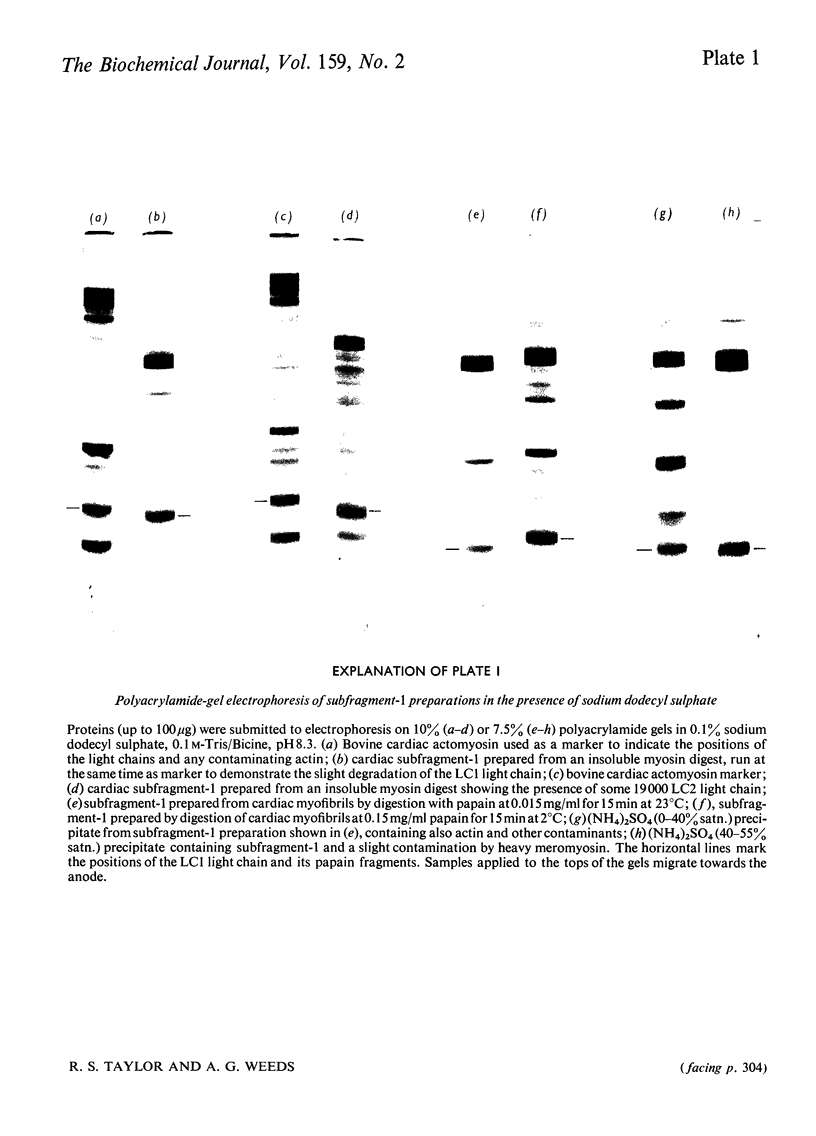
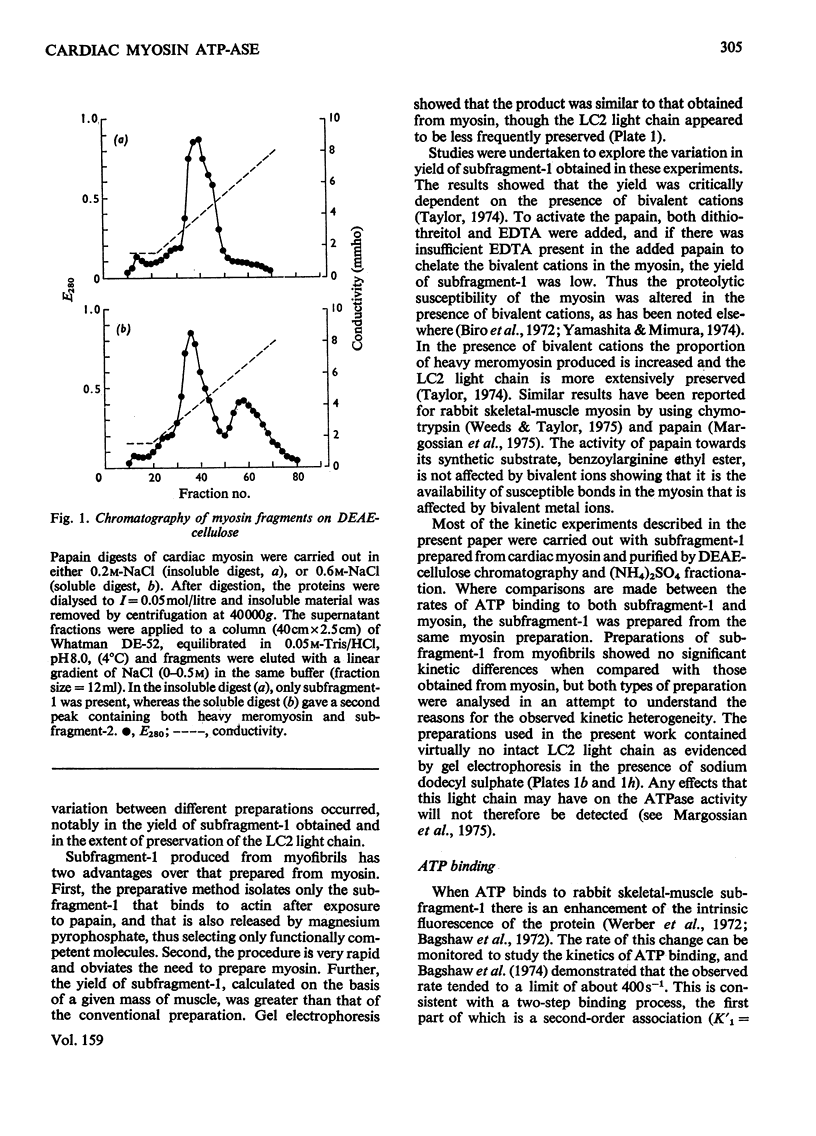
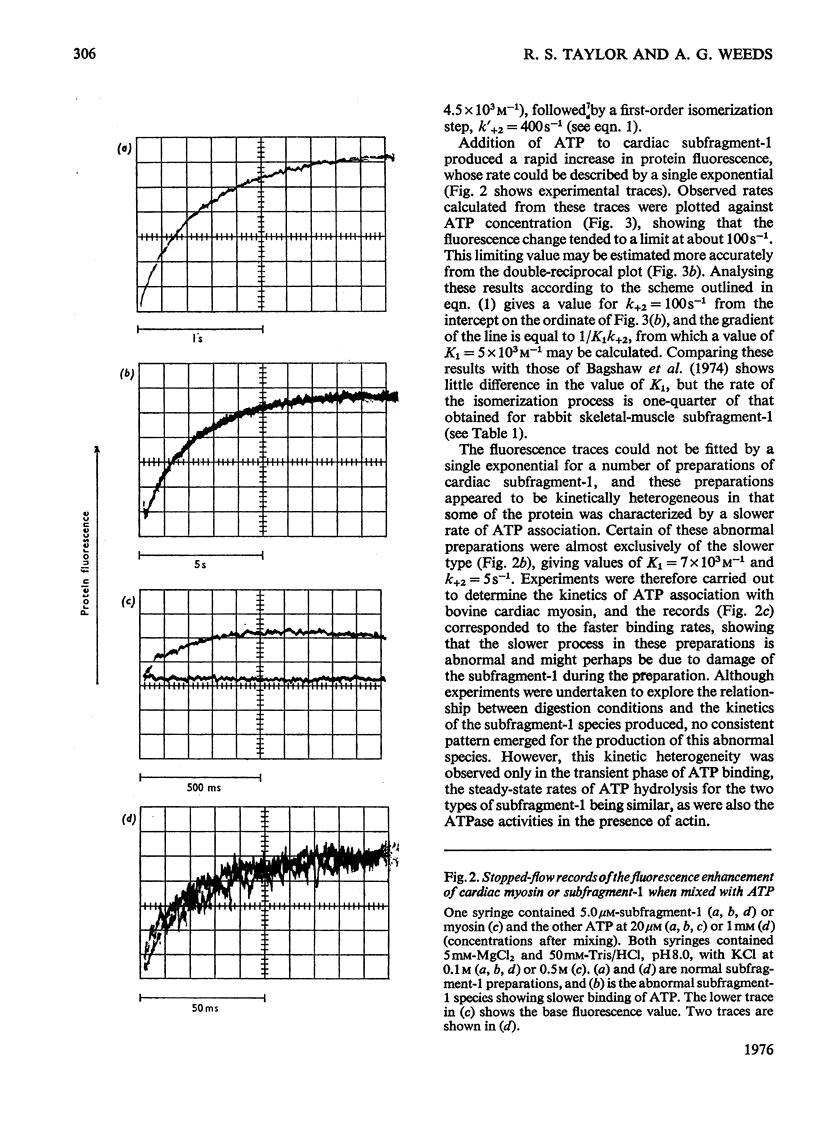
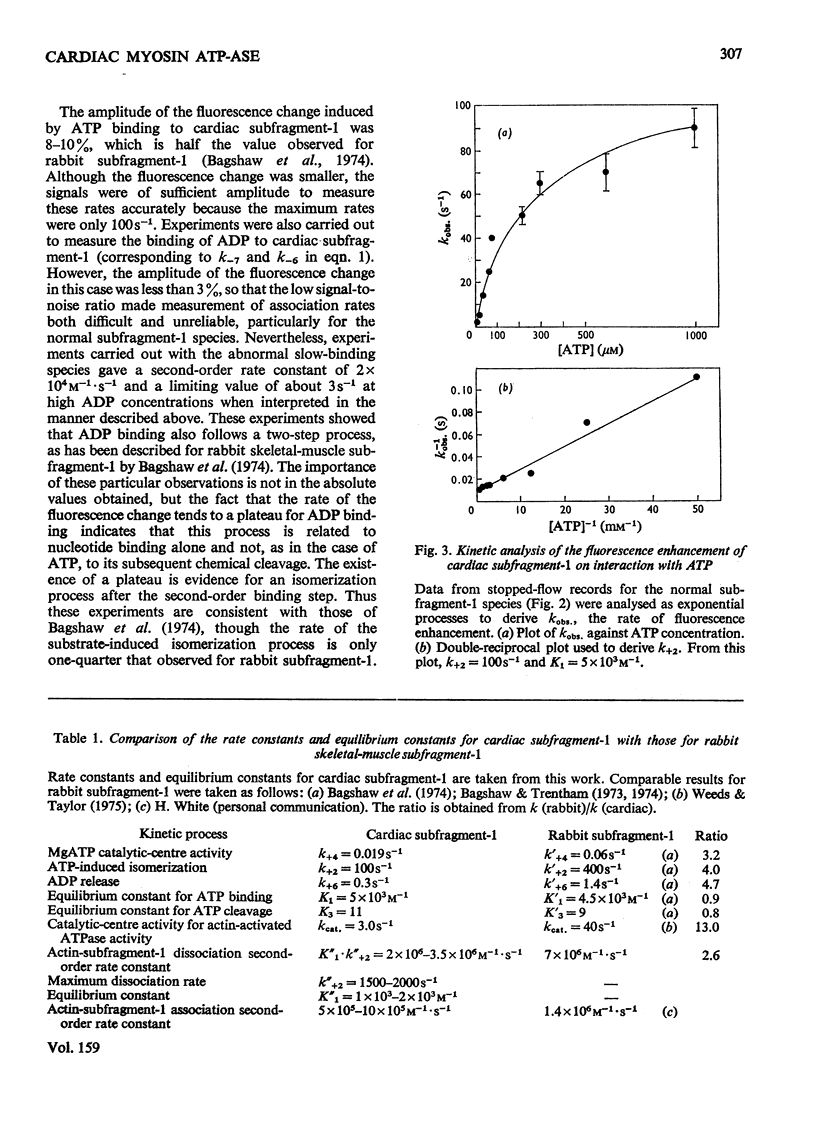
Abstract
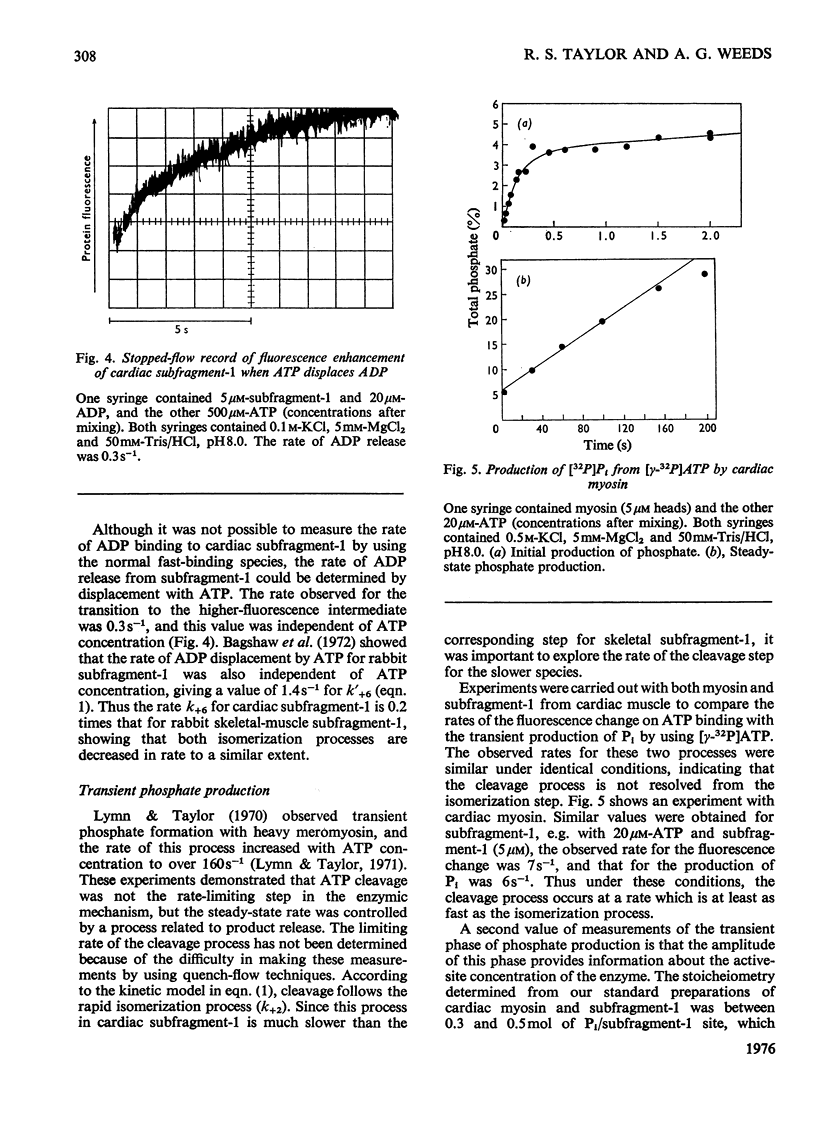
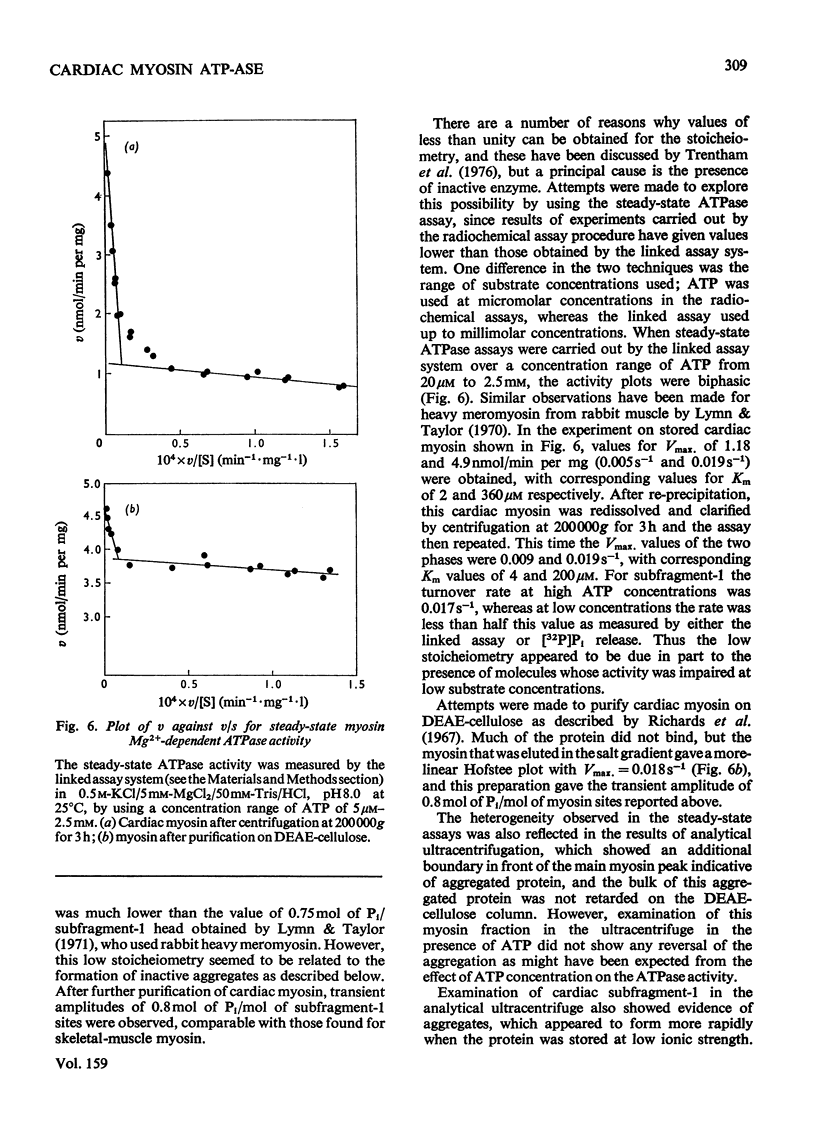
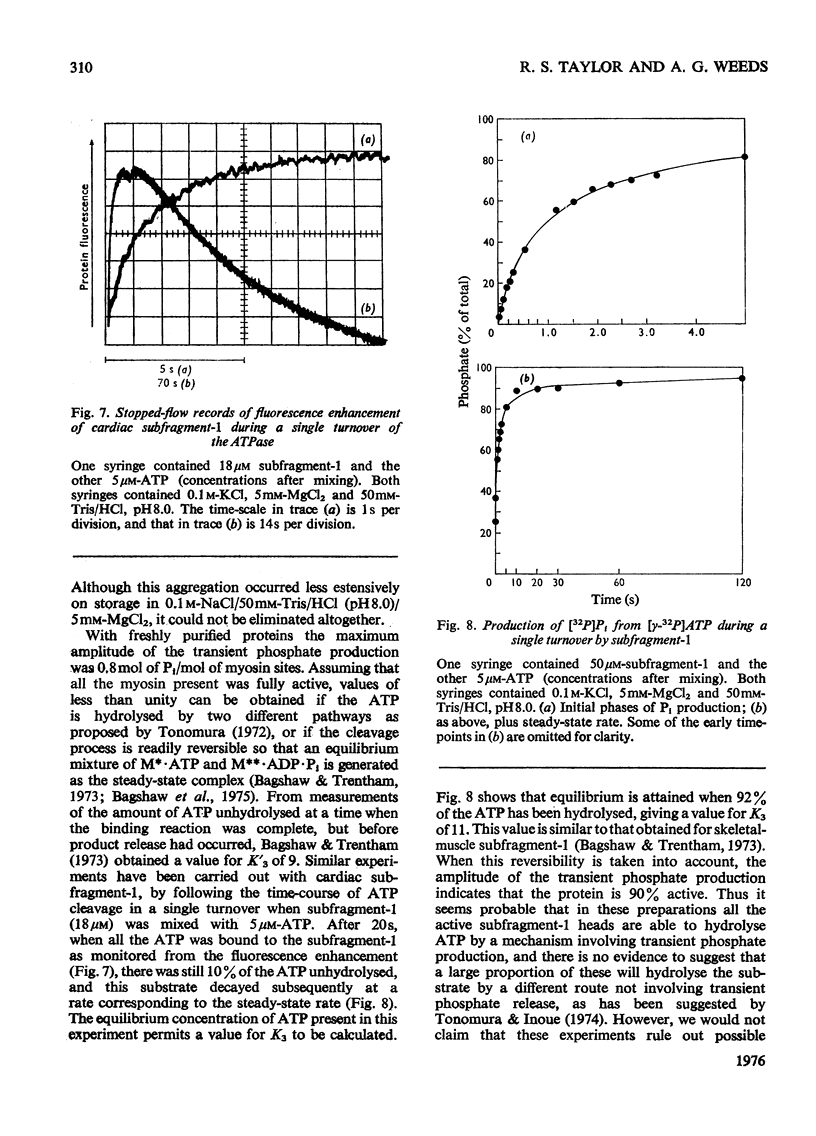
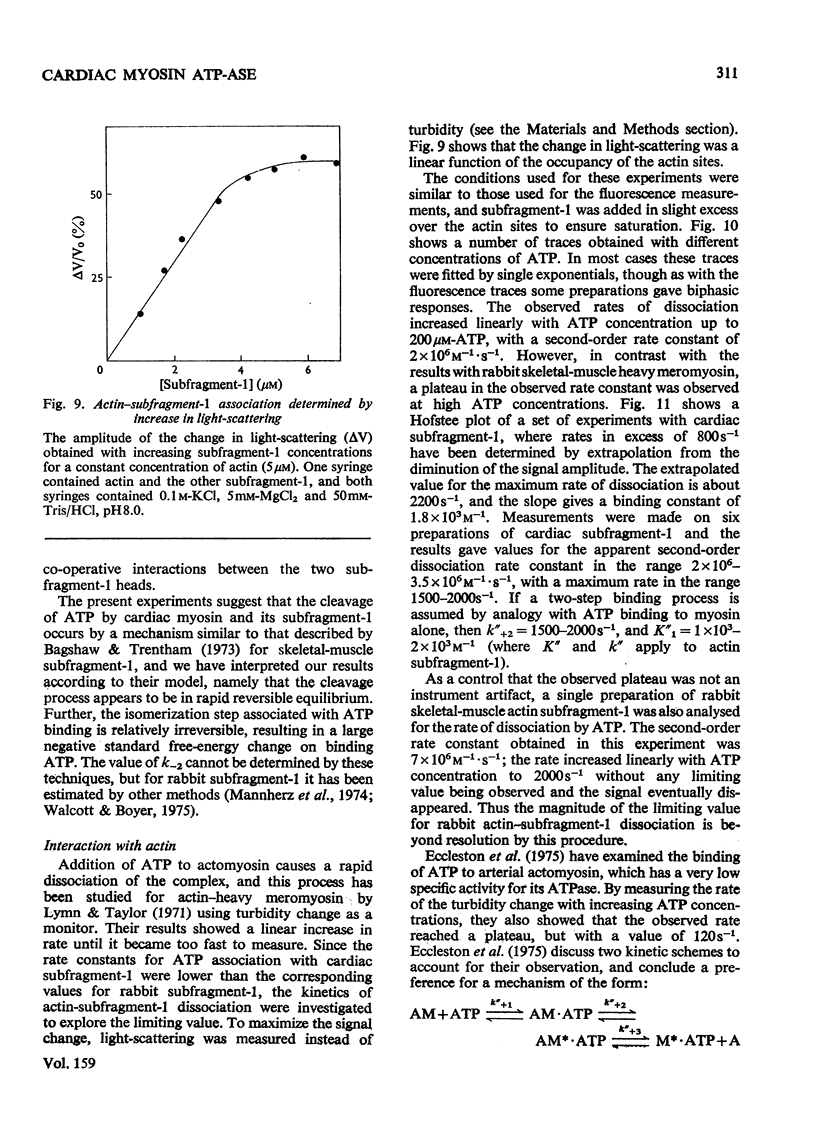
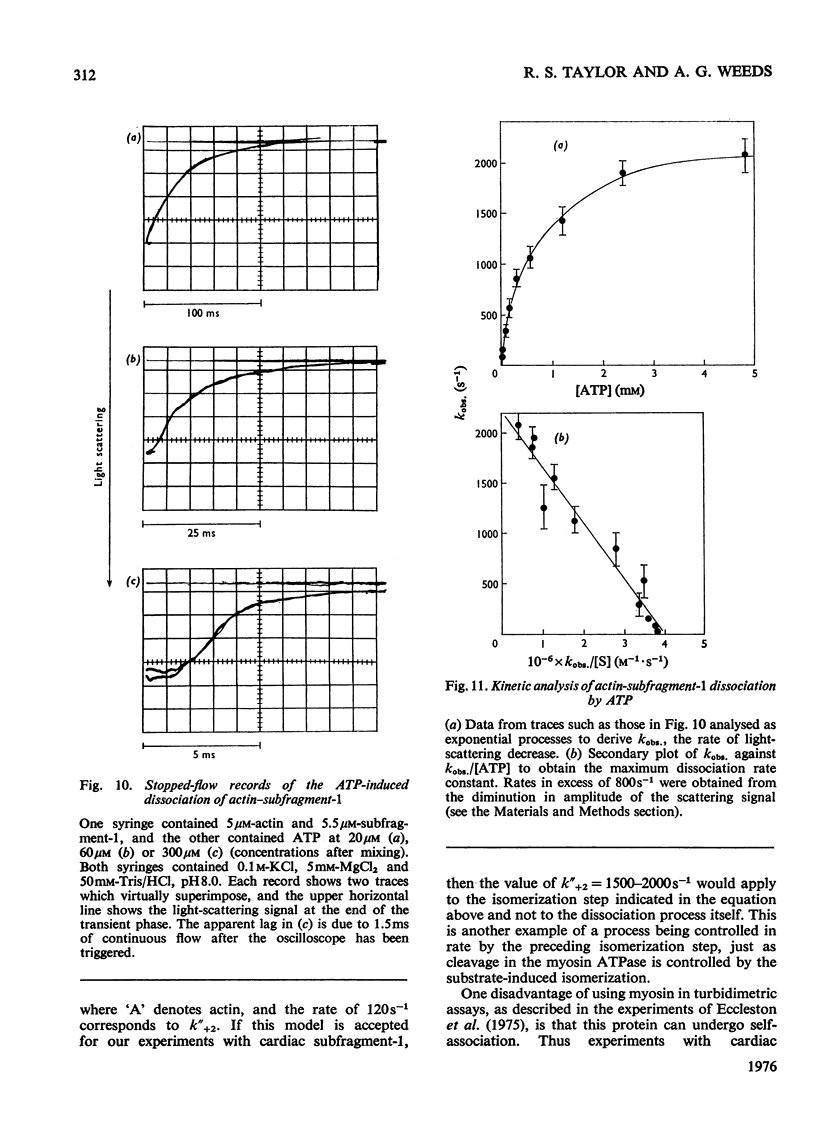
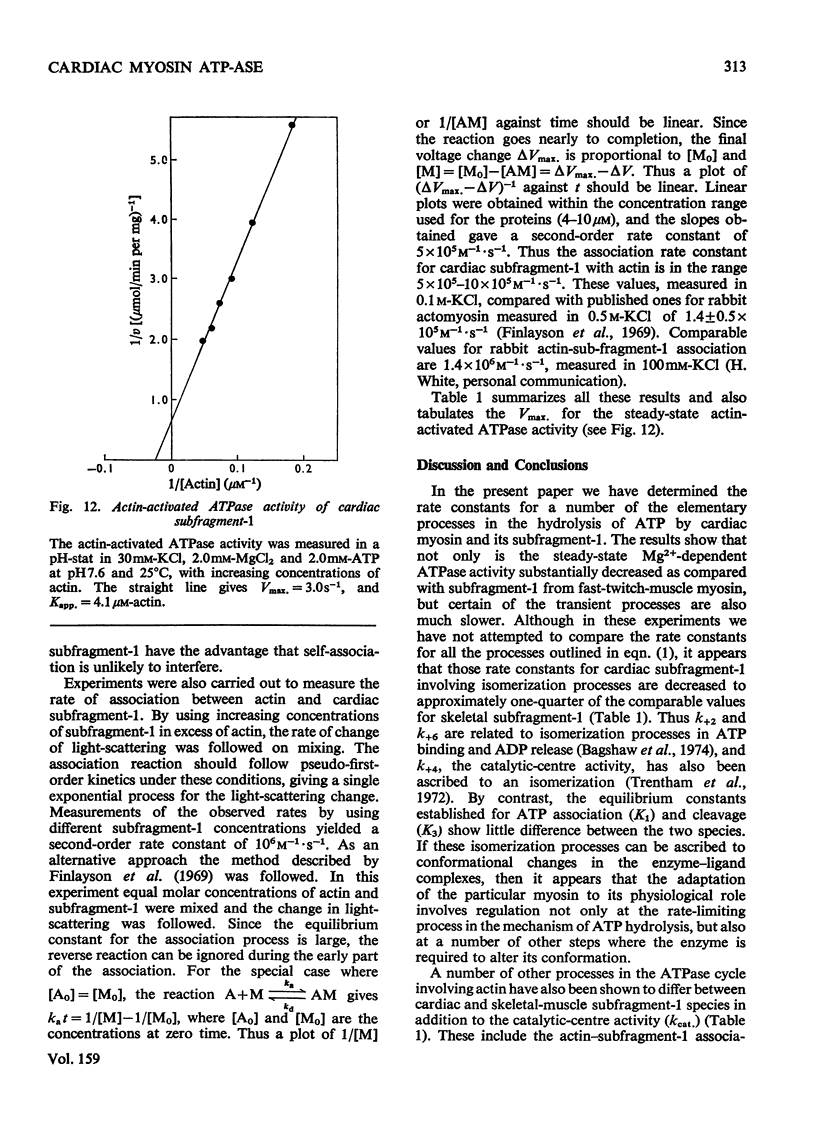
The kinetics of the Mg2+-dependent ATPase (adenosine triphosphatase) activity of bovine cardiac myosin and its papain subfragment-1 were studied by using steady-state and pre-steady-state techniques, and results were compared with published values for the corresponding processes in the ATPase mechanism of rabbit skeletal-muscle myosin subfragment-1. The catalytic-centreactivity for cardiac subfragment-1 is 0.019s-1, which is less than one-third of that determined for the rabbit protein. The ATP-induced isomerization process, measured from enhancement