Abstract
No meta-analysis has analysed role of cagrilintide as weight-loss medication in obese individuals. Electronic databases were searched for RCTs involving obese individuals receiving cagrilintide or cagrilintide-2.4 mg with semaglutide-2.4 mg combination (Cagrisema) compared to placebo/active comparator. Primary outcomes were changes in body weight; secondary outcomes were alterations in glycemia, lipids, and adverse events. From 678 articles, data from 3 RCTs involving 430 individuals were analysed. At 20–32 weeks, patients receiving Cagrisema weekly had significantly greater percentage [mean difference (MD)−9.07% (95%CI: −11.91, −6.23); P < 0.00001;I2 = 96%] and absolute [MD-9.11 kg (95%CI: −12.84, −5.39); P < 0.00001; I2 = 98%] weight-loss, compared to semaglutide 2.4 mg weekly. At 26–32 weeks, cagrilintide 2.4 mg had a similar percentage [MD − 1.83% (95%CI: −4.08, −0.42); P = 0.11; I2 = 98%] and absolute [MD − 1.88 kg (95%CI: −4.23,0.47); P = 0.12; I2 = 98%] weight-loss, compared to semaglutide/liraglutide. Treatment-emergent and serious adverse events were comparable between groups. Gastrointestinal adverse events and vomiting were significantly higher with Cagrisema compared to semaglutide. Vomiting was significantly lower with cagrilintide compared to semaglutide/liraglutide. Cagrisema outperforms semaglutide regarding weight loss. Cagrilintide shows comparable weight loss to semaglutide/liraglutide with significantly lower vomiting.
Keywords: Cagrilintide, cagrisema, meta-analysis, obesity, weight loss
INTRODUCTION
Obesity, a global pandemic, is linked to increased risks of metabolic syndrome, cardiovascular events, and mortality.[1] Weight loss in obese individuals is associated with decreased risks of type 2 diabetes (T2D), fatty liver disease, hypertension, cardiovascular disease, sleep apnoea, and osteoarthritis. The extent of risk reduction is directly tied to the degree of weight loss.[2] The psychological aspect of obesity, influenced by disrupted gut-brain communications, emphasizes the intricate and multifaceted nature of this health condition.[3]
Amylin, a pancreatic β-cell peptide incretin hormone co-secreted with insulin, induces satiety through mechanisms such as delayed gastric emptying and actions on specific brain regions.[4] Targeting amylin for weight loss has led to the development of pramlintide, a synthetic analogue primarily used for glycaemic control in diabetes. Notably, pramlintide achieves over 10% weight loss in 40–43% of patients, emphasizing its dual benefits in managing diabetes and promoting weight reduction.[5] The newer amylin analogue AM833 (cagrilintide) is a novel long-acting acylated amylin analogue that acts as a non-selective amylin receptor (AMYR) agonist.[6] Cagrilintide has a structure similar to pramlintide, except for the differences in lipidation of the N-terminal lysine and substitutions of three amino acids (N14E, V17R, and P37Y). These differences result in cagrilintide acting as a non-selective AMYR and calcitonin G protein-coupled receptor (CTR) agonist, which can dually activate both classes of receptors. This explains the greater weight loss noted in animal studies compared to pramlintide.[6] Additionally, cagrilintide is a once weekly subcutaneous (s.c.) injection instead of the twice/thrice daily s.c. injections required for pramlintide.[5,6]
Several randomized controlled trials (RCTs) have been published evaluating the weight loss potential of cagrilintide alone and cagrilintide in combination with semaglutide (cagrisema) in different doses.[7,8,9] However, no meta-analysis has analysed this novel amylin analogue’s clinical efficacy, tolerability, safety, and positioning as an anti-obesity medicine among all the newer agents available for clinical use. Hence, this meta-analysis aimed to evaluate the efficacy and safety of cagrilintide and cagrisema as anti-obesity medications.
METHODS
The recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist were strictly followed while carrying out this meta-analysis.[10] The predefined protocol has been registered in PROSPERO, with Registration number CRD42023460291. All RCTs published till August 2023 were considered. Since ethical approval already exists for individual studies, no separate approval was required for this meta-analysis. PICOS (Patient, Intervention, Control, Outcome and Study type) criteria were used to screen and select studies. Only RCTs that evaluated cagrilintide alone or cagrilintide with semaglutide fixed-dose combination (cagrisema) in the treatment arm were considered for this meta-analysis. The studies needed to have at least two treatment arms/groups, with one of the groups on either cagrilintide alone or cagrilintide/semaglutide combination (cagrisema) and the other group receiving placebo or any other active comparator medicine. Single-arm studies and uncontrolled studies were excluded. Also, studies with patients with prior exposure to cagrilintide/cagrisema were excluded.
The primary outcome was changes in body weight. Secondary outcomes were alterations in fasting plasma glucose (FPG) and glycated haemoglobin (HbA1c), percentage of patients achieving weight loss >5%, 10%, 15%, HbA1c reduction to <6.5% and <7%, waist-circumference, hypoglycaemia, lipid-parameters, and adverse events. Analyses of primary and secondary outcomes were done based on the comparator medicine the control group received: an active comparator medicine– marked as an active-control group (ACG) or a placebo – marked as a passive-control Group (PCG).
We systematically searched PubMed (Medline) with keywords or MESH terms: (cagrilintide) OR (AM833). We then searched Embase, Cochrane database, CNKI database, clinicaltrials.gov, ctri.nic.in, and Google Scholar to ensure we had not missed any relevant articles. Methodologic details have been elaborated in previous meta-analyses published by our group.[11,12] The risk of bias assessment was done by three authors using the risk of bias assessment tool in Review Manager (RevMan) Version 5.4 software. The different types of bias looked for have been elaborated in previous metanalyses by our group.[11,12] A random effect model was used for analysis. Forest plots generated for all the different outcomes were used to assess heterogeneity. We specifically used the Chi2 test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and with the I2 test.[13] The details of heterogeneity analysis have been elaborated in previously published meta-analyses.[11,12] The grading/certainty of the evidence of the major outcome was done using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach, with procedural details elaborated in a previous publication by us.[12,14] Publication bias was assessed by plotting Funnel Plots.[14,15] The key outcomes table was generated using the GRADE software (https://gdt.gradepro.org/app/).
