Abstract
Background: Routine activated partial thromboplastin time (APTT) and prothrombin time (PT) measurements do not indicate hypercoagulability in patients with acute myocardial infarction (AMI) and acute cerebral infarction (ACI). Methods: Hypercoagulability in patients with AMI or ACI was evaluated using a clot waveform analysis of the APTT or a small amount of tissue factor activation assay (sTF/FIXa). In the CWA, the derivative peak time (DPT), height (DPH), width (DPW), and area the under the curve (AUC) were evaluated. Results: The APTT did not indicate hypercoagulability, but the second DPT of CWA-sTF/FIXa was significantly shorter in patients with ACI than in healthy volunteers (HVs). The first DPH values of CWA-APTT and CWA-sTF/FIXa in patients with ACI and AMI were significantly higher than in HVs. In the receiver operating characteristic (ROC) analyses of ACI or AMI vs. non-thrombosis, the AUC was >0.800 in the DPHs of CWA-APTT and CWA-sTF/FIXa. The AUC of CWA-APTT and CWA-sTF/FIXa in patients with AMI and ACI was significantly higher than in HVs. The AUC/second DPT of CWA-APTT and CWA-sTF/FIXa in patients with AMI and ACI was significantly higher than in HVs. Regarding the ROC analyses of ACI or AMI vs. HVs, the AUC of ROC was higher than 0.800 in the AUC and AUC/second DPT of CWA-APTT and CWA-sTF/FIXa. Conclusions: The AUC/second DPT of CWA-APTT and CWA-sTF/FIXa may be a useful parameter for detecting a hypercoagulable state in patients with AMI and ACI.
Keywords: CWA, APTT, sTF/FIXa, acute myocardial infarction, acute cerebral infarction
1. Introduction
Acute coronary syndrome (ACS) and acute myocardial infarction (AMI) are caused by atherothrombotic coronary artery disease resulting from atherosclerotic plaque rupture or erosion with a non-occlusive/occlusive thrombus [1,2]. AMI, including ST-elevation myocardial infarction (STEMI) [3,4], is generally diagnosed based on clinical symptoms, electrocardiography, biomarkers such as troponin [5] and creatine phosphokinase-MB (CKMB) [6], and coronary angiography [7]. After percutaneous coronary intervention [7], patients with AMI are usually treated with antiplatelet therapy [8,9] and are not considered hypercoagulable, except for those with cancer-related thrombosis (CAT) [10].
Acute cerebral infarction (ACI) [11,12] also includes atherosclerotic ACI [13], which is due to arterial thrombosis; this differs from cardioembolic ACI, which is due to venous thrombosis [14]. Although ACI is usually diagnosed using computed tomography, magnetic resonance imaging, and angiography in core hospitals, the differential diagnosis between cardioembolic ACI and atherosclerotic or lacunar ACI may be difficult [15]. In Japan, patients with atherosclerotic ACI are usually treated with antiplatelet agents [11,16] after treatment with argatroban [17]. Therefore, platelet activation may play an important role in the development of atherosclerosis, resulting in ACI or AMI [18]. In contrast, patients with cardioembolic ACI are treated with warfarin or DOACs [19,20] for a hypercoagulable state [21].
Regarding biomarkers, troponin T [22] and CKMB [6] are sensitive to AMI; however, these biomarkers are for myocardial injury and not for arterial thrombosis. Biomarkers for thrombosis may be more sensitive for myocardial injuries, suggesting that thrombotic biomarkers can detect the early phase of AMI. Elevated D-dimer levels [23,24,25] suggest venous thromboembolism (VTE) and disseminated intravascular coagulation (DIC). Biomarkers for platelet activation may be useful for the diagnosis of arterial thrombosis (e.g., AMI and ACI). Therefore, there have been reports on soluble C-type lectin receptor-2 [15], platelet factor 4 [26], β-thromboglobulin [27], and P-selectin [28]. Furthermore, the detection of hypercoagulability by a clot waveform analysis (CWA)-activated partial thromboplastin time (CWA-APTT) and CWA-small amount of tissue factor (TF) induced FIX activation assay (CWA-sTF/FIXa) has been recently reported in atherosclerotic ACI [21].
In this study, hypercoagulability in patients with AMI and ACI was examined using a CWA, and its usefulness in the diagnosis of AMI and ACI was analyzed by a receiver operating characteristic (ROC) analysis.
2. Materials and Methods
Patients with hemostatic abnormalities who were admitted to Mie Prefectural General Medical Center from September 1, 2020 to April 30, 2024 were investigated. Patients with AMI [n = 123; mean age ± standard deviation (SD), 68.4 ± 13.7 years; female, n = 26; male, n = 97], ACI [n = 104; mean age, 75.6 ± 5.1 years; female, n = 29; male, n = 75], chronic liver disease (CLD, n = 148; mean age, 75.6 ± 5.1 years; female, n = 73; male, 75), and cancer (n = 162; mean age, 74.4 ± 12.1 years; female, 35; male, 127) were examined using a CWA. CWA examinations were also performed on 50 healthy volunteers (HVs; mean age, 45.1 ± 18.0 years; female, n = 33; male, 17). Blood samples were obtained during days 1 and 14 post-admission. Patients who were treated with anticoagulants were excluded from the study. Patients with ACI and AMI were treated with antiplatelet therapy. ACI was diagnosed using clinical symptoms, physical examinations, medical history, and computed tomography or magnetic resonance imaging findings. AMI was diagnosed based on clinical symptoms, physical examinations, medical history, electrocardiograms, biomarkers such as troponin T and creatine phosphokinase-MB, and coronary angiography. The study protocol (O-0051) was approved by the Human Ethics Review Committee of the Mie Prefectural General Medical Center, and informed consent was obtained from each participant. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared via centrifugation at 900 rpm and 3000 rpm, respectively, for 15 min [29]. The APTT using PPP was measured using an APTT-SP® (Instrumentation Laboratory, Bedford, MA, USA) with an ACL-TOP® (Instrumentation Laboratory), as previously reported [30,31]. The sTF/FIXa assay using PRP was performed using 2000-fold diluted HemosIL RecombiPlasTin 2G (TF concentration < 0.1 pg/mL; Werfen. A CWA was performed as follows: Three curves (navy, pink, and light blue lines) are displayed on the monitor of an ACL-TOP® system [30,31]. The fibrin formation (FF) curve (navy line) corresponds to the changes in the absorbance observed while measuring the APTT. The first derivative peak (first DP) curve (pink line) corresponds to the coagulation velocity. The second derivative peak (second DP) curve (light blue line) corresponds to coagulation acceleration. The height and time of the FF, first DP, and second DP curves are abbreviated as FFH and FFT, first DPH and DPT, and second DPH and DPT, respectively.
