Abstract
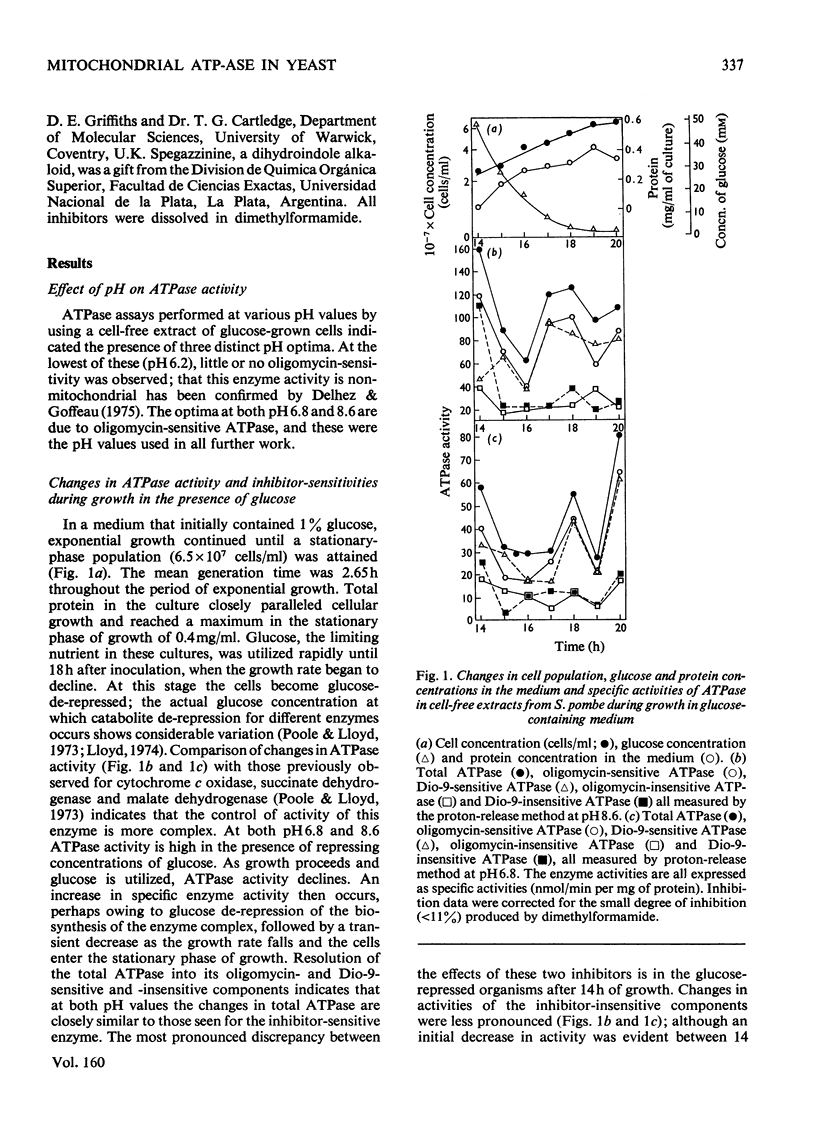
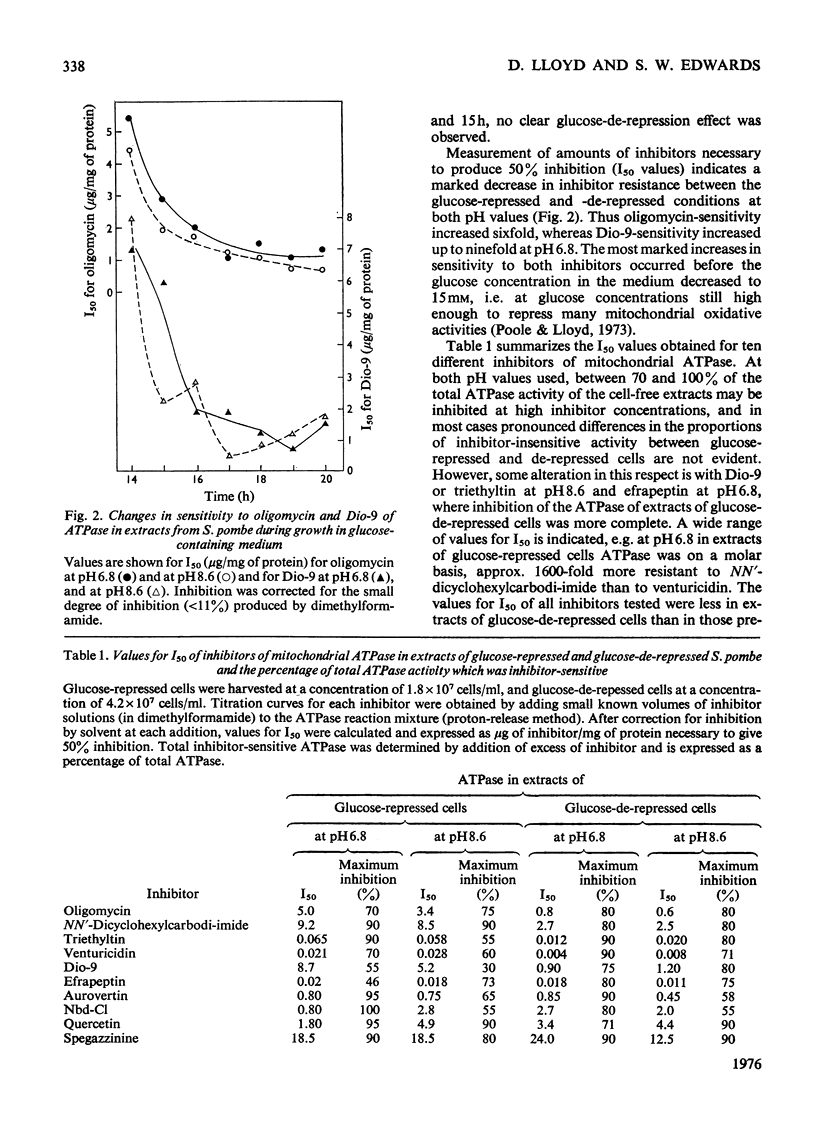
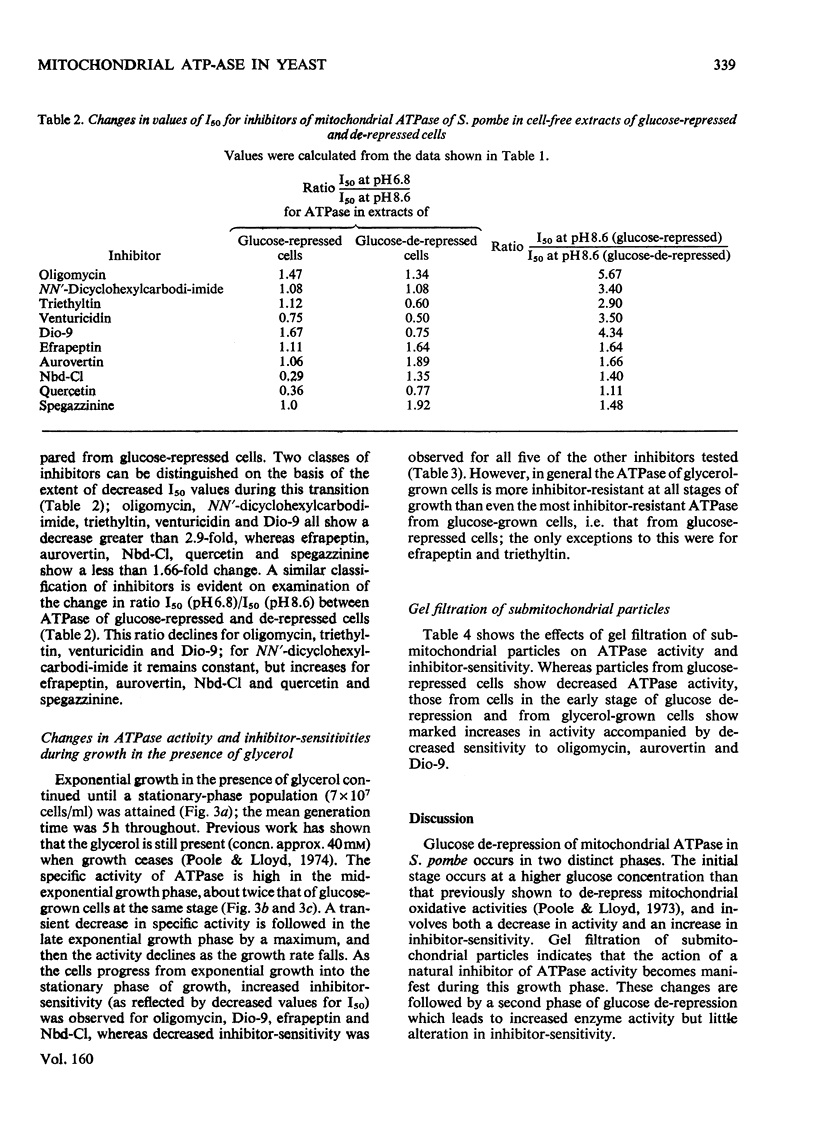
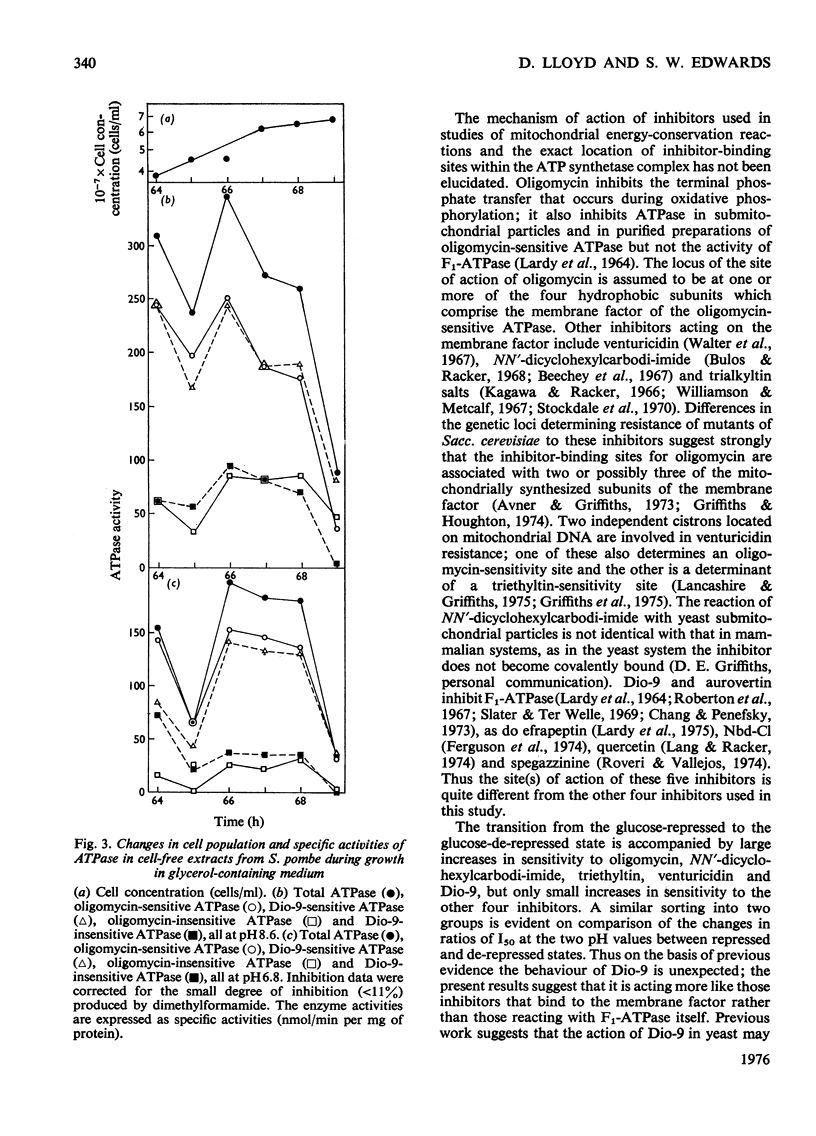
1. The specific activity of mitochondrial ATPase (adenosine triphosphatase) in extracts of Schizosaccharomyces pombe decreased 2.5-fold as the glucose concentration in the growth medium decreased from 50mM to 15mM. 2. During the late exponential phase of growth, ATPase activity doubled. 3. Sensitivity to oligomycin and Dio-9 as measured by values for I50(mug of inhibitor/mg of protein giving 50% inhibition) at pH 6.8 increased sixfold and ninefold respectively during the initial decrease in ATPase activity, and this degree of sensitivity was maintained for the remainder of the growth cycle. 4. Increased sensitivity to NN'-dicyclohexylcarbodi-imide, triethyltin and venturicidin was also observed during the early stage of glucose de-repression. 5. Smaller increases in sensitivity to efrapeptin, aurovertin, 7-chloro-4-nitrobenzo-2-oxa-1,3-diaz-le, quercetin and spegazzinine also occurred. 6. The ATPase of glycerol-grown cells was less sensitive to inhibitors than that of glucose-repressed cells; change in values for I50 were not so marked during the growth cycle of cells growing with glycerol. 7. When submitochondrial particles from glycerol-grown cells were tested by passage through Sephadex G-50, a fourfold increase in activity was accompanied by increased inhibitor resistance. 