Abstract
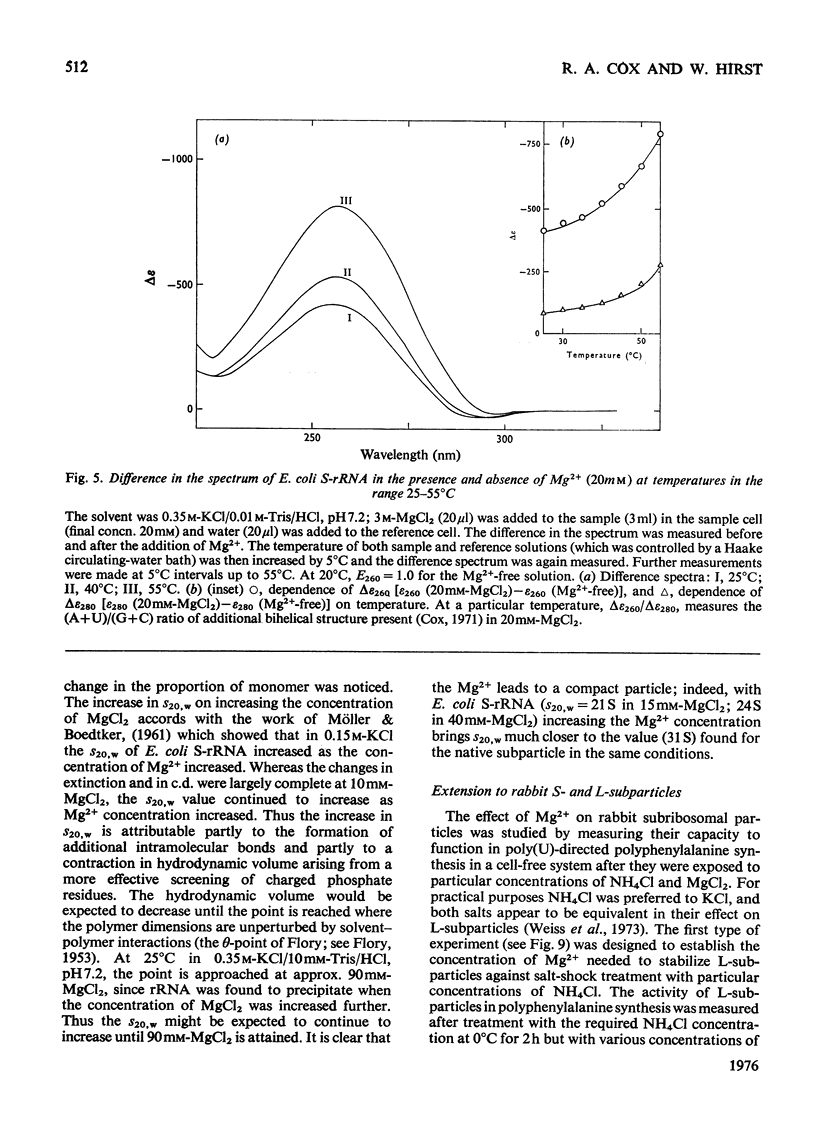
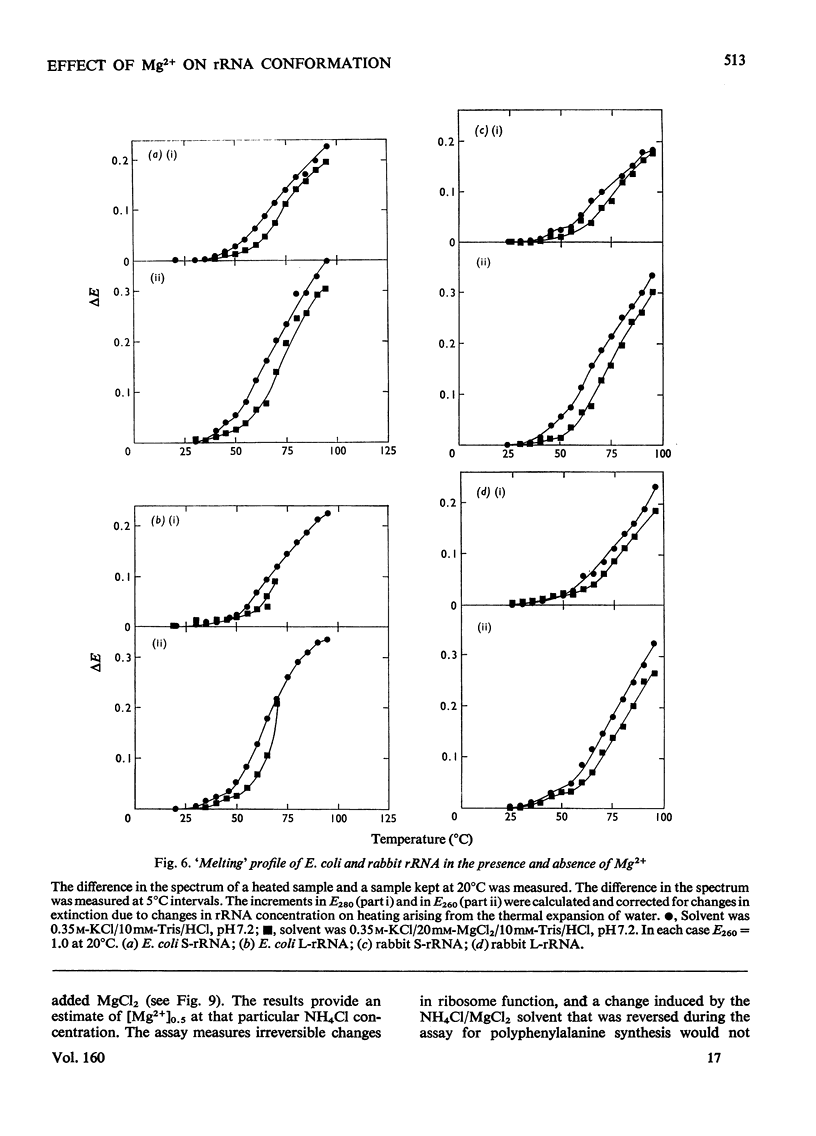
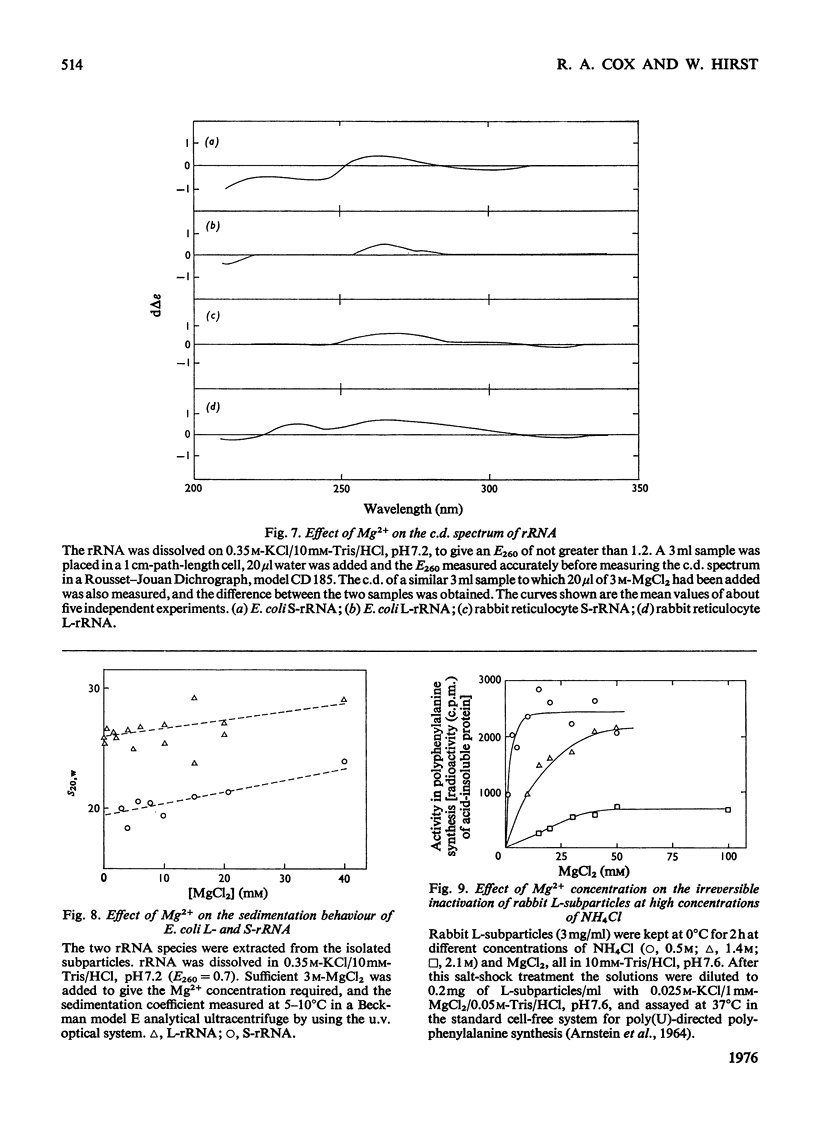
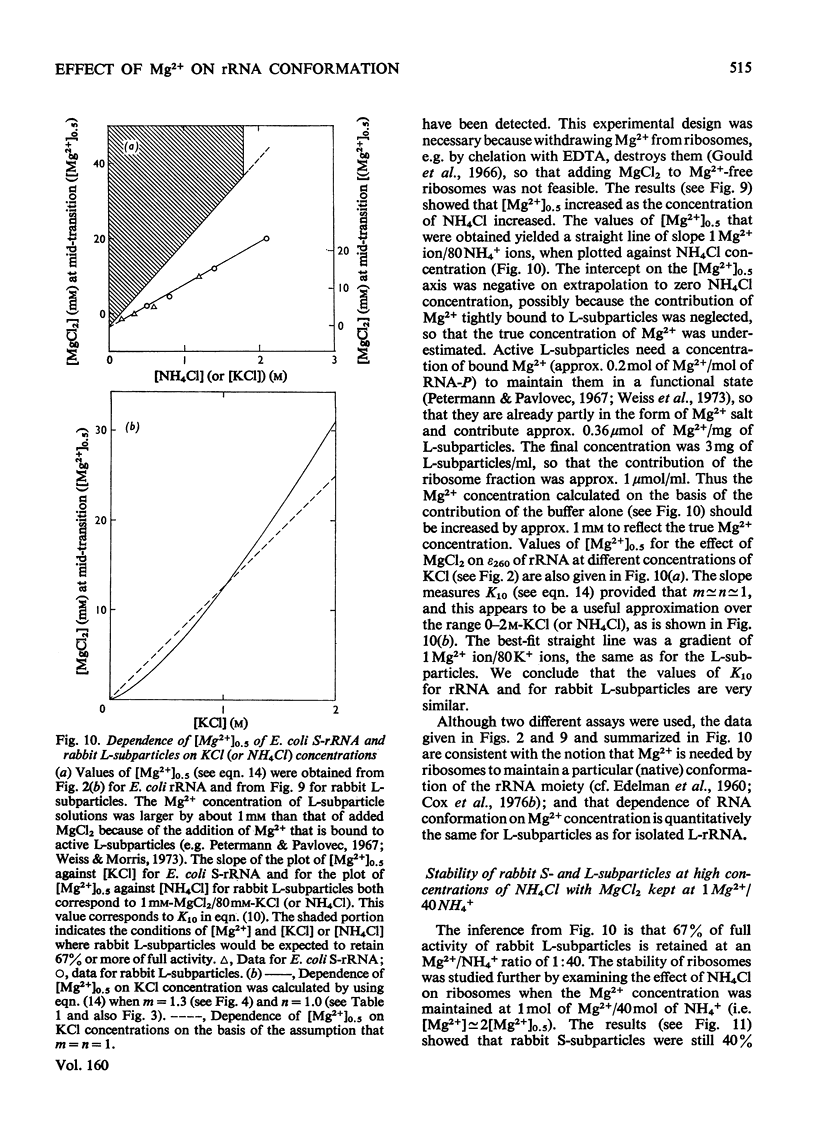
Mg2+ was shown to affect the conformation of rRNA over the range of 0.03-1.2M-KCl. The species studies were Escherichia coli S-rRNA and L-rRNA (the RNA moieties of the smaller and larger subribosomal particles respectively) and rabbits S-rRNA and L-rRNA. 2. The addition of Mg2+ to rRNA in reconstitution buffer (0.35M-KCl0.01M-Tris/HCl, pH7.2) at 20 degrees C let to an increase in bihelical secondary structure through the formation of additional (mainly A-U) base-pairs (e.g. an additional approx. 58 A-U base-pairs per molecule of E. coli S-rRNA as judged by u.v. difference spectrophotometry...