of protein fluorescence on substrate binding, is similarly decreased in rate, as is also the isomerization process associated with ADP release. However, the equilibrium constant for ATP cleavage, measured by quenched-flow by using [gamma-32P]ATP, shows little difference in the two species. Other experiments were carried out to investigate the rate of association of actin with subfragment-1 by light-scattering changes and also the rate of dissociation of the complex by ATP. The dissociation rate increases with increasing substrate concentration, to a maximum at high ATP concentrations, with a rate constant of about 2000s-1. It appears that isomerization processes which may involve conformational changes have substantially lower rate constants for the cardiac proteins, whereas equilibrium constants for substrate binding and cleavage are not significantly different. These differences may be related to the functional properties of these myosins in their different muscle types. Kinetic heterogeneity has been detected in both steady-state and transient processes, and this is discussed in relation to the apparent chemical homogeneity of cardiac myosin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Eccleston J. F., Eckstein F., Goody R. S., Gutfreund H., Trentham D. R. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J. 1974 Aug;141(2):351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. A new method for producing myosin subfragment-1. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1021–1028. doi: 10.1016/0006-291x(72)90314-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. Actin activation of heavy meromyosin adenosine triphosphatase. Dependence on adenosine triphosphate and actin concentrations. J Biol Chem. 1970 May 10;245(9):2451–2456. [PubMed] [Google Scholar]