8. Gel filtration of submitochondrial particles from glucose-de-repressed cells gave similar results, whereas loss of ATPase occurred in submitochondrial particles from glucose-repressed cells. 9. It is proposed that alterations in sensitivity to inhibitors at different stages of glucose derepression may be partly controlled by a naturally occuring inhibitor of ATPase. 10. The inhibitors tested may be classififed into two groups on the basis of alterations of sensitivity of the ATPase during physiological modification: (a) oligomycin, Dio-9, NN'-dicyclohexylcarbodi-imide, venturicidin and triethyltin, and (b) efrapeptin, aurovertin, 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole, quercetin and spegazzinine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami K., Juniti K., Ernster L. Possible regulatory function of a mitochondrial ATPase inhibitor in respiratory chain-linked energy transfer. Biochim Biophys Acta. 1970;205(2):307–311. doi: 10.1016/0005-2728(70)90261-6. [DOI] [PubMed] [Google Scholar]
- Avner P. R., Griffiths D. E. Studies on energy-linked reactions. Isolation and characterisation of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1973 Jan 15;32(2):301–311. doi: 10.1111/j.1432-1033.1973.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Senior A. E. Studies on the mitochondrial oligomycin-insensitive ATPase. II. The relationship of the specific protein inhibitor to the ATPase. Arch Biochem Biophys. 1971 Dec;147(2):467–470. doi: 10.1016/0003-9861(71)90402-4. [DOI] [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XVII. Further resolution of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1968 Jul 25;243(14):3891–3900. [PubMed] [Google Scholar]