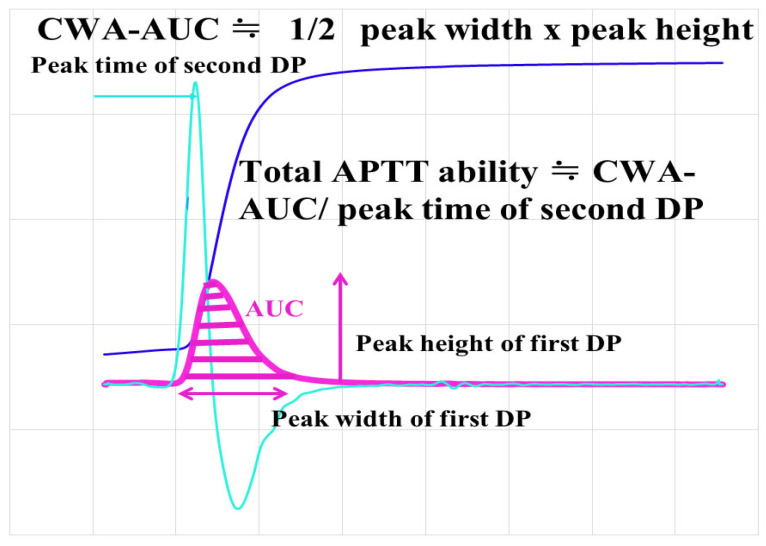
The area under the curve of the CWA (AUC-CWA) of the first DP indicates the ability of coagulation velocity instead of the peak time or height (Figure 1), and “half of the peak height × peak width” may be similar to AUC-CWA. In addition, “AUC-CWA/second peak time” may show the total coagulation ability, as shown by the APTT. The reference interval was within the 95% confidence interval of the healthy volunteers.
Figure 1.
CWA-AUC and total ability of CWA-APTT. APTT, activated partial thromboplastin time; navy line, fibrin formation curve; pink line, first derivative curve (velocity); light blue, second derivative curve (acceleration); AUC, area under the curve.
Statistical Analyses
Data are expressed as the median (range). The significance of differences between groups was examined using the Mann–Whitney U test. Cutoff values, determined as the point at which the sensitivity and specificity curves intersected, were examined using a ROC analysis. p-values of <0.05 were considered to indicate statistical significance. All statistical analyses were performed using the Stat-Flex software program (version 6; Artec Co., Ltd., Osaka, Japan).
3. Results
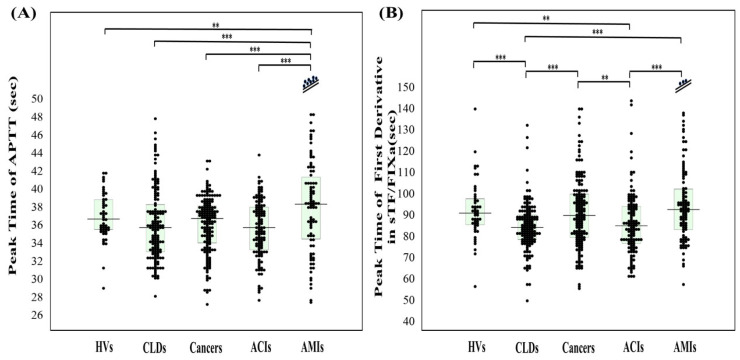
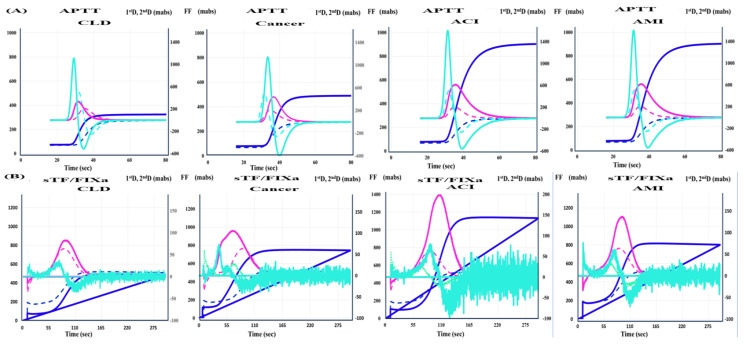
The reference interval of APTT (the second DPT of CWA-APTT) has been established as 27.3–35.8 s from 120 HVs, but the establishment of a reference interval for other parameters of CWA-APTT and CWA-sTF/FIXa is still proceeding. The 95% CI of CWA-APTT and CWA-sTF/FIXa in this study were as follows; for the second DPT, 30.7–41.9 s and 48.9–97.7 s, respectively, for the first DPT, 29.0–39.9 s and 68.1–125 s, respectively, for the fibrin formation time, 26.4–49.4 s and 74.9–124 s, respectively, for the second DPH, 434–1007 mabs and 20.4–81.1 mabs, respectively, for the first DPH, 137–317 mabs and 39.5–97.2 mabs, respectively, and for the FFH, 131–258 mabs and 175–519 mabs, respectively. The APTT was not significantly shorter in the patients with ACI and AMI than in the HVs, the patients with CLD, or the patients with cancer (Figure 2A), while it was significantly longer in the patients with AMI and was significantly longer than that in the patients with CLD, cancer, and ACI. The patients with AMI treated with percutaneous coronary intervention may have been affected by heparin. The peak time of the first derivative in CWA-sTF/FIXa was significantly shorter in the patients with CLD and the patients with ACI than in the HVs (Figure 2B). CWA-APTT showed no significant difference (p < 0.01) in peak times among the HVs and the patients with CLD, cancer, ACI, and AMI, and the peak heights in the patients with CLD, cancer, ACI, and AMI were significantly higher than those in the HVs (Figure 3A and Table 1). CWA-sTF/FIXa showed that the peak time of the second derivative in the patients with CLD, ACI, and AMI was significantly shorter than in the HVs and that the peak height of the first derivative in the patients with CLD, cancer, ACI, and AMI was significantly higher than in the HVs (Figure 3B and Table 1). In the ROC analyses of ACI or AMI vs. non-thrombosis, the AUC was >0.800 in the second DPH of CWA-APTT (ACI or AMI vs. HVs), the first DPH of CWA-APTT and CWA-sTF/FIXa (ACI or AMI vs. HVs), and the FFH of CWA-sTF/FIXa (AMI vs. HVs or CLD) (Table 2).