- Eisenberg E., Moos C. Binding of adenosine triphosphate to myosin, heavy meromyosin, and subfragment 1. Biochemistry. 1970 Oct 13;9(21):4106–4110. doi: 10.1021/bi00823a011. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Mulvey R. S., Koch G. L. Ligand binding and enzymic catalysis coupled through subunits in tyrosyl-tRNA synthetase. Biochemistry. 1975 Jan 14;14(1):13–18. doi: 10.1021/bi00672a003. [DOI] [PubMed] [Google Scholar]
- Finlayson B., Lymn R. W., Taylor E. W. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969 Mar;8(3):811–819. doi: 10.1021/bi00831a008. [DOI] [PubMed] [Google Scholar]
- Gershman L. C., Stracher A., Dreizen P. Subunit structure of myosin. 3. A proposed model for rabbit skeletal myosin. J Biol Chem. 1969 May 25;244(10):2726–2736. [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. II. Evidence for the presence of a monomer--dimer equilibrium. Biochemistry. 1970 Feb 17;9(4):894–908. doi: 10.1021/bi00806a026. [DOI] [PubMed] [Google Scholar]
- Koretz J. F., Taylor E. W. Transient state kinetic studies of proton liberation by myosin and subfragment 1. J Biol Chem. 1975 Aug 25;250(16):6344–6350. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Transient state phosphate production in the hydrolysis of nucleoside triphosphates by myosin. Biochemistry. 1970 Jul 21;9(15):2975–2983. doi: 10.1021/bi00817a007. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F. H. M. The molecular transformations of actin. I. Globular actin. J Biol Chem. 1952 Sep;198(1):445–457. [PubMed] [Google Scholar]
- Mannherz H. G., Schenck H., Goody R. S. Synthesis of ATP from ADP and inorganic phosphate at the myosin-subfragment 1 active site. Eur J Biochem. 1974 Oct 1;48(1):287–295. doi: 10.1111/j.1432-1033.1974.tb03767.x. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S., Barshop B. Effect of DTNB light chain on the interaction of vertebrate skeletal myosin with actin. Nature. 1975 Nov 13;258(5531):163–166. doi: 10.1038/258163a0. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Substructure of the myosin molecule. IV. Interactions of myosin and its subfragments with adenosine triphosphate and F-actin. J Mol Biol. 1973 Mar 5;74(3):313–330. doi: 10.1016/0022-2836(73)90376-8. [DOI] [PubMed] [Google Scholar]
- Richards E. G., Chung C. S., Menzel D. B., Olcott H. S. Chromatography of myosin on diethylaminoethyl-Sephadex A-50. Biochemistry. 1967 Feb;6(2):528–540. doi: 10.1021/bi00854a022. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D., Perry S. V. Studies on the heterogeneity of subfragment-1 preparations. Isolation of a new proteolytic fragment of the heavy chain of myosin. Biochem J. 1973 Jan;131(1):127–137. doi: 10.1042/bj1310127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Bailin G., Bárány K., Bárány M. Proteolytic fragmentation of bovine heart heavy meromyosin. Biochemistry. 1969 Dec;8(12):4842–4850. doi: 10.1021/bi00840a029. [DOI] [PubMed] [Google Scholar]
- Tonomura Y., Inoue A. The substructure of myosin and the reaction mechanism of its adenosine triphosphatase. Mol Cell Biochem. 1974 Dec 20;5(3):127–143. doi: 10.1007/BF01731376. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Burridge K. Myosin from cross-reinnervated cat muscles. Evidence for reciprocal transformation of heavy chains. FEBS Lett. 1975 Sep 15;57(2):203–208. doi: 10.1016/0014-5793(75)80717-4. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Hall R., Spurway N. C. Characterization of myosin light chains from histochemically identified fibres of rabbit psoas muscle. FEBS Lett. 1975 Jan 1;49(3):320–324. doi: 10.1016/0014-5793(75)80776-9. [DOI] [PubMed] [Google Scholar]
- Weeds A. G. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976 Jun 15;66(1):157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971 Nov 14;61(3):701–725. doi: 10.1016/0022-2836(71)90074-x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Chemical studies on light chains from cardiac and skeletal muscle myosins. Nature. 1971 Nov 12;234(5324):85–88. doi: 10.1038/234085a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Werber M. M., Szent-Györgyi A. G., Fasman G. D. Fluorescence studies on heavy meromyosin-substrate interaction. Biochemistry. 1972 Jul 18;11(15):2872–2883. doi: 10.1021/bi00765a021. [DOI] [PubMed] [Google Scholar]
- West J. J., Nagy B., Gergely J. Free adenosine diphosphate as an intermediary in the phosphorylation by creatine phosphate of adenosine diphosphate bound to actin. J Biol Chem. 1967 Mar 25;242(6):1140–1145. [PubMed] [Google Scholar]
- Wolcott R. G., Boyer P. D. Isotopic probes of catalytic steps of myosin adenosine triphosphatase. J Supramol Struct. 1975;3(2):154–161. doi: 10.1002/jss.400030208. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Mimura H. Local conformational change of myosin detected by tryptic digestibility. II. Effect of magnesium ions on the fragmentation pattern of heavy meromyosin by trypsin. J Biochem. 1974 Dec;76(6):1337–1342. doi: 10.1093/oxfordjournals.jbchem.a130687. [DOI] [PubMed] [Google Scholar]