RESULTS
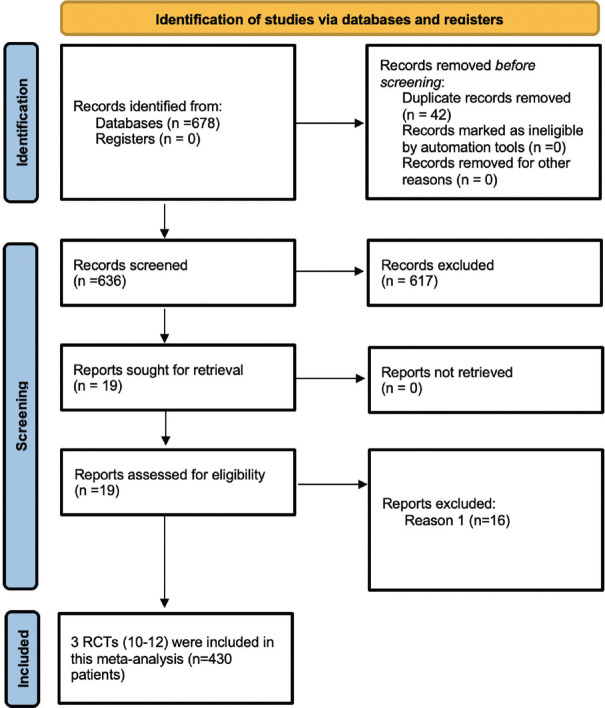
The initial search revealed 678 articles [Figure 1]. Following the screening of titles and abstracts, the search was down to 19 articles, which were evaluated in detail [Figure 1]. Data from three RCTs involving 430 obese individuals fulfilling all criteria were analysed.[7,8,9] The baseline characteristics of the included study population are elaborated in Table 1. In the RCT by Lau et al.,[7] cagrilintide was evaluated at doses ranging from 0.3-4.5 mg once weekly subcutaneous (s.c.) injections as compared to liraglutide (3 mg/day s.c.) (n = 99) and placebo (n = 101), over 26 weeks treatment period. However, we used cagrilintide 2.4 mg/week dose (n = 102) for analysis in our meta-analysis as it was the most commonly used dose across different studies. In the RCT by Enebo et al.,[8] cagrilintide was evaluated at doses ranging from 0.3-4.5 mg/week injection in combination with a dose of semaglutide 2.4 mg/week injection (cagrisema), which was compared to semaglutide 2.4 mg/week injection (n = 24), over 20 weeks treatment period. However, we used cagrilintide (2.4 mg/week) with semaglutide (2.4 mg/week) combination (cagrisema 2.4/2.4 mg) (n = 12) for analysis as it was the most commonly used dose across different studies. In the RCT by Frias et al.,[9] cagrilintide 2.4 mg/week (cagrilintide 2.4 mg) (n = 30), cagrilintide (2.4 mg/week) with semaglutide (2.4 mg/week) combination (cagrisema 2.4/2.4 mg) (n = 31), and semaglutide 2.4 mg/week (semaglutide 2.4 mg) (n = 31) were evaluated against each other in individuals with T2D and obesity (diabesity) over 32 weeks.
Figure 1.
Flowchart elaborating on study retrieval and inclusion in the meta-analysis
Table 1.
Characteristics of patients with key outcomes of the randomized controlled trials analysed in this meta-analysis
| Parameter | Lau et al.[7] | Enebo et al.[8] | Frias et al.[9] | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Cagrilintide- 2.4 mg (n=102) | Liraglutide- 3.0 mg (n=99) | Placebo (n=101) | Cagrisema- 2.4 mg/ 2.4 mg (n=12) | Semaglutide- 2.4 mg with placebo (n=24) | Cagrilintde- 2.4 mg (n=30) | Cagrisema- 2.4 mg/ 2.4 mg (n=31) | Semaglutide- 2.4 mg (n=31) | |
| Age (years) | 52·7 (9·8) | 51·5 (9·3) | 51·4 (11·9) | 43.0 (8.1) | 41.0 (8.8) | 62 (7) | 56 (10) | 57 (10) |
| Male | 45 (45%) | 27 (26%) | 42 (42%) | 5 (42%) | 16 (67%) | 23 (77%) | 18 (58%) | 18 (58%) |
| Weight (kg) | 106·8 (24·1) | 107·8 (24·1) | 106·2 (21·6) | 92.1 (11.9) | 99.6 (15.6) | 107·4 (25·0) | 104·3 (23·2) | 105·4 (24·9) |
| BMI (kg/m2) | 37·9 (7·6) | 38·4 (7·4) | 37·4 (5·7) | 32.2 (2.5) | 32.2 (3.0) | 34·4 (6·1) | 35·9 (5·7) | 36·2 (7·2) |
| HBA1c (%) | 5·6% (0·4) | 5·6% (0·4) | 5·6% (0·4) | 5.2 (0.4) | 5.4 (0.4) | 8·1 (0·8) | 8·5 (0·8) | 8·6 (0·7) |
| Duration of T2D (years) | N/A | N/A | N/A | N/A | N/A | 10·7 (9·1) | 6·4 (3·8) | 9·2 (8·3) |
| eGFR (mL/min/1·73 m2) | N/a | N/a | N/a | N/a | N/a | 92 (13) | 94 (12) | 90 (18) |
| Metformin | N/A | N/A | N/A | N/A | N/A | 21 (70%) | 23 (74%) | 23 (74%) |
| Metformin+SGLT2i | N/A | N/A | N/A | N/A | N/A | 9 (30%) | 8 (26%) | 8 (26%) |
| Percent reduction in Weight (%) | −9.7 (0.6) | −9.0 (0.6) | −3.0 (0.6) | −17.1 (1.5) | −9.5 (1.0) | −15.6 (1.26) | −8.1 (1.23) | −5.1 (1.26) |
| Absolute Weight reduction (kg) | −9.5 (0.6) | −8.4 (0.6) | −2.8 (0.6) | −15.9 (1.4) | −8.7 (1.0) | −16.3 | 8.4 | −5.3 |
