Abstract
Natalizumab is a highly effective therapy for multiple sclerosis (MS). The aim of this study was to evaluate serum neurofilament light chain (sNfL) and serum glial fibrillary acidic protein (sGFAP) in patients with relapsing–remitting MS treated with Natalizumab. sNfL and sGFAP were analyzed at baseline, 6 and 12 months post treatment using the single-molecule array (SiMoA) technique. We recruited matched healthy controls for comparison. The study included 54 patients, with a median age of 33 years (Interquartile range (IQR), 29–41), with 32 women (60%) and 76 healthy controls. A decrease in sNfL was observed at 6 (67%, p = 0.005) and 12 (72%, p < 0.0001) months compared to baseline. After two years, six patients experienced evidence of disease activity (EDA-3). The remaining ones had no evidence of disease activity (NEDA-3). NEDA-3 presented a remarkable reduction in sNfL (p < 0.0001) and sGFAP (p = 0.01) after 6 months of treatment that continued to be observed after 12 months compared to baseline. EDA-3 only reached a significant decrease in sNfL after 12 months; there were no significant changes in sGFAP values. Natalizumab leads to a decrease in sNfL, which is higher and occurs earlier in NEDA-3 patients. Patients also showed a significant reduction in sGFAP levels, which was not observed in the EDA-3 group.
Keywords: sNfL, sGFAP, SiMoA, multiple sclerosis, natalizumab
1. Introduction
Serum biomarkers play an important role in multiple sclerosis (MS), even more so since the development of new immune assays [1,2]. Neurofilaments are cytoskeletal proteins released into the cerebrospinal fluid and blood. Their quantification leads to the measurement of neuronal injury [3]. Serum neurofilament light chain (sNfL) has been validated in MS as a biomarker of disease activity. In addition, its elevation has been linked to disease progression [4,5,6,7].
Serum glial fibrillary acidic protein (sGFAP) is an intermediate filament present in the astrocytes [8]. Increases in sGFAP values have been related to future disability worsening, especially progression independent of relapse activity [9,10,11,12]. The application of these two biomarkers together seems to increase the ability to detect patients at risk of disease impairment [9,12,13].
Natalizumab is a monoclonal antibody used as a highly effective therapy for relapsing–remitting multiple sclerosis (RRMS). It is an IgG4 antibody that targets the α4 integrin, preventing harmful lymphocytes from entering the central nervous system. This is achieved by inhibiting the interaction between α4β1 integrin and vascular cell adhesion molecule-1 present in endothelial cells [14].
The way Natalizumab can make changes over time in sNfL and sGFAP values is not well known nowadays and evidence in the literature is scarce [15].
We aimed to evaluate both biomarkers in RRMS patients over their first year of treatment with Natalizumab. We compared their values with a cohort of matched healthy controls (HCs). In addition, we divided the patients into two groups according to the achievement of no evidence of disease activity status (NEDA-3) after two years of follow-up, to study differences in the evolution of sNfL and sGFAP values since the start of treatment.
2. Results
We incorporated 54 patients (32 women (60%)) into the study, all of whom initiated Natalizumab at Hospital Universitario Ramón y Cajal (Madrid, Sapin), a referral MS center. Clinical and demographic data of patients and HCs are shown in Table 1.
Table 1.
Baseline data.
| RRMS (n = 54) |
HCs (n = 76) |
p Value | |
|---|---|---|---|
| Age (years) | 33 (29–41) | 31 (26–46) | n.s. |
| Female/Male | 32/22 | 47/29 | n.s. |
| Body mass index | 25.3 (21.6–28.1) | 22.5 (20.5–24.7) | n.s. |
| Time from disease onset (years) | 3.1 (0.8–9.8) | ||
| EDSS score | 2 (1.5–2.5) | ||
| ARR 1 year before | 1 (1–2) | ||
| Previous treatment | |||
| None | 26 (48.1%) | ||
| Platform | 27 (50%) | ||
| Orals | 1 (1.8%) | ||
| Monoclonal antibody | 0 | ||
| T2 lesions (<10, 10–50, >50) | 7(13%), 39(72.2%), 8 (14.8%) | ||
| Number of gadolinium-enhancing lesions | 1 (0–4) | ||
| Patients with gadolinium-enhancing lesions | 40 (74.1%) | ||
| Oligoclonal IgG bands | 52/53 (98.1%) | ||
| Oligoclonal IgM bands against lipids | 43/53 (81.1%) | ||
| sNfL (picograms/mL) | 15 (10.2–39) | 6.11 (2–8.5) | p < 0.0001 |
| sGFAP (picograms/mL) | 203.5(138.1–268.4) | 91 (72.6–109) | p < 0.0001 |
Abbreviations: n, number of patients and controls; RRMS, relapsing–remitting multiple sclerosis; HCs, healthy controls; EDSS, Expanded Disability Status Scale; ARR, annualized relapse rate, n.s. non-significant. Platform treatments: interferon b and glatiramer acetate; oral drugs: dimethylfumarate, fingolimod, teriflunomide. Continuous variables are shown as median (IQR) and categorical variables as numbers (%).