Figure 2.
Peak times of APTT (A) and sTF/FIXa (B) in HVs, and patients with CLD, cancer, ACI, and AMI. APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor-induced FIX activation assay; HVs, healthy volunteers; CLD, chronic liver disease; ACI, acute cerebral infarction; AMI, acute myocardial infarction; **, p < 0.01; ***, p < 0.001. Navy line, fibrin formation curve; FF, fibrin formation; pink line, first derivative curve (velocity); first DPH, first derivative peak height; light blue, second derivative curve (acceleration); second DPH, second derivative peak height; solid line, patient; dotted line, control.
Figure 3.
CWA-APTT (A) and CWA-sTF/FIXa (B) in patients with CLD, cancer, ACI, and AMI. Navy line, fibrin formation curve; FF, fibrin formation; pink line, first-derivative curve (velocity); first DPH, first derivative peak height; light blue, second derivative curve (acceleration); second DPH, second-derivative peak height; solid line, patient; dotted line, control.
Table 1.
CWA-APTT and CWA-sTF/FIXa in HVs and patients with CLD, cancer, ACI, and AMI.
| Second Derivative | First Derivative | Fibrin Formation | |||||
|---|---|---|---|---|---|---|---|
| APTT | Peak Time | Peak Height | Peak Time | Peak Width | Peak Height | Peak Time | Peak Height |
| HVs | 36.8 (35.6–38.9) |
731 (600–804) |
34.7 (33.7–36.6) |
36.0 (31.1–42.0) |
231 (201–273) |
36.8 (35.6–38.9) |
196 (171–214) |
| CLD | 35.8 (33.1–38.4) |
888 *** (752–1033) |
33.8 (31.4–36.5) |
35.0 (31.5–42.1) |
292 *** (246–339) |
35.8 (33.1–38.4) |
220 *** (198–250) |
| Cancer | 36.8 (34.1–38.2) |
955 *** (824–1168) |
34.3 (31.8–36.1) |
41.1 *** (34.9–46.5) |
315 *** (268–394) |
36.8 (34.1–38.2) |
253 *** (217–308) |
| ACI | 35.8 (33.3–38.1) |
997 *** (853–1191) |
33.7 (31.4–36.1) |
37.7 (33.0–42.8) |
327 *** (276–404) |
35.8 (33.3–38.1) |
239 *** (211–282) |
| AMI | 39.2 ** (35.4–44.8) |
1069 *** (865–1374) |
37.6 ** (33.6–42.0) |
42.5 *** (37.4–50.5) |
400 *** (324–495) |
39.2 ** (35.4–44.8) |
324 *** (273–395) |
| sTF/FIXa | Peak time | Peak height | Peak time | Peak width | Peak height | Peak time | Peak height |
| HVs | 71.3 (65.1–79.2) |
31.9 (28.7–40.0) |
91.3 (85.8–98.0) |
145 (134–158) |
65.0 (55.4–71.9) |
92.2 (86.9–97.8) |
315 (255–373) |
| CLD | 68.2 * (61.5–64.7) |
44.5 *** (36.6–56.9) |
84.5 *** (78.8–90.4) |
138 *** (126–145) |
83.6 *** (71.3–101) |
85.7 *** (79.7–91.3) |
330 (277–380) |
| Cancer | 70.5 (62.7–79.3) |
43.1 *** (31.3–62.1) |
90.1 (79.8–100) |
152 (131–170) |
88.3 *** (73.2–114) |
92.4 (81.7–102) |
344 * (284–432) |
| ACI | 63.8 ** (54.3–74.0) |
47.0 *** (36.2–60.1) |
85.3 ** (77.0–94.4) |
142 (132–154) |
91.1 *** (75.6–118) |
85.8 *** (78.2–94.6) |
347 ** (302–413) |
| AMI | 68.1 (56.6–77.4) | 46.8 *** (33.0–60.2) |
93.0 (84.0–107) |
162 *** (142–183) |
112 *** (81.1–138) |
94.6 (84.5–108) |
473 *** (393–550) |
CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor activation assay; HVs, healthy volunteers; CLD, chronic liver disease, ACI, acute cerebral infarction; AMI, acute myocardial infarction; *, p < 0.05; **, p < 0.01; ***, p < 0.001 in comparison to HVs.
Table 2.
Area under the curve of ROC in CWA-APTT and CWA-sTF/FIXa (AMI or ACI vs. without thrombosis).
| ACI vs. | HVs | CLD | Cancer | AMI vs. | HVs | CLD | Cancer | ||
|---|---|---|---|---|---|---|---|---|---|
| CWA- APTT |
Second DPT | 0.658 | 0.537 | 0.538 | 0.609 | 0.652 | 0.675 | ||
| First DPT | 0.620 | 0.523 | 0.523 | 0.660 | 0.677 | 0.710 | |||
| First DPW | 0.562 | 0.573 | 0.605 | 0.741 | 0.751 | 0.584 | |||
| FFT | 0.623 | 0.509 | 0.547 | 0.650 | 0.673 | 0.689 | |||
| Second DPH | 0.859 | 0.637 | 0.519 | 0.810 | 0.655 | 0.565 | |||
| First DPH | 0.857 | 0.646 | 0.530 | 0.901 | 0.774 | 0.681 | |||
| FFH | 0.809 | 0.625 | 0.558 | 0.963 | 0.876 | 0.738 | |||
| CWA- sTF/FIX |
Second DPT | 0.658 | 0.573 | 0.619 | 0.581 | 0.501 | 0.546 | ||
| First DPT | 0.649 | 0.513 | 0.599 | 0.543 | 0.694 | 0.568 | |||
| First DPW | 0.553 | 0.597 | 0.577 | 0.681 | 0.795 | 0.619 | |||
| FFT | 0.661 | 0.509 | 0.547 | 0.548 | 0.673 | 0.689 | |||
| Second DPH | 0.747 | 0.538 | 0.551 | 0.704 | 0.510 | 0.517 | |||
| First DPH | 0.867 | 0.594 | 0.537 | 0.870 | 0.699 | 0.643 | |||
| FFH | 0.636 | 0.589 | 0.515 | 0.844 | 0.826 | 0.750 | |||
CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor activation assay; HVs, healthy volunteers; CLD, chronic liver disease, ACI, acute cerebral infarction; AMI, acute myocardial infarction; DPT, derivative peak time; DPW, derivative peak width; FFT, fibrin formation time; DPH, derivative peak height; FFH, fibrin formation height; a red number indicates area under the curve > 0.800.