| HbA1c Reduction | −0.1 (0.3) | −0.3 (0.2) | −0.1 (0.2) | −0.3 (0.2) | −0.2 (0.2) | −0.9 (0.15) | −2.2 (0.15) | −-1.8 (0.16) |
| TIR by CGM (%) | N/a | N/a | N/a | N/A | N/A | 88.9% | 71.7% | 76.2% |
| SBP reduction (mm Hg) | −8.0 (1.3) | −4.3 (1.3) | −3.6 (1.3) | N/a | N/a | −3 | −13 | 1 |
| GI side effects | 52 (51%) | 59 (60%) | 32 (32%) | 11 (92%) | 19 (79%) | 10 (33%) | 18 (58%) | 10 (32%) |
| Anti-cagrilintide antibodies at baseline | 1 (1%) | N/a | N/a | 1 (8.3%) | N/a | N/a | N/a | N/a |
N/A: not applicable; N/a: not available; BMI: body mass index; SGLT2i: sodium glucose contrasnporter-2 inhibitor; TIR: time in range; CGM: continuous glucose monitoring; GI: gastrointestinal; SBG: systolic blood pressure; T2D: type 2 diabetes; eGFR: estimated glomerular filteration rate; HbA1c: glycated haemoglobin
In the RCT by Enebo et al.,[8] cagrilintide was initiated at 0.16 mg/week and escalated incrementally every four weeks by 0.56 mg to reach the final dose of 2.4 mg/week in 16 weeks. In the RCT by Lau et al.,[7] cagrilintide was initiated at 0.6 mg/week. The dose was doubled every two weeks to reach 2.4 mg/week by four weeks of therapy (viz. started with 0.6 mg/week at Week-0, increased to 1.2 mg/week after two weeks of therapy, further increased to 2.4 mg/week after another 2 weeks of therapy). In the RCT by Frias et al.,[9] cagrilintide was initiated at 0.25 mg/week, was doubled to 0.5 mg/week after four weeks of therapy, and then again doubled to 1 mg/week after eight weeks of therapy, after that increased by 0.7 mg every four weekly to reach the full dose of 2.4 mg/week at 16 weeks of therapy.
Risk of bias in the included studies
Random sequence generation, allocation concealment bias, performance bias, detection bias, and reporting bias were found to be at low risk in all three studies. Attrition bias was found to be low in two out of three studies [Enebo (2021) et al.[8] and Frias (2023) et al.[9]]. Attrition bias was found to be high in the study by Lau (2021) et al.[7] Sources of funding, especially funding from pharmaceutical organizations and conflicts of interest, were looked into as “other bias.” All three studies had high “other bias” risk.
Effect of cagrisema 2.4/2.4 mg and cagrilintide 2.4 mg on primary outcomes
Percentage reduction in weight
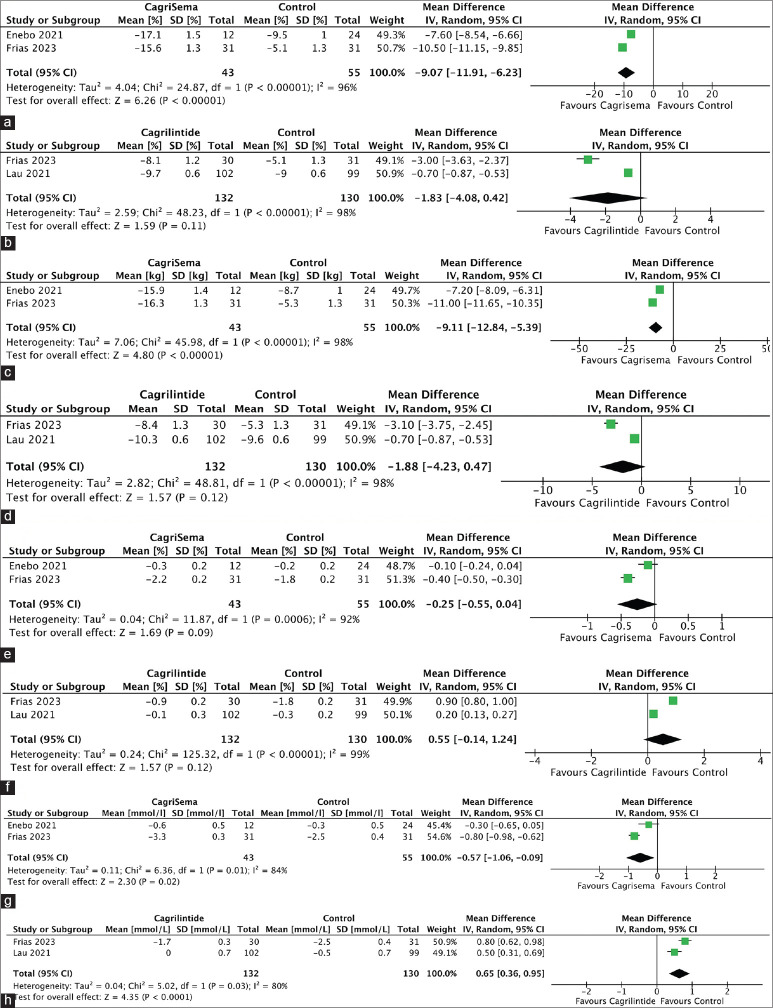
Data from 2 studies involving 98 individuals was analysed to find the impact of cagrisema 2.4/2.4 mg on the percentage reduction in body weight compared to ACG. At 20-32 weeks, cagrisema 2.4/2.4 mg had a significantly greater percentage reduction in body weight compared to semaglutide 2.4 mg [mean difference (MD) −9.07% (95% CI: −11.91, −6.23); P < 0.00001; I2 = 96% (high heterogeneity (HH)); Figure 2a]. Data from 2 studies involving 262 individuals was analysed to find the impact of cagrilintide 2.4 mg on the percentage reduction in body weight compared to ACG. At 26–32 weeks, cagrilintide 2.4 mg had a similar percentage reduction in body weight compared to liraglutide/semaglutide [MD − 1.83% (95% CI: −4.08, 0.42); P = 0.11; I2 = 98% (HH); Figure 2b]. Only one study analysed the impact of cagrilintide on the percentage reduction in body weight compared to PCG. At 26 weeks, cagrilintide 2.4 mg had a significantly greater percentage reduction in body weight as compared to placebo [MD − 6.70% (95% CI: −6.87, −6.53); P < 0.00001].