- Chang T., Penefsky H. S. Aurovertin, a fluorescent probe of conformational change in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1973 Apr 25;248(8):2746–2754. [PubMed] [Google Scholar]
- GUILLORY R. J. THE ACTION OF DIO-9: AN INHIBITOR AND AN UNCOUPLER OF OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1964 Aug 26;89:197–207. doi: 10.1016/0926-6569(64)90208-1. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Colson A. M., Landry Y., Foury F. Modifications of mitochondrial ATPase in chromosomal respiratory-deficient mutants of a "petite-negative" yeast: Schizosaccharomyces Pombe 972h. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1448–1454. doi: 10.1016/0006-291x(72)90876-5. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Landry Y., Foury F., Briquet M., Colson A. M. Oligomycin resistance of mitochondrial adenosine triphosphatase in a pleiotropic chromosomal mutant of a "petite-negative" yeast, Schizosaccharomyces pombe. J Biol Chem. 1973 Oct 25;248(20):7097–7105. [PubMed] [Google Scholar]
- Griffiths D. E., Houghton R. L., Lancashire W. E., Meadows P. A. Studies on energy-linked reactions: isolation and properties of mitochondrial venturicidin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1975 Feb 21;51(2):393–402. doi: 10.1111/j.1432-1033.1975.tb03939.x. [DOI] [PubMed] [Google Scholar]