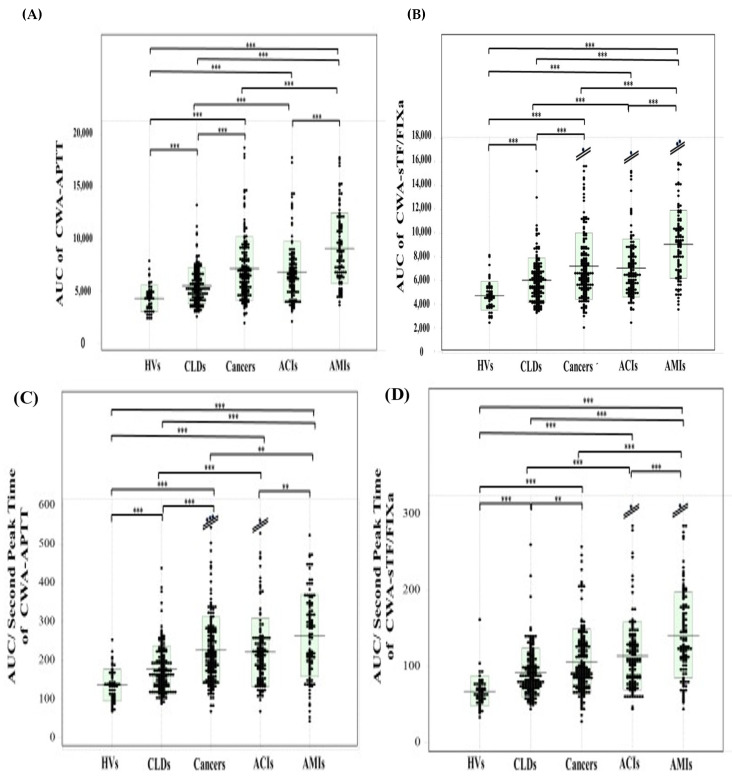
The reference interval of the AUC of CWA-APTT and CWA-sTF/FIXa was 2471–7277 mabs × sec and 2831–8031 mabs × sec, respectively. The AUC of CWA-APTT was significantly higher in the patients with cancer {6592 mabs × sec (5024–8628 mabs × sec)}, AMI {8689 mabs × sec (6518–11,243 mabs × sec)}, and ACI {8419 mabs × sec (4899–7921 mabs × sec)} than in the HVs {4343 mabs × sec (3382–4954 mabs × sec)}and the patients with CLD {5257 mabs × sec (4213–6632 mabs × sec)}, and it was significantly higher in the patients with AMI than in the HVs and the patients with CLD and cancer (Figure 4A). The AUC of CWA-sTF/FIXa was significantly higher in the patients with AMI {9216 mabs × sec (6890–10,748 mabs × sec)} than in the HVs {4643 mabs × sec (3908–5357 mabs × sec)} and the patients with ACI {6530 mabs × sec (5288–8265 mabs × sec)}, cancer {6626 mabs × sec (5377–8601 mabs × sec)}, and CLD {5883 mabs × sec (4710–6955 mabs × sec)}, and it was significantly higher in the patients with ACI than in the HVs and patients with CLD (Figure 4B). Regarding the AUC value in the ROC analyses of ACI or AMI vs. non-thrombosis, the AUC of CWA-APTT and CWA-sTF/FIXa was >0.800 in AMI vs. HVs or CLD (Table 3A).
Figure 4.
AUC {(A,B)} AUC/second peak time {(C,D)} of CWA-APTT {(A,C)} and CWA-sTF/FIXa {(B,D)} in HVs and patients with CLD, cancer, ACI, and AMI. APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor-induced FIX activation assay; HVs, healthy volunteers; CLD, chronic liver disease; ACI, acute cerebral infarction; AMI, acute myocardial infarction; **, p < 0.01; ***, p < 0.001.
Table 3.
ROC analysis of the AUC (A) and AUC/second DPT (B) of CWA-APTT and CWA-sTF/FIXa (AMI or ACI vs. without thrombosis).
| (A) AUC | Cutoff | Sensitivity | Odds Ratio | |
|---|---|---|---|---|
| CWA-APTT | ||||
| AMI vs. HVs | 0.934 | 5362 | 85.6% | 31.2 |
| AMI vs. CLD | 0.821 | 6526 | 74.3% | 8.68 |
| AMI vs. Cancer | 0.679 | 7281 | 63.6% | 3.03 |
| ACI vs. HVs | 0.812 | 4392 | 74.0% | 4.82 |
| ACI vs. CLD | 0.648 | 5801 | 62.8% | 2.93 |
| ACI vs. Cancer | 0.534 | 6479 | 51.2% | 1.05 |
| CWA-sTF/FIXa | ||||
| AMI vs. HVs | 0.929 | 5774 | 87.4% | 42.5 |
| AMI vs. CLD | 0.816 | 6890 | 74.3% | 7.25 |
| AMI vs. Cancer | 0.696 | 7712 | 67.0% | 4.18 |
| ACI vs. HVs | 0.833 | 5336 | 74.0% | 8.10 |
| ACI vs. CLD | 0.635 | 6157 | 58.5% | 2.01 |
| ACI vs. Cancer | 0.506 | 6587 | 51.6% | 1.16 |
| (B) AUC/Second DPT | Cutoff | Sensitivity | Odds Ratio | |
| CWA-APTT | ||||
| AMI vs. HVs | 0.867 | 169 | 78.8% | 13.2 |
| AMI vs. CLD | 0.783 | 106 | 74.3% | 8.57 |
| AMI vs. Cancer | 0.603 | 222 | 55.8% | 1.12 |
| ACI vs. HVs | 0.836 | 158 | 74.0% | 8.09 |
| ACI vs. CLD | 0.665 | 188 | 62.0% | 2.66 |
| ACI vs. Cancer | 0.526 | 212 | 49.6% | 1.00 |
| CWA-sTF/FIXa | ||||
| AMI vs. HVs | 0.912 | 79.8 | 85.4% | 36.0 |
| AMI vs. CLD | 0.771 | 107 | 73.8% | 7.86 |
| AMI vs. Cancer | 0.691 | 116 | 65.4% | 3.62 |
| ACI vs. HVs | 0.867 | 76.5 | 78.0% | 13.2 |
| ACI vs. CLD | 0.662 | 94.5 | 62.2% | 2.75 |
| ACI vs. Cancer | 0.553 | 102 | 57.2% | 1.83 |
AUC, area under the curve; CWA, clot waveform analysis; APTT, activated partial thromboplastin time; sTF/FIXa, small amount of tissue factor activation assay; HVs, healthy volunteers; CLD, chronic liver disease; ACI, acute cerebral infarction; AMI, acute myocardial infarctions; DPT, derivative peak time; a red number indicates area under the curve > 0.800.