Figure 2.
Forest plot highlighting the impact of (a): Cagrisema 2.4 mg/2.4 mg on percent reduction in body weight as compared to semaglutide 2.4 mg; (b): Cagrilintide 2.4 mg on percent reduction in body weight as compared to semaglutide 2.4 mg/liraglutide 3 mg; (c): Cagrisema 2.4 mg/2.4 mg on absolute reduction in body weight (kg) as compared to semaglutide 2.4 mg; (d): Cagrilintide 2.4 mg on absolute reduction in body weight as compared to semaglutide 2.4 mg/liraglutide 3 mg; (e): Cagrisema 2.4 mg/2.4 mg on HbA1c as compared to semaglutide 2.4 mg; (f): Cagrilintide 2.4 mg on HbA1c as compared to semaglutide 2.4 mg/liraglutide 3 mg; (g): Cagrisema 2.4 mg/2.4 mg on fasting plasma glucose as compared to semaglutide 2.4 mg; (h): Cagrilintide 2.4 mg on fasting plasma glucose as compared to semaglutide 2.4 mg/liraglutide 3 mg
Absolute reduction in weight
Data from 2 studies involving 98 individuals was analysed to find the impact of cagrisema 2.4/2.4 mg on reduction in body weight compared to ACG. At 20-32 weeks, cagrisema 2.4/2.4 mg had a significantly greater reduction in body weight compared to semaglutide 2.4 mg [MD − 9.11 kg (95%CI: −12.84, −5.39); P < 0.00001; I2 = 98% (HH); Figure 2c]. Data from 2 studies involving 262 individuals was analysed to find the impact of cagrilintide 2.4 mg on reduction in body weight compared to ACG. At 26–32 weeks, cagrilintide 2.4 mg had a similar decrease in body weight compared to liraglutide/semaglutide [MD -1.88 kg (95% CI: −4.23, 0.47); P = 0.12; I2 = 98% (HH); Figure 2d]. Only one study analysed the impact of cagrilintide on body weight compared to PCG. At 26 weeks, cagrilintide 2.4 mg had a significantly greater reduction in body weight as compared to placebo [MD − 7.00 kg (95% CI: −7.17, −6.83); P < 0.00001].
Effect of cagrisema 2.4/2.4 and cagrilintide 2.4 on secondary outcomes
HbA1c
At 20–32 weeks, cagrisema 2.4/2.4 mg had a similar change in HbA1c as compared to semaglutide 2.4 mg [MD − 0.25% (95% CI: −0.55, 0.04); P = 0.09; I2 = 92% (HH); Figure 2e]. At 26–32 weeks, cagrilintide 2.4 mg had similar change in HbA1c as compared to liraglutide/semaglutide [MD 0.55% (95% CI: −0.14, 1.24); P = 0.12; I2 = 99% (HH); Figure 2f]. At 26 weeks, cagrilintide 2.4 mg had a similar change in HbA1c as compared to placebo [MD 0% (95% CI: −0.07, 0.07); P = 1.00].
Fasting plasma glucose
At 20–32 weeks, cagrisema 2.4/2.4 mg had a greater reduction in fasting plasma glucose (FPG) as compared to semaglutide 2.4 mg [MD -0.57 mmol/L (95% CI: −1.06, −0.09); P = 0.02; I2 = 84% (HH); Figure 2g]. At 26–32 weeks, cagrilintide 2.4 mg had a greater reduction in FPG as compared to liraglutide/semaglutide [MD 0.65 mmol/L (95% CI: 0.36, 0.95); P < 0.0001; I2 = 80% (HH); Figure 2h]. At 26 weeks, cagrilintide 2.4 mg had a similar change in FPG compared to placebo [MD 0 mmol/L (95% CI: −0.18, 0.18)].
Lipid parameters
At 20–32 weeks, cagrisema 2.4/2.4 mg had similar changes in total cholesterol [MD 0.33 mmol/L (95% CI: −0.03, 0.69); P = 0.07; I2 = 0% (Low heterogeneity (LH))], high density lipoprotein cholesterol (HDL-C) [MD 0.10 mmol/L (95% CI: −0.09, 0.29); P = 0.29; I2 = 0% (LH)], low density lipoprotein cholesterol (LDL-C) [MD 0.20 mmol/L (95% CI: −0.12, 0.52); P = 0.23; I2 = 0% (LH)], very low density lipoprotein cholesterol (VLDL-C) [MD − 0.08 mmol/L (95% CI: −0.22, 0.06); P = 0.27; I2 = 0% (LH)] and triglycerides [MD 1.71 mmol/L (95% CI: −1.20, 4.62); P = 0.25] as compared to semaglutide 2.4 mg. At 26–32 weeks, cagrilintide 2.4 mg had similar changes in total cholesterol [MD 0.17 mmol/L (95% CI: −0.02, 0.36); P = 0.07; I2 = 0% (LH)], HDL-C [MD 0 mmol/L (95% CI: −0.04, 0.04); P = 0.97; I2 = 0% (LH)], LDL-C [MD 0.10 mmol/L (95% CI: −0.06, 0.27); P = 0.23; I2 = 0% (LH)], VLDL-C [MD 6.38 mmol/L (95% CI: −6.60, 19.35); P = 0.34; I2 = 99% (HH)] and triglycerides [MD 0.07 mmol/L (95% CI: −0.07, 0.21); P = 0.34] as compared to liraglutide/semaglutide. At 26 weeks, cagrilintide 2.4 mg had similar changes in total cholesterol [MD − 0.07 mmol/L (95% CI: −0.25, 0.11); P = 0.44], HDL-C [MD 0 mmol/L (95% CI: −0.04, 0.04); P = 1.00], LDL-C [MD 0 mmol/L (95% CI: −0.17, 0.17); P = 1.00] as compared to placebo. However, VLDL-C [MD − 0.12 mmol/L (95% CI: −0.21, -0.03); P = 0.01] and triglycerides [MD − 0.31 mmol/L (95% CI: −0.56, -0.06); P = 0.01] was significantly lower in the cagrilintide group as compared to placebo.
