Abstract
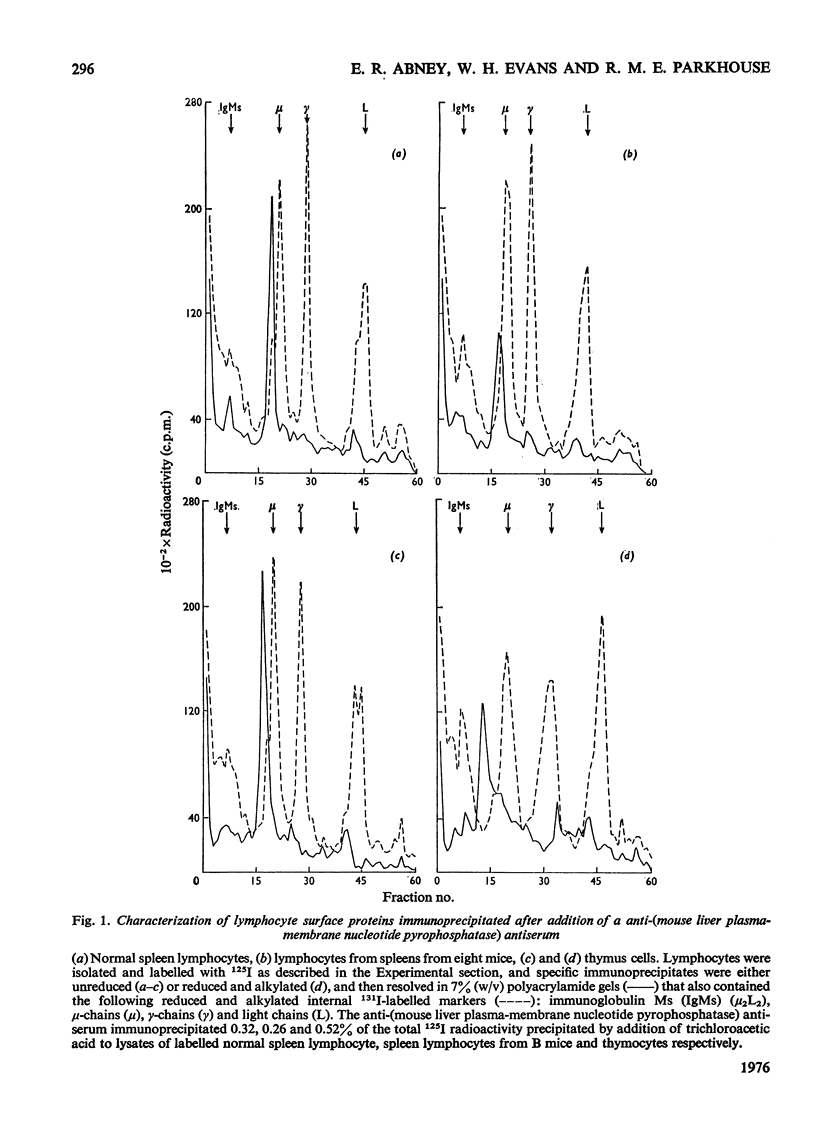
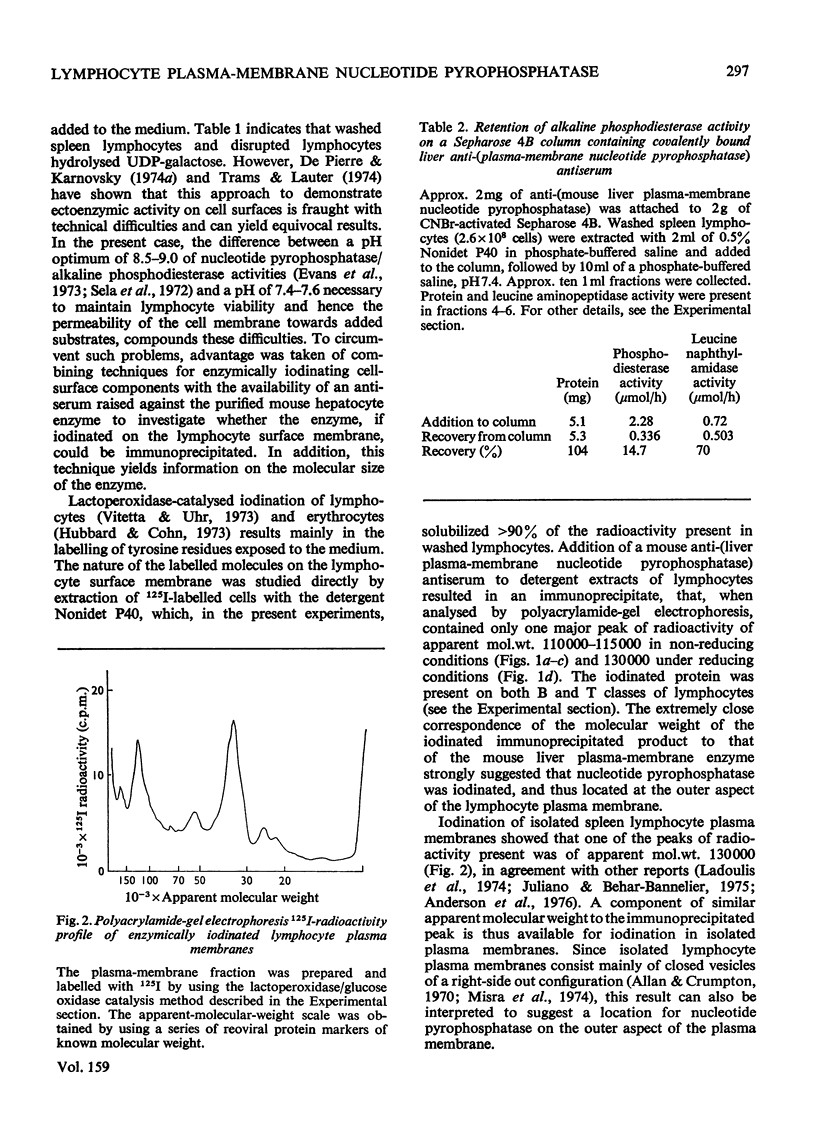
1. Isolated mouse spleen lymphocytes hydrolysed UDP-galactose added to the medium. Nucleotide pyrophosphatase activity that accounted for this hydrolysis was enriched to a similar extent as alkaline phosphodiesterase and 5'-nucleotidase in a lymphocyte plasma-membrane fraction. 2. The cell surfaces of mouse spleen and thymus lymphocytes were iodinated with 125I by using the lactoperoxidase-catalysis method. Detergent extracts of the cells were mixed with a purified anti-(mouse liver plasma-membrane nucleotide pyrophosphatase) antiserum and the immunoprecipitates analysed by polyacrylamide-gel electrophoresis. Only one major radioactive component, similar in size (apparent mol.wt 110000-130000) to the liver enzyme, was observed. 3. Electrophoresis of an iodinated spleen plasma-membrane fraction indicated peaks of radioactivity, including one of apparent mol.wt 110000-130000. 4. When detergent extracts of spleen lymphocytes were passed through a Sepharose-bead column containing covalently attached anti-(nucleotide pyrophosphatase) antiserum, the nucleotide pyrophosphatase activity was retained by the beads, whereas protein and leucine naphthylamidase activity were eluted. 5. The results indicate that nucleotide pyrophosphatase and alkaline phosphodiesterase activities are due to the location of the same or similar enzymes at the outer aspect of the lymphocyte plasma membrane. Some possible functions of enzymes at this location are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Parkhouse R. M. Candidate for immunoglobulin D present on murine B lymphocytes. Nature. 1974 Dec 13;252(5484):600–602. doi: 10.1038/252600a0. [DOI] [PubMed] [Google Scholar]
- Allan D., Crumpton M. J. Preparation and characterization of the plasma membrane of pig lymphocytes. Biochem J. 1970 Nov;120(1):133–143. doi: 10.1042/bj1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Wasastjerna C., Gahmberg C. G. Different surface glycoprotein patterns on human T-, B- and leukemic-lymphocytes. Int J Cancer. 1976 Jan 15;17(1):40–46. doi: 10.1002/ijc.2910170107. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Membrane transport of purine and pyrimidine bases and nucleosides in animal cells. Int Rev Cytol. 1975;42:287–336. doi: 10.1016/s0074-7696(08)60983-3. [DOI] [PubMed] [Google Scholar]