The reference interval of the AUC/second DPT of CWA-APTT and CWA-sTF/FIXa was 70.8–231 mabs and 37.2–118 mabs, respectively. The AUC/second DPT of CWA-APTT was significantly higher in the patients with cancer {212 mabs (165–278 mabs)}, AMI {265 mabs (186–342 mabs)}, and ACI {212 mabs (156–258 mabs)} than in the HVs {135 mabs (105–163 mabs)} and CLDs {165 mabs (133–210 mabs)}, and it was significantly higher in the patients with AMIs than in the HVs, the patients with cancers, and the patients with CLD (Figure 4C). The AUC/second DPT of CWA-sTF/FIXa was significantly higher in the patients with AMI {128 mabs (103–176 mabs)} than in the HVs {64.4 mabs (57.0–75.9 mabs)} and the patients with ACI {107 mabs (80.9–133 mabs)}, cancer {95.2 mabs (77.0–128 mabs)}, and CLD {86.3 mabs (72.0–100 mabs)}, and it was significantly higher in the patients with ACI than in the HVs and the patients with CLD (Figure 4D). Regarding the AUC value from the ROC analyses of the CWA-AUC/second DPT for ACI or AMI vs. non-thrombosis, the CWA-APTT showed that it was >0.800 in patients with AMI or ACI vs. HVs, and the CWA-sTF/FIXa showed that it was >0.900 in patients with AMI or ACI vs. HVs (Table 3B).
4. Discussion
Routine measurements of clotting time, such as APTT and PT, are used to evaluate hemostatic abnormality, clotting factor deficiencies [32], inhibitors of clotting factor [33], DIC [34], and lupus anticoagulant (LA) [35] and to monitor anticoagulants such as heparin or warfarin [36,37,38]; however, these measurements are not able to detect a hypercoagulable state as reliably as a thrombin generation test [39] or thromboelastography [40]. Our findings show no significant difference in the peak times of CWA-APTT between patients with and without thrombosis. However, the evaluation of the APTT or PT has been updated since the development of the CWA, including the modified CWA [30,31]. The second peak time of CWA-sTF/FIXa was significantly shorter in the patients with ACI than in the HVs. In addition, a CWA shows not only the peak time but also the peak height, peak width, and AUC [30,31]. The detection of a hypercoagulable state using the elevated peak height of CWA-APTT and CWA-sTF/FIXa has been reported in patients with malignant neoplasm [41] and ACI [21]. Our findings also show that the patients with AMI were in a hypercoagulable state, as were the patients with cancer or ACI. In the thrombin generation test [42,43], a shortened peak time and increased AUC indicate a hypercoagulable state. Therefore, the AUC and AUC/second DPT of CWA-APTT or CWA-sTF/FIXa may indicate a hypercoagulable state or thrombogenicity, respectively. A ROC analysis of the patients with AMI or ACI vs. the patients with non-thrombotic diseases showed that the usefulness in detecting a hypercoagulable state was in the order of AUC/second DPT, AUC, and peak height; CWA-sTF/FIXa was better than CWA-APTT. As these AMI patients with hypercoagulability by CWA had low D-dimer levels, CWA-APTT and CWA-sTF/FIXa can be independent markers for hypercoagulability from fibrin-related markers.
Patients with AMI or atherosclerotic ACI are generally treated with antiplatelet therapy [44,45], and hypercoagulability has not been considered important in these patients. Our CWA-APTT and CWA-sTF/FIXa results showed that the patients with ACI or AMI were markedly hypercoagulable. Indeed, atherosclerotic ACI patients are treated with antithrombin agents such as argatroban [46]. Many relationships between the hypercoagulable state and AMI have also been reported [47,48,49]. During atherosclerotic plaque rupture, the hypercoagulable state may increase the size of the coronary artery thrombosis, causing severe AMI. However, a hypercoagulable state might not be observed in patients with AMI induced by coronary spasm [50]. It has been reported that oral anticoagulants with or without aspirin do not reduce mortality, reinfarction, or stroke but significantly increase major bleeding [51]. However, when percutaneous coronary intervention is performed in patients with AMI, they are usually treated with unfractionated heparin and antiplatelets [52]. Therefore, the peak times of CWA-APTT and CWA-sTF/FIXa were slightly prolonged in the AMI patients in our study. In these patients, the effect of heparin was confirmed based on thrombin time and an anti-Xa assay. Patients with lupus anticoagulant were excluded from this study. sTF/FIXa using PRP can be used to evaluate the effect of platelets. AMI is considered to have major effects via platelet activation. There was no significant difference in platelet count between the patients with AMI and CLD. There was a significant difference in sTF/FIXa between the patients with and without thrombocytopenia (platelet count < 8 × 109/L) [29,30,31]. This thrombocytopenia, which was observed in liver cirrhosis, was excluded from CLD in this study.
The mechanism underlying the development of a hypercoagulable state in AMI is considered to involve various underlying diseases, such as diabetes mellitus [53], hypertension [54], hyperlipidemia [55], inflammation [56], malignant neoplasm [57], and obesity [58]. These risk factors cause mild or strong hypercoagulable states owing to the increased expression of TF [59], the activation of the coagulation system [60], inflammatory cytokines [61], atherosclerosis [55], platelet activation [62], and thrombin burst [63]. These factors increase the peak heights of CWA-APTT and CWA-sTF/FIXa and shorten the peak time of CWA-sTF/FIXa [31,64]. “The peak height × peak width” half of the velocity curve is similar to the AUC of the velocity curve. Although there was no significant difference in the peak widths of CWA-APTT and CWA-sTF/FIXa between the patients with and without thrombosis, the AUC and AUC/peak time of the acceleration curve were strongly reflected in the hypercoagulable state.
In terms of clinical implication, this study demonstrates hypercoagulability in patients with AMI or ACI by using CWA-APTT or CWA-sTF/FIXa, thus suggesting that anticoagulant therapy may be useful for AMI or ACI. Evidence of hypercoagulability in AMI or ACI indicates a thrombotic risk for these diseases, suggesting the possibility of the prophylaxis of AMI or ACI.