The median age (Interquartile range, IQR) of Natalizumab-treated patients was 33 (29–41) years. The annualized relapse rate (ARR) the year before treatment was 1 (1–2). The time from disease onset was 3.1 years (0.8–9.8). Twenty-six patients (48.1%) had not previously received disease-modifying treatment, and twenty-eight patients (51.9%) had received other treatment and needed a change due to lack of efficacy. Eight patients (14.8%) had at least 50 T2 lesions on baseline MRI; forty patients (74.1%) had gadolinium-enhancing lesions.
The data showed that 76% of patients had high (≥10 picograms/mL) sNfL values at treatment onset. Patients with high sNfL at treatment onset had more T2 lesions in MRI performed at baseline (p = 0.007). sNfL and sGFAP values were higher in RRMS patients at baseline compared to HCs (p ≤ 0.001 for both biomarkers).
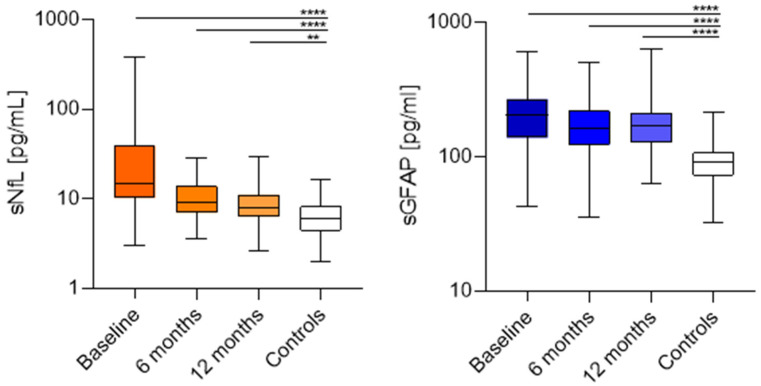
A significant reduction in sNfL values was observed at 6 (67%, p = 0.005) and 12 (72%, p < 0.0001) months compared to baseline. However, the decrease between 6 and 12 months was not significant. Compared to HCs, differences persisted at 6 (p > 0.0001) and 12 (p = 0.006) months. We did not find significant changes in sGFAP values in the whole cohort (Figure 1).
Figure 1.
sNfL (picograms/mL) of RRMS patients and healthy controls at baseline, 6 and 12 months of Natalizumab; sGFAP (picograms/mL) of RRMS patients and healthy controls at baseline, 6 and 12 months of Natalizumab. ** (p = 0.006), **** (p < 0.0001).
Patients who switched from other DMTs had higher sNfL values (34.4 (14.2–73.4) vs. 12.5 (8.9–19.7), p = 0.0003) compared with naïve patients. These differences persisted at 6 months (12.9 (7–28.2) vs. 8.1 (6.7–10.9), p = 0.04), and disappeared at one year of follow-up (8.2 (6.4–14.3) vs. 8 (6.3–10), p = 0.4). However, we did not find differences in sGFAP values between these groups.