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnstein H. R., Cox R. A., Hunt J. A. The function of high-molecular-weight ribonucleic acid from rabbit reticulocytes in haemoglobin biosynthesis. Biochem J. 1964 Sep;92(3):648–661. doi: 10.1042/bj0920648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX R. A., LITTAUER U. Z. Ribonucleic acid from Escherichia coli. III. The influence of ionic strength and temperature on hydrodynamic and optical properties. Biochim Biophys Acta. 1962 Aug 20;61:197–208. [PubMed] [Google Scholar]
- Cohn M., Danchin A., Grunberg-Manago M. Proton magnetic relaxation studies of marganous complexes of transfer RNA and related compounds. J Mol Biol. 1969 Jan 14;39(1):199–217. doi: 10.1016/0022-2836(69)90342-8. [DOI] [PubMed] [Google Scholar]
- Cox R. A. A spectrophotometric study of the secondary structure of ribonucleic acid isolated from the smaller and larger ribosomal subparticles of rabbit reticulocytes. Biochem J. 1970 Mar;117(1):101–118. doi: 10.1042/bj1170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. Conformation of nucleic acids and the analysis of the hypochromic effect. Biochem J. 1970 Dec;120(3):539–547. doi: 10.1042/bj1200539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Greenwell P., Hirst W. Re-activation of the peptidyltransferase centre of rabbit reticulocyte ribosomes after inactivation by exposure to low concentrations of magnesium ion. Biochem J. 1976 Dec 15;160(3):521–531. doi: 10.1042/bj1600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Greenwell P. Reassembly of the peptidyltransferase centre of larger subparticles of rabbit reticulocyte ribosomes from a core-particle and split-protein fraction. Biochem J. 1976 Dec 15;160(3):533–546. doi: 10.1042/bj1600533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Hirst W., Godwin E., Kaiser I. The circular dichroism of ribosomal ribonucleic acids. Biochem J. 1976 May 1;155(2):279–291. doi: 10.1042/bj1550279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A., Pratt H., Huvos P., Higginson B., Hirst W. A study of the thermal stability of ribosomes and biologically active subribosomal particles. Biochem J. 1973 Jul;134(3):775–793. doi: 10.1042/bj1340775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Bollen A., Herzog A. Ionic effects on the ribosomal quaternary structure. Eur J Biochem. 1970 Mar 1;13(1):132–136. doi: 10.1111/j.1432-1033.1970.tb00908.x. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Cox R. A. Structure of Escherichia coli ribosomes: effect of ribonuclease on the 30-S and 50-S subunits. Biochim Biophys Acta. 1970 Apr 15;204(2):489–501. doi: 10.1016/0005-2787(70)90169-3. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Arnstein H. R., Cox R. A. The dissociation of reticulocyte polysomes into subunits and the location of messenger RNA. J Mol Biol. 1966 Feb;15(2):600–618. doi: 10.1016/s0022-2836(66)80130-4. [DOI] [PubMed] [Google Scholar]
- Hultin T., Näslund P. H., Sjöqvist A. Conditions of structural and functional destabilization of mammalian ribosomes by magnesium ions. Biochim Biophys Acta. 1973 Aug 10;319(1):81–90. doi: 10.1016/0005-2787(73)90043-9. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Dahlberg J. E., Dahlberg A. E. Detection of cation-specific conformational changes in ribosomal RNA by gel electrophoresis. Nucleic Acids Res. 1975 Apr;2(4):447–458. doi: 10.1093/nar/2.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann M. L., Pavlovec A. The effect of temperature on the magnesium binding and ultracentrifugal properties of rat liver ribosomes. Biochemistry. 1967 Sep;6(9):2950–2958. doi: 10.1021/bi00861a040. [DOI] [PubMed] [Google Scholar]
- Pratt H., Cox R. A. Dissociation of ribosomes from oocytes of Xenopus laevis into active subparticles. Biochem J. 1971 Oct;124(5):897–903. doi: 10.1042/bj1240897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer R., Hach R. tRNA conformation and magnesium binding. A study of a yeast phenylalanine-specific tRNA by a fluorescent indicator and differential melting curves. Eur J Biochem. 1975 Jun 16;55(1):271–284. doi: 10.1111/j.1432-1033.1975.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30 s ribosomes studied in vitro. J Mol Biol. 1969 Mar 28;40(3):391–413. doi: 10.1016/0022-2836(69)90161-2. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Ehresmann C., Stiegler P., Garrett R. Evidence for tertiary structural RNA-RNA interactions within the protein S4 binding site at the 5'-end of 16S ribosomal RNA of Escherichia coli.+. Nucleic Acids Res. 1975 Oct;2(10):1867–1888. doi: 10.1093/nar/2.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Kimes B. W., Morris D. R. Cations and ribosome structure. 3. Effects on the 30S and 50S subunits of replacing bound Mg 2+ by inorganic cations. Biochemistry. 1973 Jan 30;12(3):450–456. doi: 10.1021/bi00727a014. [DOI] [PubMed] [Google Scholar]
- Weiss R. L., Morris D. R. Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry. 1973 Jan 30;12(3):435–441. doi: 10.1021/bi00727a012. [DOI] [PubMed] [Google Scholar]
- Willick G. E., Kay C. M. Magnesium-induced conformational change in transfer ribonucleic acid as measured by circular dichroism. Biochemistry. 1971 Jun 8;10(12):2216–2222. doi: 10.1021/bi00788a005. [DOI] [PubMed] [Google Scholar]