Limitation of Reference Interval of CWA
APTT (the second DPT of CWA-APTT) has sufficiently been established; however, the establishment of a reference interval for other parameters of CWA-APTT and CWA-sTF/FIXa is proceeding. APTT is automatically obtained from a fully automatic blood coagulometer, whereas the other parameters of CWA-APTT and all parameters of CWA-sTF/FIXa are manually measured in each patient, suggesting that large-scale study using CWA may be slightly difficult. In addition, there are a few HVs who have normal coagulation ability at old age. Therefore, it is difficult to use age-matched controls in a CWA study.
5. Conclusions
An increased peak height and AUC of CWA-sTF/FIXa and a shortened second DPT of CWA-sTF/FIXa were observed in patients with AMI and ACI. The CWA-AUC/second DPT of CWA-sTF/FIXa may be useful for detecting a hypercoagulable state in AMI or ACI.
Acknowledgments
We would like to thank Hiroko Nishii and Yumi Sakano (Department of Molecular Pathobiology and Cell Adhesion Biology, Mie University Graduate School of Medicine) for their support with CWA-APTT and CWA-sTF/FIXa.
Author Contributions
Conceptualization, H.W.; methodology, Y.I.; software, T.K. (Takashi Kato); validation, Y.T., T.K. (Toshitaka Kamon) and N.I.; formal analysis, K.H.; investigation, K.Y. and T.O.; resources, K.N. and K.O.; data curation, H.O.; writing—original draft preparation, J.M.; writing—review and editing, H.W.; visualization, M.S.; supervision, K.D. and H.S.; project administration, K.S.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol (O-0051/1 August 2020) was approved by the Human Ethics Review committee of Mie Prefectural General Medical Center, and informed consent was obtained from each patient.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by a Grant-in-Aid from the Ministry of Health, Labor and Welfare of Japan (grant number 21FC1008).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018) J. Am. Coll. Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 2.Ceasovschih A., Mantzouranis E., Dimitriadis K., Sorodoc V., Vlachakis P.K., Karanikola A.-E., Theofilis P., Koutsopoulos G., Drogkaris S., Andrikou I., et al. Coronary artery thromboembolism as a cause of myocardial infarction with non-obstructive coronary arteries (MINOCA) Hell. J. Cardiol. 2024;79:70–83. doi: 10.1016/j.hjc.2024.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K., Kimura T., Ishihara M., Nakagawa Y., Nakao K., Miyauchi K., Sakamoto T., Tsujita K., Hagiwara N., Miyazaki S., et al. JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. Circ. J. 2019;83:1085–1196. doi: 10.1253/circj.CJ-19-0133. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Alpert J.S., Thygesen K., Antman E., Bassand J.P. Myocardial infarction redefined: A consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur. Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 6.Bruins Slot M.H., van der Heijden G.J., Stelpstra S.D., Hoes A.W., Rutten F.H. Point-of-care tests in suspected acute myocardial infarction: A systematic review. Int. J. Cardiol. 2013;168:5355–5362. doi: 10.1016/j.ijcard.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt D.L., Lopes R.D., Harrington R.A. Diagnosis and Treatment of Acute Coronary Syndromes: A Review. JAMA. 2022;327:662–675. doi: 10.1001/jama.2022.0358. [DOI] [PubMed] [Google Scholar]
- 8.Khan S.U., Singh M., Valavoor S., Khan M.U., Lone A.N., Khan M.Z., Khan M.S., Mani P., Kapadia S.R., Michos E.D., et al. Dual Antiplatelet Therapy After Percutaneous Coronary Intervention and Drug-Eluting Stents: A Systematic Review and Network Meta-Analysis. Circulation. 2020;142:1425–1436. doi: 10.1161/CIRCULATIONAHA.120.046308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelbenegger G., Jilma B. Clinical pharmacology of antiplatelet drugs. Expert Rev. Clin. Pharmacol. 2022;15:1177–1197. doi: 10.1080/17512433.2022.2121702. [DOI] [PubMed] [Google Scholar]
- 10.Pernod G., Cohen A., Mismetti P., Sanchez O., Mahé I., INNOVTE CAT Working Group Cancer-related arterial thromboembolic events. Arch. Cardiovasc. Dis. 2024;117:101–113. doi: 10.1016/j.acvd.2023.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Phipps M.S., Cronin C.A. Management of acute ischemic stroke. Br. Med. J. 2020;368:l6983. doi: 10.1136/bmj.l6983. [DOI] [PubMed] [Google Scholar]
- 12.World Stroke Organization (WSO) Global Stroke Fact Sheet. [(accessed on 1 August 2024)]. Available online: https://www.world-stroke.org/
- 13.Ministrini S., Carbone F., Montecucco F. Updating concepts on atherosclerotic inflammation: From pathophysiology to treatment. Eur. J. Clin. Investig. 2020;51:e13467. doi: 10.1111/eci.13467. [DOI] [PubMed] [Google Scholar]
- 14.Strandberg M., Mustonen P., Taina M., Korpela J., Vanninen S., Hedman M. Etiology, diagnostics and treatment of cardiogenic stroke. Duodecim Laaketieteellinen Aikakauskirja. 2016;132:1625–1633. [PubMed] [Google Scholar]
- 15.Nishigaki A., Ichikawa Y., Ezaki M., Yamamoto A., Suzuki K., Tachibana K., Kamon T., Horie S., Masuda J., Makino K., et al. Soluble C-Type Lectin-Like Receptor 2 Elevation in Patients with Acute Cerebral Infarction. J. Clin. Med. 2021;10:3408. doi: 10.3390/jcm10153408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarenco P., Denison H., Evans S.R., Himmelmann A., James S., Knutsson M., Ladenvall P., Molina C.A., Wang Y., Johnston S.C., et al. Ticagrelor added to aspirin in acute ischemic stroke or transient ischemic attack in prevention of disabling stroke: A randomized clinical trial. JAMA Neurol. 2020;78:177–185. doi: 10.1001/jamaneurol.2020.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang P., He X.-Y., Xu M. Effect of Argatroban Injection on Clinical Efficacy in Patients with Acute Cerebral Infarction: Preliminary Findings. Eur. Neurol. 2021;84:38–42. doi: 10.1159/000512813. [DOI] [PubMed] [Google Scholar]