- Bischoff E., Liersch M., Keppler D., Decker K. Fate of intravenously administered UDPglucose. Hoppe Seylers Z Physiol Chem. 1970 Jun;351(6):729–736. doi: 10.1515/bchm2.1970.351.1.729. [DOI] [PubMed] [Google Scholar]
- Bischoff E., Tran-Thi T. A., Decker K. F. Nucleotide pyrophosphatase of rat liver. A comparative study on the enzymes solubilized and purified from plasma membrane and endoplasmic reticulum. Eur J Biochem. 1975 Feb 21;51(2):353–361. doi: 10.1111/j.1432-1033.1975.tb03935.x. [DOI] [PubMed] [Google Scholar]
- Bischoff E., Wilkening J., Tran-Thi T. A., Decker K. Differentiation of the nucleotide pyrophosphatases of rat-liver plasma membranes and endoplasmic reticulum by enzymic iodination. Eur J Biochem. 1976 Feb 16;62(2):279–283. doi: 10.1111/j.1432-1033.1976.tb10158.x. [DOI] [PubMed] [Google Scholar]
- Cacan R., Verbert A., Montreuil J. New evidence for cell surface galactosyltransferase. FEBS Lett. 1976 Mar 15;63(1):102–106. doi: 10.1016/0014-5793(76)80203-7. [DOI] [PubMed] [Google Scholar]
- Crumpton M. J., Snary D. Preparation and properties of lymphocyte plasma membrane. Contemp Top Mol Immunol. 1974;3:27–56. doi: 10.1007/978-1-4684-2838-4_2. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. II. Properties and suitability as markers for the plasma membrane. J Biol Chem. 1974 Nov 25;249(22):7121–7129. [PubMed] [Google Scholar]
- Deppert W., Werchau H., Walter G. Differentiation between intracellular and cell surface glycosyl transferases: galactosyl transferase activity in intact cells and in cell homogenate. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3068–3072. doi: 10.1073/pnas.71.8.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson J. G., Jr, Rubio R., Berne R. M. Role of adenine nucleotides, adenosine, and inorganic phosphate in the regulation of skeletal muscle blood flow. Circ Res. 1971 Oct;29(4):375–384. doi: 10.1161/01.res.29.4.375. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Nucleotide pyrophosphatase, a sialoglycoprotein located on the hepatocyte surface. Nature. 1974 Aug 2;250(465):391–394. doi: 10.1038/250391a0. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H. Distribution of liver plasma membrane 5' nucleotidase as indicated by its reaction with anti-plasma membrane serum. Arch Biochem Biophys. 