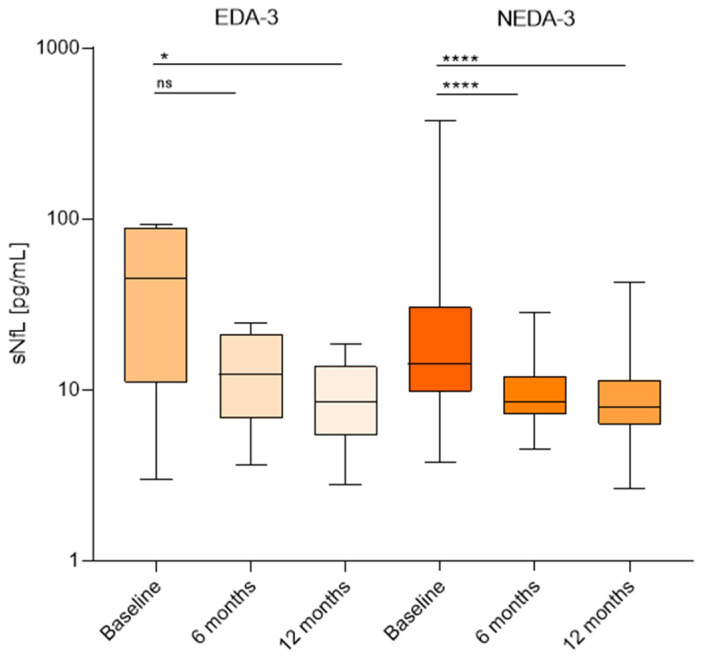
Patients were followed for two years. During this time, six patients experienced EDA-3. In four cases this was due to radiological activity, and in the other two to a clinical relapse. The remaining patients achieved NEDA-3. No significant differences were found in the baseline sNfL values of EDA-3 and NEDA-3 patients. These were 44.7 (11–89.2) picograms/mL and 12.4 (6.7–30.5) picograms/mL, respectively. NEDA-3 patients exhibited a reduction in sNfL after 6 months to 8.5 (7.2–12) picograms/mL, p < 0.0001, and after 12 months to 8 (6.2–11.4) picograms/mL, p < 0.0001, compared to baseline. By contrast, in EDA-3 patients the median sNfL value at six months of treatment was 15 (8.8–22.4) picograms/mL with no significant differences with baseline levels. Only after 12 months of treatment did we find a clear decrease in this group to 8.6 (5.4–13.7) picograms/mL, p = 0.01 (Figure 2).
Figure 2.
Comparison between sNfL (picograms/mL) values at baseline and after 6 and 12 months in EDA-3 and NEDA-3 patients after 2 years of Natalizumab. * (p = 0.01) **** (p = 0.0001) ns (non significant).
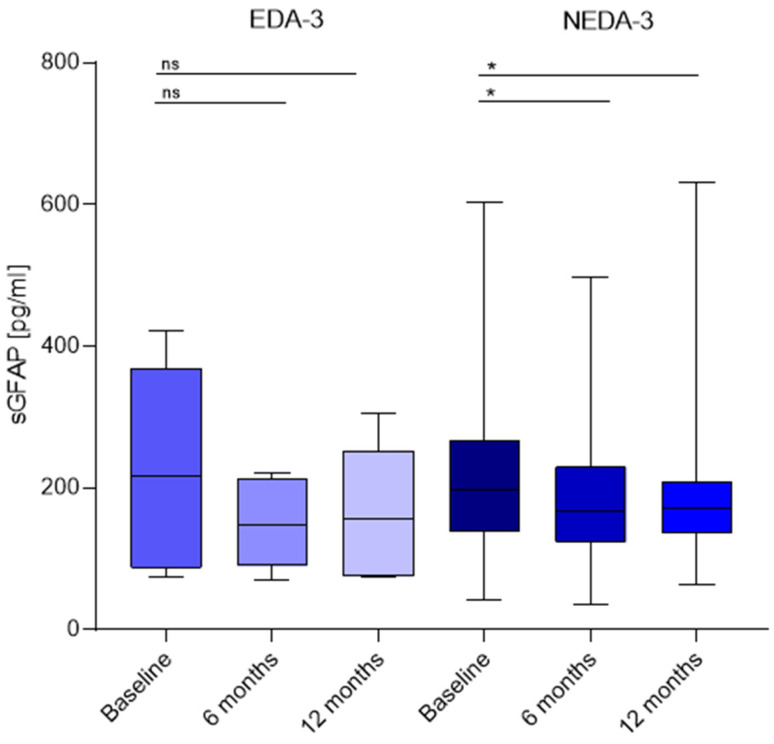
We did not find differences in baseline sGFAP between EDA-3 and NEDA-3 patients. The values were 216.9 (86.1–369) picograms/mL and 197.7 (138.7–266.9) picograms/mL, respectively. EDA-3 patients did not experience significant changes during the first year of treatment. Nevertheless, sGFAP values decreased in NEDA-3 patients after 6 months of treatment to 165.9 (123.7–229.5) picograms/mL, p = 0.01, and remained low after 12 months (171.9 (136.3–208.9) picograms/mL, p =0.02) compared to baseline (Figure 3).