- 18.Macha K., Marsch A., Siedler G., Breuer L., Strasser E.F., Engelhorn T., Schwab S., Kallmünzer B. Cerebral ischemia in patients on direct oral anticoagulants. Stroke. 2019;50:873–879. doi: 10.1161/STROKEAHA.118.023877. [DOI] [PubMed] [Google Scholar]
- 19.Pircher J., Engelmann B., Massberg S., Schulz C. Platelet–Neutrophil Crosstalk in Atherothrombosis. Thromb. Haemost. 2019;119:1274–1282. doi: 10.1055/s-0039-1692983. [DOI] [PubMed] [Google Scholar]
- 20.Streifler J.Y., Katz M. Cardiogenic cerebral emboli: Diagnosis and treatment. Curr. Opin. Neurol. 1995;8:45–54. doi: 10.1097/00019052-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kamon T., Horie S., Inaba T., Ito N., Shiraki K., Ichikawa Y., Ezaki M., Shimpo H., Shimaoka M., Nishigaki A., et al. The Detection of Hypercoagulability in Patients with Acute Cerebral Infarction Using a Clot Waveform Analysis. Clin. Appl. Thromb. 2023;29:10760296231161591. doi: 10.1177/10760296231161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandoval Y., Lewis B.R., Mehta R.A., Ola O., Knott J.D., De Michieli L., Akula A., Lobo R., Yang E.H., Gharacholou S.M., et al. Rapid Exclusion of Acute Myocardial Injury and Infarction With a Single High-Sensitivity Cardiac Troponin T in the Emergency Department: A Multicenter United States Evaluation. Circulation. 2022;145:1708–1719. doi: 10.1161/CIRCULATIONAHA.122.059235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson E.D., Schell J.C., Rodgers G.M. The D-dimer assay. Am. J. Hematol. 2019;94:833–839. doi: 10.1002/ajh.25482. [DOI] [PubMed] [Google Scholar]
- 24.Linkins L.A., Lapner T. Review of D-dimer testing: Good, bad, and ugly. Int. J. Lab. Hematol. 2017;39:98–103. doi: 10.1111/ijlh.12665. [DOI] [PubMed] [Google Scholar]
- 25.Adelborg K., Larsen J.B., Hvas A.M. Disseminated intravascular coagulation: Epidemiology, biomarkers, and management. Br. J. Haematol. 2021;192:803–818. doi: 10.1111/bjh.17172. [DOI] [PubMed] [Google Scholar]
- 26.Wang D., Zhao P., Lv Y., Ming J., Wang Z., Yang E., Li Y., Wang M., Niu J., Zhang Y., et al. Proteomic-Based Platelet Activation-Associated Protein SELP May Be a Novel Biomarker for Coagulation and Prognostic in Essential Thrombocythemia. J. Clin. Med. 2023;12:1078. doi: 10.3390/jcm12031078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matowicka-Karna J. Markers of inflammation, activation of blood platelets and coagulation disorders in inflammatory bowel dis eases. Postępy Hig. Med. Doświadczalnej. 2016;70:305–312. doi: 10.5604/17322693.1199305. [DOI] [PubMed] [Google Scholar]
- 28.Petito E., Franco L., Falcinelli E., Guglielmini G., Conti C., Vaudo G., Paliani U., Becattini C., Mencacci A., Tondi F., et al. COVIR-Study Investigators. COVIR-study investigators: COVID-19 infection-associated platelet and neutrophil activation is blunted by previous anti-SARS-CoV-2 vaccination. Br. J. Haematol. 2023;201:851–856. doi: 10.1111/bjh.18726. [DOI] [PubMed] [Google Scholar]
- 29.Wada H., Shiraki K., Matsumoto T., Ohishi K., Shimpo H., Shimaoka M. Effects of platelet and phospholipids on clot formation activated by a small amount of tissue factor. Thromb. Res. 2020;193:146–153. doi: 10.1016/j.thromres.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Wada H., Matsumoto T., Ohishi K., Shiraki K., Shimaoka M. Update on the Clot Waveform Analysis. Clin. Appl. Thromb. 2020;26:1076029620912027. doi: 10.1177/1076029620912027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada H., Shiraki K., Matsumoto T., Shimpo H., Shimaoka M. Clot waveform analysis for hemostatic abnormalities. Ann. Lab. Med. 2023;43:531–538. doi: 10.3343/alm.2023.43.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto T., Nogami K., Shima M. A combined approach using global coagulation assays quickly differentiates coagulation disorders with prolonged aPTT and low levels of FVIII activity. Int. J. Hematol. 2017;105:174–183. doi: 10.1007/s12185-016-2108-x. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T., Wada H., Fujimoto N., Toyoda J., Abe Y., Ohishi K., Yamashita Y., Ikejiri M., Hasegawa K., Suzuki K., et al. An Evaluation of the Activated Partial Thromboplastin Time Waveform. Clin. Appl. Thromb. 2018;24:764–770. doi: 10.1177/1076029617724230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi M., Sivapalaratnam S. Disseminated intravascular coagulation: An update on pathogenesis and diagnosis. Expert Rev. Hematol. 2018;11:663–672. doi: 10.1080/17474086.2018.1500173. [DOI] [PubMed] [Google Scholar]
- 35.Tokutake T., Baba H., Shimada Y., Takeda W., Sato K., Hiroshima Y., Kirihara T., Shimizu I., Nakazawa H., Kobayashi H., et al. Exogenous Magnesium Chloride Reduces the Activated Partial Thromboplastin Times of Lupus Anticoagulant-Positive Patients. PLoS ONE. 2016;11:e0157835. doi: 10.1371/journal.pone.0157835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sevenet P.O., Depasse F. Clot waveform analysis: Where do we stand in 2017? Int. J. Lab Hematol. 2017;39:561–568. doi: 10.1111/ijlh.12724. [DOI] [PubMed] [Google Scholar]
- 37.Nogami K. Clot Waveform Analysis for Monitoring Hemostasis. Semin. Thromb. Hemost. 2022;49:592–599. doi: 10.1055/s-0042-1756706. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa M., Tone S., Wada H., Naito Y., Matsumoto T., Yamashita Y., Shimaoka M., Sudo A. The Evaluation of Hemostatic Abnormalities Using a CWA-Small Amount Tissue Factor Induced FIX Activation Assay in Major Orthopedic Surgery Patients. Clin. Appl. Thromb. 2021;27:10760296211012094. doi: 10.1177/10760296211012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tripodi A. Thrombin Generation Assay and Its Application in the Clinical Laboratory. Clin. Chem. 2016;62:699–707. doi: 10.1373/clinchem.2015.248625. [DOI] [PubMed] [Google Scholar]