Safety
Data from 2 studies [Enebo (2021) et al.[8] and Frias (2023) et al.[9]] was analysed to compare the adverse event profile of patients receiving cagrisema 2.4/2.4 mg as compared to semaglutide 2.4 mg [Table 2]. After 20–32 weeks, treatment-emergent adverse events (TAEs), serious adverse events (SAEs), injection site reactions, nervous system disorders, nausea and diarrhoea were similar between the cagrisema 2.4/2.4 mg and semaglutide 2.4 mg groups [Table 2]. The occurrence of gastrointestinal adverse events as a whole and vomiting was significantly higher in the cagrisema 2.4/2.4 mg group as compared to the semaglutide group [Table 2]. In the study by Frias et al.,[9] hypoglycemia was similar between the cagrisema 2.4/2.4 mg and semaglutide 2.4 mg groups [OR 5.34 (95% CI: 0.25, 115.89); P = 0.29].
Table 2.
The results of safety outcomes of Cagrisema 2.4/2.4mg and cagrilintide 2.4mg vs. control in the meta-analysis
| Safety variables | Cagrisema 2.4/2.4mg vs. semaglutide 2.4mg groups | Cagrilintide 2.4mg vs. semaglutide 2.4mg groups | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No. of RCTs (Participants) | Pooled effect size, OR [95% CI] | I2 (%) | P | No. of RCTs (Participants) | Pooled effect size, RR [95% CI] | I2 (%) | P | |
| TAEs | 2 (98) | 0.91 (95% CI: 0.33, 2.55) | 0% | 0.86 | 2 (262) | 0.97 (95% CI: 0.54, 1.75) | 0% | 0.92 |
| SAEs | 2 (98) | 0.33 (95% CI: 0.04, 3.11) | 0% | 0.33 | 2 (262) | 0.95 (95% CI: 0.47, 1.94) | 7% | 0.89 |
| Injection site reactions | 2 (98) | 3.51 (95% CI: 0.85, 14.46) | 9% | 0.08 | 2 (262) | 1.90 (95% CI: 1.00, 3.62) | 0% | 0.05 |
| Nervous system disorders | 2 (98) | 0.33 (95% CI: 0.10, 1.10) | 0% | 0.07 | 2 (262) | 1.07 (95% CI: 0.45, 2.55) | 0% | 0.88 |
| Nausea | 2 (98) | 3.34 (95% CI: 0.49 22.74) | 71% | 0.22 | 2 (262) | 0.72 (95% CI: 0.42, 1.23) | 0% | 0.22 |
| Diarrhoea | 2 (98) | 0.97 (95% CI: 0.12, 7.74) | 66% | 0.97 | 2 (262) | 0.97 (95% CI: 0.49, 1.92) | 0% | 0.93 |
| GAEs | 2 (98) | 2.91 (95% CI: 1.13, 7.46) | 0% | 0.03 | 2 (262) | 0.67 (95% CI: 0.41, 1.09) | 0% | 0.10 |
| Vomiting | 2 (98) | 9.57 (95% CI: 1.54, 59.30) | 37% | 0.02 | 2 (262) | 0.38 (95% CI: 0.17, 0.86) | 0% | 0.02 |
TEAE=Treatment-emergent adverse events; SAE=Serious Adverse events; GAEs: gastrointestinal adverse events RCT=Randomized controlled trials; RR=Risk ratio; CI=Confidence interval; I2: heterogeneity; P<0.05 considered statistically significant and highlighted in bold
Data from 2 studies [Frias (2023) et al.[9] and Lau (2021) et al.[7]] was analysed to compare the adverse event profile of patients receiving cagrisema 2.4/2.4 mg as compared to semaglutide 2.4 mg [Table 2]. After 26-32 weeks, TAEs, SAEs, injection site reactions, nervous system disorders, gastrointestinal disorders, nausea and diarrhoea were similar between the cagrilintide 2.4 mg and the semaglutide/liraglutide groups [Table 2]. The occurrence of vomiting was significantly lower in the cagrilintide 2.4 mg group as compared to the semaglutide/liraglutide group [Table 2]. In the study by Frias et al.,[9] the occurrence of hypoglycaemia was similar between the cagrilintide 2.4 mg and semaglutide 2.4 mg groups [OR 5.53 (95% CI: 0.25, 120.05); P = 0.28].
At 26 weeks, the occurrence of TAEs [OR 1.74 (95% CI: 0.94, 3.24); P = 0.08], SAEs [OR 0.99 (95% CI: 0.20, 5.02); P = 0.99], cardiovascular disorders [OR 1.63 (95% CI: 0.52, 5.18); P = 0.40], psychiatric disorders [OR 0.99 (95% CI: 0.36 2.75); P = 0.98] and neoplasms [OR 0.99 (95% CI: 0.06, 16.05); P = 0.99] was similar between the cagrilintide 2.4 mg and the placebo groups. However, the occurrence of injection site reactions [OR 28.04 (95% CI: 1.64, 1.75); P = 0.92], gastrointestinal disorders [OR 0.97 (95% CI: 0.54, 1.75); P = 0.92], nausea [OR 0.97 (95% CI: 0.54, 480.32); P = 0.02], vomiting [OR 3.16 (95% CI: 0.83, 12.04); P = 0.09] and diarrhoea [OR 2.19 (95% CI: 0.93, 5.14); P = 0.92] was significantly higher in the cagrilintide group as compared to placebo.