Figure 3.
Comparison between sGFAP (picograms/mL) values at baseline and after 6 and 12 months in EDA-3 and NEDA-3 patients after 2 years of Natalizumab. * (p = 0.01, p = 0.02), ns (non-significant).
3. Discussion
Acute axonal damage generated by highly inflammatory diseases can manifest as clinical (relapses) or radiological (increases in T2/gadolinium-enhancing lesions) activity. This is associated with high sNfL levels in RRMS patients [7]. According to this, sNFL levels are used as a biomarker for monitoring inflammation [7] and sGFAP seems to be linked to disease progression not associated with acute inflammation [9]. The combination of both biomarkers, sNfL, and sGFAP, may help to ameliorate the ability to detect patients at risk of worsening [10,13].
Early initiation of high-efficacy disease-modifying treatments in RRMS patients with high sNfL values at disease onset has been linked with a reduction in inflammatory activity and disease progression [7,13]. Nevertheless, there are only a small number of studies focused on variations in sNfL over time in RRMS patients who start a disease-modifying treatment. Furthermore, even fewer studies have analyzed both biomarkers (sNfL and sGFAP) together.
We aimed to analyze the role of sNfL and sGFAP in a group of highly active RRMS patients who started treatment with Natalizumab. First, we explored sNfL and sGFAP during the first year and compared the results to a cohort of matched HCs. We observed that sNfL decreased progressively, as described in other cohorts [16,17,18], but did not reach similar values to those of the HCs after a year of treatment. Natalizumab was administered every 4 weeks since, at that time, there was still no evidence of the 6-week dosing [19].
In another cohort [20], sNfL values were measured at 3 months and then after a year of treatment; consequently, they described nadir at one year of treatment. However, we observed that the decrease in sNfL values occurs mainly during the first six months, mostly in patients reaching NEDA-3 after follow-up. This decrease was maintained after one year of treatment. By contrast, the decrease was only observed after a year in EDA-3 patients, showing that an early reduction in sNfL levels was associated with an optimal response. This is in line with previous findings describing an association with sNfL ratio at 12 months and the risk of new MRI activity after two years of Natalizumab treatment [21].
Different data have been published about sNfL levels and their correlation with clinical and radiological activity in patients treated with Natalizumab. Thus, an increase in sNfL levels was linked to the recurrence of disease activity, defining these high levels as an early biomarker to predict the presence of disease before clinical or radiological signs appear [22]. From another perspective, a different research study [23] found no association between the wearing-off symptoms and sNfL and sGFAP levels in patients treated with Natalizumab, thus suggesting that it may not be associated with new disease activity, at least in all cases. However, research has focused more on the role of neurofilaments as predictors of the development of progressive multifocal leukoencephalopathy in patients treated with Natalizumab [24,25,26,27]. In our cohort, we observed that the reduction in sNfL values after six months of treatment could serve as a predictor of disease activity at two years, as only patients who reached NEDA-3 status after two years experienced a significant reduction in sNfL levels at this point.
Less evidence is available for sGFAP in Natalizumab-treated patients [15]. The possible effect of this drug on activated astroglia is not well known [14]. Our data showed no differences in this variable after 6 and 12 months of treatment in the entire cohort, but we found significant decreases at these points in the NEDA-3 group. These data strongly suggest that a reduction in sGFAP values in conjunction with that of sNfL levels can identify patients who will reach a NEDA-3 status in the long term during Natalizumab treatment.
The main limitation of our study was the sample size. These findings should be validated in larger, multicenter cohorts followed for a more prolonged period of time.
In conclusion, our data show that an early decrease in sNfL and sGFAP values could identify patients at low risk of disease activity during Natalizumab treatment.