- 40.Konstantinidi A., Sokou R., Parastatidou S., Lampropoulou K., Katsaras G., Boutsikou T., Gounaris A.K., Tsantes A.E., Iacovidou N. Clinical Application of Thromboelastography/Thromboelastometry (TEG/TEM) in the Neonatal Population: A Narrative Review. Semin. Thromb. Hemost. 2019;45:449–457. doi: 10.1055/s-0039-1692210. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi M., Wada H., Fukui S., Mizutani H., Ichikawa Y., Shiraki K., Moritani I., Inoue H., Shimaoka M., Shimpo H. A Clot Waveform Analysis Showing a Hypercoagulable State in Patients with Malignant Neoplasms. J. Clin. Med. 2021;10:5352. doi: 10.3390/jcm10225352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dargaud Y., Delavenne X., Hart D., Meunier S., Mismetti P. Individualized PK-based prophylaxis in severe haemophilia. Haemophilia. 2018;24:3–17. doi: 10.1111/hae.13397. [DOI] [PubMed] [Google Scholar]
- 43.Hofer S., Ay C., Rejtö J., Wolberg A.S., Haslacher H., Koder S., Pabinger I., Gebhart J. Thrombin-generating potential, plasma clot formation, and clot lysis are impaired in patients with bleeding of unknown cause. J. Thromb. Haemost. 2019;17:1478–1488. doi: 10.1111/jth.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed G.W., Rossi J.E., Cannon C.P. Acute myocardial infarction. Lancet. 2017;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 45.Deguchi I., Takahashi S.J. Pathophysiology and Optimal Treatment of Intracranial Branch Atheromatous Disease. Atheroscler. Thromb. 2023;30:701–709. doi: 10.5551/jat.RV22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urabe T., Tanaka R., Noda K., Mizuno Y. Anticoagulant therapy with a selective thrombin inhibitor for acute cerebral infarction: Usefulness of coagulation markers for evaluation of efficacy. J. Thromb. Thrombolysis. 2002;13:155–160. doi: 10.1023/A:1020426906956. [DOI] [PubMed] [Google Scholar]
- 47.Lee S.H., Kim H.K., Ahn J.-H., Kang M.G., Kim K.-H., Bae J.S., Cho S.Y., Koh J.-S., Park Y., Hwang S.J., et al. Prognostic impact of hypercoagulability and impaired fibrinolysis in acute myocardial infarction. Eur. Heart J. 2023;44:1718–1728. doi: 10.1093/eurheartj/ehad088. [DOI] [PubMed] [Google Scholar]
- 48.Camaj A., Fuster V., Giustino G., Bienstock S.W., Sternheim D., Mehran R., Dangas G.D., Kini A., Sharma S.K., Halperin J., et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022;79:1010–1022. doi: 10.1016/j.jacc.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Siegerink B., Maino A., Algra A., Rosendaal F.R. Hypercoagulability and the risk of myocardial infarction and ischemic stroke in young women. J. Thromb. Haemost. 2015;13:1568–1575. doi: 10.1111/jth.13045. [DOI] [PubMed] [Google Scholar]
- 50.Haq S.A., Heitner J.F., Sacchi T.J., Brener S.J. Long-term Effect of Chronic Oral Anticoagulation with Warfarin after Acute Myocardial Infarction. Am. J. Med. 2010;123:250–258. doi: 10.1016/j.amjmed.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Sueda S., Sakaue T. Coronary artery spasm-induced acute myocardial infarction in patients with myocardial infarction with non-obstructive coronary arteries. Heart Vessel. 2021;36:1804–1810. doi: 10.1007/s00380-021-01878-z. [DOI] [PubMed] [Google Scholar]
- 52.Braun M., Kassop D. Acute Coronary Syndrome: Management. FP Essent. 2020;490:20–28. [PubMed] [Google Scholar]
- 53.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqi T.J., Usman M.S., Rashid A.M., Javaid S.S., Ahmed A., Clark D., 3rd, Flack J.M., Shimbo D., Choi E., Jones D.W., et al. Clinical Outcomes in Hypertensive Emergency: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2023;12:e029355. doi: 10.1161/JAHA.122.029355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zubielienė K., Valterytė G., Jonaitienė N., Žaliaduonytė D., Zabiela V. Familial Hypercholesterolemia and Its Current Diagnostics and Treatment Possibilities: A Literature Analysis. Medicina. 2022;58:1665. doi: 10.3390/medicina58111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 57.Shobayo F., Bajwa M., Koutroumpakis E., Hassan S.A., Palaskas N.L., Iliescu C., Abe J.-I., Mouhayar E., Karimzad K., Thompson K.A., et al. Acute coronary syndrome in patients with cancer. Expert Rev. Cardiovasc. Ther. 2022;20:275–290. doi: 10.1080/14779072.2022.2063840. [DOI] [PubMed] [Google Scholar]
- 58.Shao C., Wang J., Tian J., Tang Y.D. Coronary Artery Disease: From Mechanism to Clinical Practice. Adv. Exp. Med. Biol. 2020;1177:1–36. doi: 10.1007/978-981-15-2517-9_1. [DOI] [PubMed] [Google Scholar]
- 59.Grover S.P., Mackman N. Intrinsic Pathway of Coagulation and Thrombosis. Arter. Thromb. Vasc. Biol. 2019;39:331–338. doi: 10.1161/ATVBAHA.118.312130. [DOI] [PubMed] [Google Scholar]
- 60.Grover S.P., Mackman N. Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis. 2020;307:80–86. doi: 10.1016/j.atherosclerosis.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Weber C., Habenicht A.J.R., von Hundelshausen P. Novel mechanisms and therapeutic targets in atherosclerosis: Inflammation and beyond. Eur. Heart J. 2023;44:2672–2681. doi: 10.1093/eurheartj/ehad304. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki-Inoue K. Platelets and cancer-associated thrombosis: Focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134:1912–1918. doi: 10.1182/blood.2019001388. [DOI] [PubMed] [Google Scholar]
- 63.Monroe D.M., Hoffman M., Roberts H.R. Platelets and Thrombin Generation. Arter. Thromb. Vasc. Biol. 2002;22:1381–1389. doi: 10.1161/01.ATV.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 64.Matsumoto T., Wada H., Shiraki K., Suzuki K., Yamashita Y., Tawara I., Shimpo H., Shimaoka M. The Evaluation of Clot Waveform Analyses for Assessing Hypercoagulability in Patients Treated with Factor VIII Concentrate. J. Clin. Med. 2023;12:6320. doi: 10.3390/jcm12196320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.