1974 Sep;164(1):305–311. doi: 10.1016/0003-9861(74)90035-6. [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H., Perkins H. R. The distribution of surface antigens during fractionation of mouse liver plasma membranes. Biochem J. 1972 Nov;130(1):271–280. doi: 10.1042/bj1300271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Behar-Bannelier M. Surface polypeptides of the cultured Chinese hamster ovary cell. Biochemistry. 1975 Aug 26;14(17):3816–3825. doi: 10.1021/bi00688a014. [DOI] [PubMed] [Google Scholar]
- Ladoulis C. T., Misra D. N., Estes L. W., Gill T. J., 3rd Lymphocyte plasma membranes. I. Thymic and splenic membranes from inbred rats. Biochim Biophys Acta. 1974 Jul 12;356(1):27–35. doi: 10.1016/0005-2736(74)90291-0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Misra D. N., Gill T. J., 3rd, Estes L. W. Lymphocyte plasma membranes. II. Cytochemical localization of 5'-nucleotidase in rat lymphocytes. Biochim Biophys Acta. 1974 Jun 29;352(3):455–461. [PubMed] [Google Scholar]
- Monneron A. Ultrastructural study of membrane-bound enzymes in thymocytes. J Histochem Cytochem. 1974 Dec;22(12):1128–1134. doi: 10.1177/22.12.1128. [DOI] [PubMed] [Google Scholar]
- Mookerjea S., Yung J. W. Studies on uridine diphosphate-galactose pyrophosphatase and uridine diphosphate-galactose: glycoprotein galactosyltransferase activities in microsomal membranes. Arch Biochem Biophys. 1975 Jan;166(1):223–226. doi: 10.1016/0003-9861(75)90383-5. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer B. L., Widnell C. C. The demonstration of a specific 5'-nucleotidase activity in rat tissues. Arch Biochem Biophys. 1975 Nov;171(1):343–347. doi: 10.1016/0003-9861(75)90041-7. [DOI] [PubMed] [Google Scholar]
- Sela B. A., Lis H., Sachs L. Enzymatic hydrolysis of uridine diphosphate-N-acetyl-D-galactosamine and uridine diphosphate-N-acetyl-D-glucosamine by normal cells, and blocks in this hydrolysis in transformed cells and their revertants. J Biol Chem. 1972 Dec 10;247(23):7585–7590. [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H., Cumming J., Verrier-Jones J. A set of surface proteins common to the circulating human platelet and lymphocyte. Biochem J. 1974 Sep;141(3):909–911. doi: 10.1042/bj1410909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Synthesis, transport, dynamics and fate of cell surface Ig and alloantigens in murine lymphocytes. Transplant Rev. 1973;14:50–75. doi: 10.1111/j.1600-065x.1973.tb00102.x. [DOI] [PubMed] [Google Scholar]


