Abstract
Genome-wide association studies performed in patients with coronavirus disease 2019 (COVID-19) have uncovered various loci significantly associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. However, the underlying cis-regulatory genetic factors that contribute to heterogeneity in the response to SARS-CoV-2 infection and their impact on clinical phenotypes remain enigmatic. Here, we used single-cell RNA-sequencing to quantify genetic contributions to cis-regulatory variation in 361,119 peripheral blood mononuclear cells across 63 COVID-19 patients during acute infection, 39 samples collected in the convalescent phase, and 106 healthy controls. Expression quantitative trait loci (eQTL) mapping across cell types within each disease state group revealed thousands of cis-associated variants, of which hundreds were detected exclusively in immune cells derived from acute COVID-19 patients. Patient-specific genetic effects dissipated as infection resolved, suggesting that distinct gene regulatory networks are at play in the active infection state. Further, 17.2% of tested loci demonstrated significant cell state interactions with genotype, with pathways related to interferon responses and oxidative phosphorylation showing pronounced cell state-dependent variation, predominantly in CD14+ monocytes. Overall, we estimate that 25.6% of tested genes exhibit gene-environment interaction effects, highlighting the importance of environmental modifiers in the transcriptional regulation of the immune response to SARS-CoV-2. Our findings underscore the importance of expanding the study of regulatory variation to relevant cell types and disease contexts and argue for the existence of extensive gene-environment effects among patients responding to an infection.
Keywords: single-cell RNA-seq, expression quantitative trait loci (eQTL), SARS-CoV-2 infection, COVID-19, gene-environment interactions
Susceptibility to viral infection varies widely among individuals, influenced by a combination of host genetics and environmental factors. However, the precise contribution of each to immune response variation and disease progression remains unclear. Recent advances have demonstrated the considerable role of host genetics in shaping human immune response variation through expression quantitative trait loci (eQTL) mapping, applied to various immune cell subsets both at baseline and after exposure to immune stimuli and live pathogens. These ‘immune response eQTL’ studies have identified numerous genetic variants that underlie differences in immune responses to infection, including both cell type-specific eQTL and eQTL induced only upon infection (i.e., response eQTL)1–5. However, a significant limitation of these studies is that immune response measurements were largely collected in vitro, raising questions about the role of gene-environment interactions during viral infection in vivo.
More recently, efforts have expanded to explore other forms of genetic interaction effects, facilitated by the availability of population-scale cohorts genotyped and characterized by single-cell RNA sequencing6. Continuous cell state-dependent eQTL—eQTL that interact with specific cellular contexts defined at single-cell resolution—have been shown to explain more variation in gene expression than conventional, non-interacting eQTL7. Notably, autoimmune risk variants were enriched in these state-dependent loci7,8, highlighting the critical importance of cellular context in understanding disease-relevant genetic variants.
The global COVID-19 pandemic highlighted the possible consequences of the spread of a novel virus in a naïve population. Particularly in the initial waves of the pandemic, substantial immune response variation and disease heterogeneity was observed among individuals infected with SARS-CoV-2, the virus that causes COVID-19. While a fraction of individuals succumbed to severe disease, some developed typical influenza-like symptoms, while others harbored asymptomatic SARS-CoV-2 infections9. Although much of this variation can be attributed to environmental and social determinants10, genetic factors also clearly play a role.
Genome-wide association studies (GWAS) conducted for SARS-CoV-2 susceptibility and COVID-19 severity phenotypes revealed a handful of genome-wide significant loci associated with these traits11–13, often in genes related to viral immunity, including IFNAR2 and OAS113. An eQTL mapping study performed in peripheral blood mononuclear cells (PBMCs) collected from healthy individuals exposed to SARS-CoV-2 in vitro also found that response eQTL were highly cell type-dependent, often specific to the SARS-CoV-2 infection condition in the myeloid compartment5. Despite these findings, few studies have examined how genome-wide cis-regulatory genetic variation influences immune response diversity directly in patients during active viral infection14.