Lau et al.[7] had a faster titration regimen (up-titration to cagrilintide 2.4 mg by four weeks of therapy) compared to the study by Frias et al.[9] (up-titration to cagrilintide 2.4 mg by 16 weeks of treatment). We compared the gastrointestinal side effects among the two studies. The occurrence of gastrointestinal side effects was not significantly different among both the studies (rapid vs. slow up-titration of cagrilintide) [OR 2.08 (95% CI: 0.89, 4.88); P = 0.09].
Funnel plots assessing the publication bias for key outcomes of this meta-analysis were plotted and have been elaborated in Supplementary Figure 1 (128.6KB, tif) . Due to the presence of one or more studies outside the funnel plot, publication bias was considered to be high for percent reduction in body weight with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg; absolute reduction in body weight with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg; percent reduction in body weight with cagrilintide 2.4 mg as compared to semaglutide/liraglutide; and absolute reduction in body weight with cagrilintide 2.4 mg as compared to semaglutide/liraglutide. The summary of findings of the key outcomes of this systematic review with the grading of the outcomes has been elaborated in Table 3.
Table 3.
Summary of findings of the key outcomes of this systematic review and meta-analysis
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|
|
| |||||
| Risk with Control (Semaglutide-2.4mg) | Risk with Cagrisema (2.4mg/2.4mg) | ||||
| Body Weight percent reduction | The mean body weight percent reduction was -7.3% | MD 9.07 lower (11.91 lower to 6.23 lower) | - | 98 (2 RCTs) | ⊕⊕◯◯ Lowa,b |
| Body weight Reduction | The mean body weight reduction was -7 kg | MD 9.11 lower (12.84 lower to 5.39 lower) | - | 98 (2 RCTs) | ⊕⊕◯◯ Lowa,b |
| Treatment-emergent Adverse Events | 818 per 1,000 | 804 per 1,000 (598 to 920) | OR 0.91 (0.33 to 2.55) | 98 (2 RCTs) | ⊕⊕⊕⊕ High |
| Severe adverse events | 55 per 1,000 | 19 per 1,000 (2 to 152) | OR 0.33 (0.04 to 3.11) | 98 (2 RCTs) | ⊕⊕⊕⊕ High |
| Gastrointestinal adverse events | 527 per 1,000 | 764 per 1,000 (558 to 893) | OR 2.91 (1.13 to 7.46) | 98 (2 RCTs) | ⊕⊕⊕⊕ High |
|
| |||||
| Anticipated absolute effects* (95% CI) | |||||
|
| |||||
| Risk with control (Semaglutide/liraglutide) | Risk with cagrilintide 2.4 mg | ||||
|
| |||||
| Body weight percent reduction | The mean body weight Percent Reduction was -7.05% | MD 1.83 lower (4.08 lower to 0.42 higher) | - | 262 (2 RCTs) | ⊕⊕◯◯ Lowa,b |
| Body weight | The mean body weight reduction was -7.45 kg | MD 1.88 lower (4.23 lower to 0.47 higher) | - | 262 (2 RCTs) | ⊕⊕◯◯ Lowa,b |
| Treatment-emergent Adverse Events | 785 per 1,000 | 779 per 1,000 (663 to 864) | OR 0.97 (0.54 to 1.75) | 262 (2 RCTs) | ⊕⊕⊕⊕ High |
| Severe adverse events | 631 per 1,000 | 619 per 1,000 (445 to 768) | OR 0.95 (0.47 to 1.94) | 262 (2 RCTs) | ⊕⊕⊕⊕ High |
| Gastrointestinal adverse events ACG | 615 per 1,000 | 517 per 1,000 (396 to 636) | OR 0.67 (0.41 to 1.09) | 262 (2 RCTs) | ⊕⊕⊕⊕ High |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); CI: confidence interval; MD: mean difference; OR: odds ratio; Explanations: a: I2 is >75% suggestive of considerable heterogeneity in data; b. A funnel plot is suggestive of the presence of most of the studies outside the plot, hence it is likely that significant publication bias is present
DISCUSSION
Obesity has severe health consequences, with each 5 kg/m2 increase in BMI beyond the normal range linked to a 30% increase in mortality and a potential reduction in life expectancy by up to 10 years.[16] Conversely, significant weight loss is associated with metabolic and non-metabolic benefits. A 5 kg weight loss can reduce the need for hypertension and diabetes medications, while a 5–10 kg loss improves conditions like steatotic liver disease, dyslipidaemia, and polycystic ovary syndrome. Further weight loss (10–15 kg) extends benefits to conditions like sleep apnoea, acid reflux, and osteoarthritis while exceeding 15 kg is strongly linked to type 2 diabetes remission and improved cardiovascular function.[17]
Approved anti-obesity medications, like orlistat, liraglutide, naltrexone-bupropion, and phentermine-topiramate, offer modest weight loss (3–5 kg).[17] Liraglutide’s 3 mg/day injection leads to an 8% reduction after one year.[18] Semaglutide achieves up to 16% weight loss with a weekly 2.4 mg subcutaneous injection over a year.[19,20,21] Tirzepatide, a dual GIP and GLP1RA, exhibits an 11.9% to 12.4% weight reduction at 10 mg and 15 mg doses per week over 6–18 months.[22,23] In this context, our meta-analysis highlights the impressive weight loss achieved with cagrilintide-based therapies, particularly with cagrisema − 2.4/2.4 mg, which was noted to be superior to semaglutide 2.4 mg in terms of both percentage and absolute weight loss over a 6-month clinical use period, albeit at the expense of increased gastrointestinal side effects. Cagrisema-2.4/2.4 mg was associated with an additional 9% weight loss over established weight loss medications like semaglutide injections 2.4 mg/weekly, which has traditionally been associated with up to 16% weight loss as compared to placebo (STEP trials; vide supra). This indirectly translates into more than 20% weight loss with cagrisema 2.4/2.4 mg, better than even tirzepatide. When used as a standalone treatment, cagrilintide 2.4 mg/week resulted in weight loss comparable to that achieved with weekly semaglutide 2.4 mg or daily liraglutide 3 mg. Additionally, it offered the added advantage of significantly reduced vomiting. Data on common gastrointestinal side effects of different incretin-based therapies compared to placebo, pooled from other published systematic reviews and meta-analyses, highlights the lowest OR for nausea, vomiting, and diarrhoea with cagrilintide [Table 4].[22,24] Therefore, cagrilintide can be considered a promising therapeutic option for weight loss in obese individuals who may not tolerate GLP1RAs or have a lower tolerance for nausea and vomiting. Also, cagrilintide-based therapies, tirzepatide, and semaglutide can be an alternative to bariatric surgery in people with diabesity. This is because restrictive procedures like sleeve gastrectomy have weight loss potential similar to that noted with cagrilintide-based therapies, tirzepatide and semaglutide.