4. Materials and Methods
4.1. Study Design
This was an observational study with prospective data collection, following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Patients were recruited at the Hospital Universitario Ramón y Cajal in Madrid, Spain. We enrolled patients with RRMS who started Natalizumab treatment between March 2011 and August 2016. These patients were followed for two years. Treatment-naïve and previously treated patients were included. Patients received 300 mg of Natalizumab intravenously every 4 weeks. Age-, sex- and body mass index-matched HCs were recruited between August 2023 and February 2024.
4.2. Patient Consent
All patients and HCs signed an informed consent prior to participation. Anonymized data, which support the findings of this study, will be available to any qualified investigator upon request for 3 years following the publication of the study.
4.3. Data Collection
Clinical, radiological, and demographic variables were collected at onset. Experienced neurologists in the field conducted all Expanded Disability Status Scale (EDSS) evaluations every 3 months. Additional examinations were conducted in case of a relapse. A baseline MRI was performed within a month before treatment onset following established clinical protocols. Control MRI studies were performed annually.
4.4. Sample Collection
Patient blood specimens were collected just before initiating Natalizumab treatment and again at 6 and 12 months after that. Serum sample aliquots were stored at −80° until they were processed.
4.5. Serum sNfL and sGFAP Quantification
sNfL and sGFAP were quantified using an HD-X instrument (Quanterix, Lexington, MA, USA) with the single-molecule array (SIMoA) technique (Quanterix, Billerica, MA, USA). We employed a Neurology 2-Plex B Kit (Quanterix, Billerica, MA, USA), following the manufacturer’s instructions. The mean inter- and intra-assay coefficients equaled 5.6% and 4.4% for sNfL and 5.8% and 5% for sGFAP, respectively. The research team handling the evaluation of the serum samples remained unaware of the clinical data.
4.6. Definitions
We used the 2017 McDonald criteria for diagnosis [28]. Disability was assessed with the EDSS score [29]. Confirmed disability worsening was defined as a rise of at least 1.5 points in the EDSS if the baseline score was 0, an increase of at least 1 point if the previous EDSS was between 1 and 5, and a minimum 0.5 point increase for patients with a baseline EDSS of 5.5 or higher [30]. NEDA-3 was defined as the absence of relapses, disability worsening and new and/or enlarged T2 lesions or gadolinium-enhancing lesions on MRI. Patients experiencing a relapse, MRI activity, or an exacerbation of neurological disability were classified as having evidence of disease activity-3 (EDA-3) [31].
The cut-off applied for sNfL and sGFAP levels was established at the 90th percentile value of the corresponding HC, which was 10 picograms/mL for sNfL and 140 picograms/mL for sGFAP, in line with the benchmarks used in previous studies [5,7,31,32,33].
4.7. Statistical Analyses
Descriptive analyses were summarized using absolute and relative proportions for categorical variables, and differences were examined using χ² or Fisher’s exact test. The median with an Interquartile range (IQR) was employed to describe continuous variables, and associations between groups were evaluated using the Friedman and Mann–Whitney U tests. We performed statistical analyses using the GraphPad Prism 9.0 software (GraphPad Prism Inc., San Diego, CA, USA). All tests were two-tailed, and a significance level of p < 0.05 was deemed significant.
Acknowledgments
We would like to acknowledge Ana María Pérez Macias for their labor in collecting blood samples and daily care of patients with MS as a nurse specialist and to Sonia Ortega Sánchez for her technical assistance with sample analyses.
Author Contributions
Conceptualization, R.S.-A., A.R.-R. and L.M.V.; methodology, R.S.-A., A.R.-R. and L.M.V.; formal analysis, R.S.-A., A.R.-R. and L.M.V.; investigation R.S.-A., A.R.-R. and L.M.V.; writing—original draft preparation: R.S.-A., A.R.-R. and L.M.V.; writing—review and editing R.S.-A., A.R.-R., L.M.V., E.M., J.L.C.-G., F.R.-J., J.I.F.-V., N.V., J.L.V.-G., S.S.d.l.M., J.M. and L.C.-F.; visualization, L.M.V.; supervision, L.M.V.; funding acquisition L.M.V. and N.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Ramón y Cajal University Hospital in Madrid (protocol code 044/23).
Informed Consent Statement
Written informed consent was obtained from the subjects involved in the study to publish this paper.
Data Availability Statement
The raw data can be obtained from the corresponding author upon reasonable request in the three years following the manuscript’s publication.
