976 Nov;159(2):221–234. doi: 10.1042/bj1590221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murachi T., Okumura K. Normal apparent pKa value for the ionization of the histidine residue of papain and stem bromelain as determined by photooxidation reaction. FEBS Lett. 1974 Mar 15;40(1):127–129. doi: 10.1016/0014-5793(74)80909-9. [DOI] [PubMed] [Google Scholar]
- Price N. C., Cohn M., Schirmer R. H. Fluorescent and spin label probes of the environments of the sulfhydryl groups of porcine muscle adenylate kinase. J Biol Chem. 1975 Jan 25;250(2):644–652. [PubMed] [Google Scholar]
- Radda G. K. Enzyme and membrane conformation in biochemical control. Biochem J. 1971 May;122(4):385–396. doi: 10.1042/bj1220385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Kierstan M. P., Malthouse J. P., Stuchbury T., Brocklehurst K. The case for assigning a value of approximately 4 to pKa-i of the essential histidine-cysteine interactive systems of papain, bromelain and ficin. FEBS Lett. 1975 Feb 15;50(3):365–368. doi: 10.1016/0014-5793(75)80529-1. [DOI] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]