Table 4.
Data on common gastrointestinal side effects of different incretin-based therapies as compared to placebo, pooled from different published systematic reviews and meta-analyses
| Number of Trials | Nausea OR (95%CI) | Vomiting OR (95%CI) | Diarrhoea OR (95%CI) | |
|---|---|---|---|---|
| Cagrilintide vs Placebo * | This meta-analysis | 0.97 (0.54–480.32) | 3.16 (0.83–12.04) | 2.19 (0.93–5.14) |
| Semaglutide vs Placebo (25) | 17 (n=6756) | 2.82 (2.56–3.81) | 3.79 (2.77–5.19) | 1.90 (1.65–2.19) |
| Tirzepatide vs Placebo (7) | 3 (n=594) | 3.02 (1.51–6.05) | 3.63 (1.13–11.67) | 3.17 (1.64–6.15) |
*Data comparing Cagrisema to placebo is currently not available, hence not presented here; OR: Odds ratio; CI: confidence interval
Cagrilintide 2.4 mg, alone or as a part of cagrisema, has a half-life of 184 ± 7.4 hours.[8] Cagrilintide is slowly absorbed after administration, with a median time to achieve Cmax of around 24 hours.[8] Semaglutide, in comparison, has a corresponding half-life of 158 ± 10.9 hours and a median time to reach Cmax of 18 hours. These values are almost similar to those of cagrilintide at the same doses, making the cagrisema combination easy to initiate and titrate doses without the fear of a differential response between the two components. It is reassuring to note that the use of cagrilintide and cagrisema was not associated with an increased risk of hypoglycaemia in individuals with obesity without diabetes. Both cagrilintide and cagrisema had a similar impact on lipid parameters compared to semaglutide and liraglutide. Cagrilintide use was associated with significant reductions in triglycerides and VLDL-C compared to a placebo. The increased occurrence of injection site reactions with cagrilintide when compared to placebo requires further evaluation.
The only new agent on the horizon that can match or even surpass the initial performance of cagrisema is retatrutide, a triple hormone receptor agonist. Retatrutide is a single peptide with agonist activity at the glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide (GLP)-1, and glucagon receptors. Initial phase 2 RCTs have documented a 16.81% and 16.94% weight loss with retatrutide at doses of 8 mg/week and 12 mg/week, respectively, escalated over 24 weeks of therapy.[25] Another RCT documented a 22.8% and 24.2% weight loss with retatrutide at 8 mg/week doses and 12 mg/week over 48 weeks of therapy.[26] Again, gastrointestinal adverse events were the most common issues noted, which were found to be dose-dependent and primarily seen at higher doses used for weight loss. This impressive weight reduction with the various long-acting gut peptide-based therapies is largely believed to result from a reduced food/calorie intake, which results from decreased appetite and earlier and increased satiety, coupled with controlled and mindful eating. A lot of research is currently happening in the field of long-acting amylin analogues, to develop novel weight loss medications with reduced gastrointestinal side effects. As of now, apart from cagrilintide, all other agents are at the stage of phase-1 clinical trials. These include oral amycretin (Novo Nordisk), ZP8396 (Zealand Pharma), AZD6234 (Astra Zeneca), and an unnamed long-acting amylin agonist by Eli Lily.[27]
Limitations of this meta-analysis include the relatively short duration of follow-up, with data available only from 3 RCTs with a limited number of patients for analysis. Hence, there remains an urgent need for bigger multi-centric RCTs evaluating the durability of weight loss with cagrisema and cagrilintide over many years of clinical use. Also, the mean BMI of patients assessed in the 3 RCTs ranged from 32.2-37.9 kg/m2. Hence, there remains a need for evaluation of cagrilintide-based therapies in extreme obesity (BMI > 40 mg/m2), who are more likely to receive this treatment in the real-world scenario, and also in people with obesity having lower BMI in the range of 27–32 kg/m2 which would be relevant from south Asian point of view.
CONCLUSIONS
This meta-analysis provides exciting data on the impressive weight loss observed with cagrisema 2.4/2.4 mg. Cagrisema 2.4/2.4 mg appears superior to semaglutide 2.4 mg in weight loss over a six-month therapy period. Cagrilintide 2.4 mg demonstrates similar efficacy to semaglutide 2.4 mg and liraglutide 3 mg, with a reduced incidence of vomiting. Cagrilintide-based therapies offer the advantage of substantial weight loss with fewer gastrointestinal side effects, likely a major factor in determining long-term compliance and sustained weight loss. However, the need for more long-term efficacy, tolerability, and safety data regarding cagrilintide-based therapies, especially Cagrisema, for weight loss remains.
Approval of the research protocol
The meta-analysis was registered in PROSPERO, with registration number CRD42023460291. The review protocol summary can be accessed at the PROSPERO website.
Approval date of Registry and the Registration No. of the study/trial
CRD42023460291, Date: 14/09/2023.