Conflicts of Interest
RS reports receiving research travel support from Roche and Janssen outside the submitted work. AR reports receiving research travel support from Roche. EM reports receiving research grants, travel support or honoraria for speaking engagements from Biogen, Sanofi, Merck, Novartis, Almirall, Roche, Bristol Myers Squibb and Janssen outside the submitted work. JF reports receiving research travel support from Roche and Janssen outside the submitted work. SS reports receiving personal fees from Almirall, Bristol Myers Squibb and Teva outside the submitted work and receiving compensation for lectures or travel expenses from Merck Serono, Biogen, Sanofi Genzyme, Roche, Janssen and Novartis. JC reports receiving personal fees from Sanofi outside the submitted work and receiving speaker honoraria from Biogen Idec and Sanofi. FR reports receiving personal fees from Sanofi outside the submitted work and receiving speaker honoraria from Biogen Idec and Sanofi. LC reports receiving speaker fees and travel support and/or serving on advisory boards for Biogen, Sanofi, Merck, Bayer, Novartis, Roche, Teva, Celgene, Ipsen, Biopas, Bristol Myers Squibb, Janssen and Almirall. LV reports receiving grants and personal fees from Merck, Roche, Sanofi Genzyme, Bristol Myers Squibb, Celgene, Biogen and Novartis outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This study was funded by Instituto de Salud Carlos III (ISCIII) through the project “PI21/00828” and by grant RD21/0002/0053 from La Red Española de Enfermedades Inflamatorias, and was co-funded by the European Union.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Khalil M., Teunissen C.E., Otto M., Piehl F., Sormani M.P., Gattringer T., Barro C., Kappos L., Comabella M., Fazekas F., et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 2.Petzold A. Glial fibrillary acidic protein is a body fluid biomarker for glial pathology in human disease. Brain Res. 2015;1600:17–31. doi: 10.1016/j.brainres.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Gafson A.R., Jiang X., Shen C., Kapoor R., Zetterberg H., Fox R.J., Belachew S. Serum neurofilament light and multiple sclerosis progression independent of acute inflammation. JAMA Netw. Open. 2022;5:e2147588. doi: 10.1001/jamanetworkopen.2021.47588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disanto G., Barro C., Benkert P., Naegelin Y., Schädelin S., Giardiello A., Zecca C., Blennow K., Zetterberg H., Leppert D., et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 2017;81:857–870. doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benkert P., Meier S., Schaedelin S., Manouchehrinia A., Yaldizli Ö., Maceski A., Oechtering J., Achtnichts L., Conen D., Derfuss T., et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol. 2022;21:246–257. doi: 10.1016/S1474-4422(22)00009-6. [DOI] [PubMed] [Google Scholar]
- 6.Thebault S., Abdoli M., Fereshtehnejad S.M., Tessier D., Tabard-Cossa V., Freedman M.S. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 2020;10:10381. doi: 10.1038/s41598-020-67504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monreal E., Fernández-Velasco J.I., García-Sánchez M.I., de la Maza S.S., Llufriu S., Álvarez-Lafuente R., Casanova B., Comabella M., Ramió-Torrentà L., Martínez-Rodríguez J.E., et al. Association of Serum Neurofilament Light Chain Levels at Disease Onset With Disability Worsening in Patients With a First Demyelinating Multiple Sclerosis Event Not Treated With High-Efficacy Drugs. JAMA Neurol. 2023;80:397–403. doi: 10.1001/jamaneurol.2023.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z., Wang K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38:364–374. doi: 10.1016/j.tins.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barro C., Healy B.C., Liu Y., Saxena S., Paul A., Polgar-Turcsanyi M., Guttmann C.R., Bakshi R., Kropshofer H., Weiner H.L., et al. Serum GFAP and NfL Levels Differentiate Subsequent Progression and Disease Activity in Patients With Progressive Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflammation. 