- Griffiths D. E., Houghton R. L. Studies on energy-linked reactions: modified mitochondrial ATPase of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):157–167. doi: 10.1111/j.1432-1033.1974.tb03608.x. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lancashire W. E., Griffiths D. E. Studies on energy-linked reactions: isolation, characterisation and genetic analysis of trialkyl-tin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1975 Feb 21;51(2):377–392. doi: 10.1111/j.1432-1033.1975.tb03938.x. [DOI] [PubMed] [Google Scholar]
- Lardy H., Reed P., Lin C. H. Antibiotic inhibitors of mitochondrial ATP synthesis. Fed Proc. 1975 Jul;34(8):1707–1710. [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- Poole R. K., Lloyd D. Changes in respiratory activities during the cell-cycle of the fission yeast Schizosaccharomyces pompe 972h--growing in the presence of glycerol. Biochem J. 1974 Oct;144(1):141–148. doi: 10.1042/bj1440141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Lloyd D. Oscillations of enzyme activities during the cell-cycle of a glucose-repressed fission-yeast Schizosaccharomyces pombe 972h-. Biochem J. 1973 Sep;136(1):195–207. doi: 10.1042/bj1360195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Horstman L. L. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 13. Structure and function of submitochondrial particles completely resolved with respect to coupling factor. J Biol Chem. 1967 May 25;242(10):2547–2551. [PubMed] [Google Scholar]