Authors contributions
The meta-analysis was conceptualized by DD and LN. The literature search was done by HBG, MS, AJ, HB, and ABMK. Detailed reviews of articles were done by HBG, MS, AJ, and HB. Data entry was done by DD, LN, and ABMK. Statistical analysis was done by DD, LN, and ABMK. All authors contributed equally to the manuscript preparation and approval for submission. The manuscript has been read and approved by all the authors for submission to this journal for consideration for publication.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Funnel plot assessing the publication bias for key outcomes of this meta-analysis (a) Percent reduction in body weight with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (b) Absolute reduction in body weight with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (c) Treatment emergent adverse events (TAEs) with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (d) Serious adverse events (SAEs) with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (e) Gastrointestinal adverse events with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (f) Percent reduction in body weight with cagrilintide 2.4 mg as compared to semaglutide/liraglutide, (g) Absolute reduction in body weight with cagrilintide 2.4 mg as compared to semaglutide/liraglutide, (h) Treatment emergent adverse events (TAEs) with cagrilintide 2.4 mg as compared to semaglutide/liraglutide
Acknowledgements
None
REFERENCES
- 1.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 2.Dutta D, Jaisani R, Khandelwal D, Ghosh S, Malhotra R, Kalra S. Role of metformin, Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors, Glucagon-Like Peptide-1 (GLP-1) receptor agonists, and orlistat based multidrug therapy in glycemic control, weight loss, and euglycemia in diabesity: A real-world experience. Indian J Endocr Metab. 2019;23:460–7. doi: 10.4103/ijem.IJEM_185_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10:575–84. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- 4.Boyle CN, Lutz TA, Le Foll C. Amylin—its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol Metab. 2018;8:203–10. doi: 10.1016/j.molmet.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SR, Aronne LJ, Burns CM, Kesty NC, Halseth AE, Weyer C. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care. 2008;31:1816–23. doi: 10.2337/dc08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehestani B, Stratford NR, le Roux CW. Amylin as a future obesity treatment. J Obes Metab Syndr. 2021;30:320–5. doi: 10.7570/jomes21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau DCW, Erichsen L, Francisco AM, Satylganova A, le Roux CW, McGowan B, et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: A multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet. 2021;398:2160–72. doi: 10.1016/S0140-6736(21)01751-7. [DOI] [PubMed] [Google Scholar]
- 8.Enebo LB, Berthelsen KK, Kankam M, Lund MT, Rubino DM, Satylganova A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet. 2021;397:1736–48. doi: 10.1016/S0140-6736(21)00845-X. [DOI] [PubMed] [Google Scholar]
- 9.Frias JP, Deenadayalan S, Erichsen L, Knop FK, Lingvay I, Macura S, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: A multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402:720–30. doi: 10.1016/S0140-6736(23)01163-7. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing the risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutta D, Bhattacharya S, Surana V, Aggarwal S, Singla R, Khandelwal D, et al. Efficacy and safety of saroglitazar in managing hypertriglyceridemia in type-2 diabetes: A meta-analysis. Diabetes Metab Syndr. 2020;14:1759–68. doi: 10.1016/j.dsx.2020.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Dutta D, Agarwal A, Maisnam I, Singla R, Khandelwal D, Sharma M. Efficacy and safety of the novel dipeptidyl peptidase-4 inhibitor gemigliptin in the management of type 2 diabetes: A meta-analysis. Endocrinol Metab (Seoul) 2021;36:374–87. doi: 10.3803/EnM.2020.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions:explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating the quality of evidence and strength of recommendations. BMJ (Clinical research ed) 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000;4:1–115. [PubMed] [Google Scholar]
- 16.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colin IM, Gérard KM. Once-weekly 2.4 mg semaglutide for weight management in obesity: A game changer?touch. REV Endocrinol. 2022;18:35–42. doi: 10.17925/EE.2022.18.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 19.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 randomized clinical trial. JAMA. 2021;325:1414–25. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two-year effect of semaglutide 2.4 mg vs placebo in adults with overweight or obesity: STEP 5. Obesity. 2021;29:43. Oral abstract#96. [Google Scholar]
- 21.Wharton S, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two-year effect of semaglutide 2.4 mg on control of eating in adults with overweight/obesity: STEP 5. Obesity (Silver Spring) 2023;31:703–15. doi: 10.1002/oby.23673. [DOI] [PubMed] [Google Scholar]
- 22.Dutta D, Surana V, Singla R, Aggarwal S, Sharma M. Efficacy and safety of novel twincretin tirzepatide a dual GIP and GLP-1 receptor agonist in the management of type-2 diabetes: A Cochrane meta-analysis. Indian J Endocrinol Metab. 2021;25:475–89. doi: 10.4103/ijem.ijem_423_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Mesquita YLL, Pera Calvi I, Reis Marques I, Almeida Cruz S, Padrao EMH, Carvalho PEP, et al. Efficacy and safety of the dual GIP and GLP-1 receptor agonist tirzepatide for weight loss: A meta-analysis of randomized controlled trials. Int J Obes (Lond) 2023;47:883–92. doi: 10.1038/s41366-023-01337-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Hou QC, Li BH, Deng L, Yang YM, Li TX, et al. Efficacy and safety of subcutaneous semaglutide in adults with overweight or obese: A subgroup meta-analysis of randomized controlled trials. Front Endocrinol. 2023;14:1132004. doi: 10.3389/fendo.2023.1132004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402:529–44. doi: 10.1016/S0140-6736(23)01053-X. [DOI] [PubMed] [Google Scholar]
- 26.Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity-A phase 2 trial. N Engl J Med. 2023;389:514–26. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 27.Melson E, Ashraf U, Papamargaritis D, Davies MJ. What is the pipeline for future medications for obesity? Int J Obes (Lond) 2024 doi: 10.1038/s41366-024-01473-y. doi:10.1038/s41366-024-01473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plot assessing the publication bias for key outcomes of this meta-analysis (a) Percent reduction in body weight with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (b) Absolute reduction in body weight with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (c) Treatment emergent adverse events (TAEs) with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (d) Serious adverse events (SAEs) with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (e) Gastrointestinal adverse events with cagrisema-2.4 mg/2.4 mg as compared to semaglutide 2.4 mg, (f) Percent reduction in body weight with cagrilintide 2.4 mg as compared to semaglutide/liraglutide, (g) Absolute reduction in body weight with cagrilintide 2.4 mg as compared to semaglutide/liraglutide, (h) Treatment emergent adverse events (TAEs) with cagrilintide 2.4 mg as compared to semaglutide/liraglutide