2022;10:e200052. doi: 10.1212/NXI.0000000000200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier S., Willemse E.A.J., Schaedelin S., Oechtering J., Lorscheider J., Melie-Garcia L., Cagol A., Barakovic M., Galbusera R., Subramaniam S., et al. Serum Glial Fibrillary Acidic Protein Compared with Neurofilament Light Chain as a Biomarker for Disease Progression in Multiple Sclerosis. JAMA Neurol. 2023;80:287–297. doi: 10.1001/jamaneurol.2022.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelhak A., Foschi M., Abu-Rumeileh S., Yue J.K., D’anna L., Huss A., Oeckl P., Ludolph A.C., Kuhle J., Petzold A., et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022;18:158–172. doi: 10.1038/s41582-021-00616-3. [DOI] [PubMed] [Google Scholar]
- 12.Högel H., Rissanen E., Barro C., Matilainen M., Nylund M., Kuhle J., Airas L. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult. Scler. 2020;26:210–219. doi: 10.1177/1352458518819380. [DOI] [PubMed] [Google Scholar]
- 13.Monreal E., Fernández-Velasco J.I., Álvarez-Lafuente R., de la Maza S.S., García-Sánchez M.I., Llufriu S., Casanova B., Comabella M., Martínez-Yélamos S., Galimberti D., et al. Serum biomarkers at disease onset for personalized therapy in multiple sclerosis. Brain. 2024;147:4084–4093. doi: 10.1093/brain/awae260. [DOI] [PubMed] [Google Scholar]
- 14.Morrow S.A., Clift F., Devonshire V., Lapointe E., Schneider R., Stefanelli M., Vosoughi R. Use of natalizumab in persons with multiple sclerosis: 2022 update. Mult. Scler. Relat. Disord. 2022;65:103995. doi: 10.1016/j.msard.2022.103995. [DOI] [PubMed] [Google Scholar]
- 15.Wessels M.H., Wessels M.H., Van Lierop Z.Y., Van Lierop Z.Y., Noteboom S., Noteboom S., Strijbis E.M., Strijbis E.M., Heijst J.A., Heijst J.A., et al. Serum glial fibrillary acidic protein in natalizumab-treated relapsing-remitting multiple sclerosis: An alternative to neurofilament light. Mult. Scler. 2023;29:1229–1239. doi: 10.1177/13524585231188625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Lierop Z.Y., Noteboom S., Steenwijk M.D., van Dam M., A Toorop A., LE van Kempen Z., Moraal B., Barkhof F., Uitdehaag B.M., Schoonheim M.M., et al. Neurofilament-light and contactin-1 association with long-term brain atrophy in natalizumab-treated relapsing-remitting multiple sclerosis. Mult. Scler. 2022;28:2231–2242. doi: 10.1177/13524585221118676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalla Costa G., Martinelli V., Moiola L., Sangalli F., Colombo B., Finardi A., Cinque P., Kolb E., Haghikia A., Gold R., et al. Serum neurofilaments increase at progressive multifocal leukoencephalopathy onset in natalizumab-treated multiple sclerosis patients. Ann. Neurol. 2019;85:606–610. doi: 10.1002/ana.25437. [DOI] [PubMed] [Google Scholar]
- 18.Gunnarsson M., Malmeström C., Axelsson M., Sundström P., Dahle C., Vrethem M., Olsson T., Piehl F., Norgren N., Rosengren L., et al. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011;69:83–89. doi: 10.1002/ana.22247. [DOI] [PubMed] [Google Scholar]
- 19.Foley J.F., Defer G., Ryerson L.Z., A Cohen J., Arnold D.L., Butzkueven H., Cutter G., Giovannoni G., Killestein J., Wiendl H., et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): A randomised, controlled, open-label, phase 3b trial. Lancet Neurol. 2022;21:608–619. doi: 10.1016/S1474-4422(22)00143-0. [DOI] [PubMed] [Google Scholar]
- 20.Bridel C., Leurs C.E., van Lierop Z.Y.G.J., van Kempen Z.L., Dekker I., Twaalfhoven H.A., Moraal B., Barkhof F., Uitdehaag B.M., Killestein J., et al. Serum Neurofilament Light Association with Progression in Natalizumab-Treated Patients With Relapsing-Remitting Multiple Sclerosis. Neurology. 2021;97:e1898–e1905. doi: 10.1212/WNL.0000000000012752. [DOI] [PubMed] [Google Scholar]