- Roberton A. M., Beechey R. B., Holloway C. T., Knight I. G. The effect of aurovertin on a soluble mitochondrial adenosine triphosphatase. Biochem J. 1967 Sep;104(3):54C–55C. doi: 10.1042/bj1040054c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre M., de Jerphanion M. B., Huet J., Vignais P. V. ATPase inhibitor from yeast mitochondria. Purification and properties. Biochim Biophys Acta. 1975 May 15;387(2):241–255. doi: 10.1016/0005-2728(75)90107-3. [DOI] [PubMed] [Google Scholar]
- Schatz G., Penefsky H. S., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XIV. J Biol Chem. 1967 May 25;242(10):2552–2560. [PubMed] [Google Scholar]
- Stockdale M., Dawson A. P., Selwyn M. J. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970 Aug;15(2):342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. II. Synthesis of the mitochondrial adenosine triphosphatase. F1. J Biol Chem. 1969 Sep 25;244(18):5027–5033. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Walter P., Lardy H. A., Johnson D. Antibiotics as tools for metabolic studies. X. Inhibition of phosphoryl transfer reactions in mitochondria by peliomycin, ossamycin, and venturicidin. J Biol Chem. 1967 Nov 10;242(21):5014–5018. [PubMed] [Google Scholar]
- Watson K., Houghton R. L., Bertoli E., Griffiths D. E. Membrane-lipid unsaturation and mitochondrial function in Saacharomyces cerevisiae. Biochem J. 1975 Feb;146(2):409–416. doi: 10.1042/bj1460409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Stadt R. J., de Boer B. L., van Dam K. The interaction between the mitochondrial ATPase (F 1 ) and the ATPase inhibitor. Biochim Biophys Acta. 1973 Feb 22;292(2):338–349. doi: 10.1016/0005-2728(73)90040-6. [DOI] [PubMed] [Google Scholar]