- 21.Højsgaard Chow H., Petersen E.R., Olsson A., Laursen J.H., Hansen M.B., Oturai A.B., Sørensen P.S., Søndergaard H.B., Sellebjerg F. Age-corrected neurofilament light chain ratio decreases but does not predict relapse in highly active multiple sclerosis patients initiating natalizumab treatment. Mult. Scler. Relat. Disord. 2024;88:105701. doi: 10.1016/j.msard.2024.105701. [DOI] [PubMed] [Google Scholar]
- 22.Proschmann U., Inojosa H., Akgün K., Ziemssen T. Natalizumab Pharmacokinetics and -Dynamics and Serum Neurofilament in Patients with Multiple Sclerosis. Front. Neurol. 2021;12:650530. doi: 10.3389/fneur.2021.650530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toorop A.A., Wessels M.H., Boonkamp L., Gelissen L.M., Hoitsma E., Zeinstra E.M., van Rooij L.C., van Munster C.E., Vennegoor A., Mostert J.P., et al. Serum neurofilament light and glial fibrillary acidic protein levels are not associated with wearing-off symptoms in natalizumab-treated multiple sclerosis patients. Mult. Scler. 2024;30:1683–1688. doi: 10.1177/13524585241293940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalla Costa G., Leocani L., Pisa M., Croese T., Martinelli V., Moiola L., Sangalli F., Colombo B., Haghikia A., Gold R., et al. Neuroaxonal damage in natalizumab-treated MS patients: The role of JCV antibody titres. Mult. Scler. Mult Scler. 2024;30:1561–1565. doi: 10.1177/13524585241260977. [DOI] [PubMed] [Google Scholar]
- 25.Valentino P., Malucchi S., Bava C.I., Martire S., Capobianco M., Malentacchi M., Sperli F., Oggero A., Di Sapio A., Bertolotto A. Serum Neurofilaments are a reliable biomarker to early detect PML in Multiple Sclerosis patients. Mult. Scler. Relat. Disord. 2023;77:104893. doi: 10.1016/j.msard.2023.104893. [DOI] [PubMed] [Google Scholar]
- 26.Fissolo N., Pignolet B., Rio J., Vermersch P., Ruet A., Desèze J., Labauge P., Vukusic S., Papeix C., Martinez-Almoyna L., et al. Serum Neurofilament Levels and PML Risk in Patients With Multiple Sclerosis Treated With Natalizumab. Neurol. Neuroimmunol. Neuroinflamm. 2021;8:e1003. doi: 10.1212/NXI.0000000000001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loonstra F.C., Verberk I.M.W., Wijburg M.T., Wattjes M.P., Teunissen C.E., van Oosten B.W., Uitdehaag B.M.J., Killestein J., van Kempen Z.L.E. Serum neurofilaments as candidate biomarkers of natalizumab associated progressive multifocal leukoencephalopathy. Ann. Neurol. 2019;86:322–324. doi: 10.1002/ana.25523. [DOI] [PubMed] [Google Scholar]
- 28.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., Correale J., Fazekas F., Filippi M., Freedman M.S., et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 29.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 30.Weinshenker B.G., Issa M., Baskerville J. Meta-analysis of the placebo-treated groups in clinical trials of progressive MS. Neurology. 1996;46:1613–1619. doi: 10.1212/WNL.46.6.1613. [DOI] [PubMed] [Google Scholar]
- 31.Giovannoni G., Turner B., Gnanapavan S., Offiah C., Schmierer K., Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult. Scler. Relat. Disord. 2015;4:329–333. doi: 10.1016/j.msard.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Tybirk L., Hviid C.V.B., Knudsen C.S., Parkner T. Serum GFAP—Reference interval and preanalytical properties in Danish adults. Clin. Chem. Lab. Med. 2022;60:1830–1838. doi: 10.1515/cclm-2022-0646. [DOI] [PubMed] [Google Scholar]
- 33.Rodero-Romero A., Monreal E., Sainz-Amo R., Domínguez J.M.G., Villarrubia N., Veiga-González J.L., Fernández-Velasco J.I., Goicochea-Briceño H., Rodríguez-Jorge F., de la Maza S.S., et al. Establishing Normal Serum Values of Neurofilament Light Chains and Glial Fibrillary Acidic Protein Considering the Effects of Age and Other Demographic Factors in Healthy Adults. Int. J. Mol. Sci. 2024;25:7808. doi: 10.3390/ijms25147808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data can be obtained from the corresponding author upon reasonable request in the three years following the manuscript’s publication.