In this study, we explore the nature of genetic interaction effects in the context of bona fide SARS-CoV-2 infection, using patient cells sampled prior to the rollout of COVID-19 vaccines and during longitudinal follow-up. We specifically investigate cell type-specific, disease state-specific, and cell state-dependent gene regulatory heterogeneity, providing new insights into how genetic variation shapes immune responses in vivo.
Single-cell profiling reveals severity-dependent cellular restructuring in COVID-19 patients
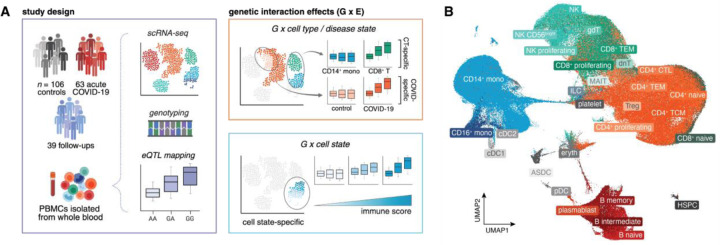
In this study, we used single-cell RNA-sequencing to profile the transcriptomes of PBMCs collected from 106 healthy control donors, 63 hospitalized COVID-19 patients during the acute stage of infection (days after symptom onset [DSO] ≤ 20 days, mean DSO at time of sampling = 12.1 days), and 39 samples obtained from a subset of recovered COVID-19 patients resampled at various time points following their initial primary infection (“follow-ups”, DSO > 20 days, mean DSO = 128.8 days) (Fig. 1A, Fig. S1A, Table S1). Across individuals, we captured 361,119 high-quality single-cell transcriptomes (n = 163,639 cells from controls, n = 131,457 cells from acute patients, and n = 66,023 cells from follow-ups). Clustering followed by cell type label transfer annotation from a multimodal human PBMC reference dataset (Hao et al.15, detailed in Methods) revealed 30 distinct immune cell types at fine-scale resolution (Fig. 1B).
Fig. 1. Summary of the study cohort and aims.
(A) Study design (left) and examples of various gene-environment interactions, including cell type-, disease state-, and cell state-dependent effects, evaluated in this study (right). (B) UMAP visualization of all cells (n = 361,119) collected across healthy control, acute COVID-19 patient, and follow-up samples (n = 208 samples). ASDC: AXL+SIGLEC6+ dendritic cells, CD4+ CTL: cytotoxic CD4+ T cells, cDC: conventional dendritic cells, dnT: double-negative T cells, Eryth: erythrocytes, gdT: gamma delta T cells, HSPC: hematopoietic stem and progenitor cells, ILC: innate lymphoid cells, MAIT: mucosal associated invariant T cells, mono: monocytes, NK: natural killer, pDC: plasmacytoid dendritic cells, TEM: T effector memory, TCM: T central memory.
We next sought to dissect the extent to which SARS-CoV-2 infection induces shifts in underlying cell type composition across acutely-infected individuals compared to non-infected healthy controls and recovered donors. Although all COVID-19 patients included in this study were hospitalized at the time of sample collection, these patients spanned a range of clinical disease severity, allowing us to evaluate the effect of severity on various molecular phenotypes. Disease severity was assessed using a five-point scale of respiratory support needed at the time of acute patient sampling, encompassing the following categories: Moderate (MOD, n = 16), Severe (SEV, n = 17), 2-Critical (CRIT2, n = 9), 3-Critical (CRIT3, n = 20), and 4-Critical (CRIT4, n = 16). A summary of basic demographic information stratified by disease severity can be found in Table S1. Non-critical patients were defined as those requiring no oxygen supplementation (moderate disease) or oxygen supplementation through a nasal cannula (severe disease), whereas critical patients required mechanical ventilation, ranging from non-invasive ventilation (CRIT2) and intubation (CRIT3) to extracorporeal membrane oxygenation (CRIT4).
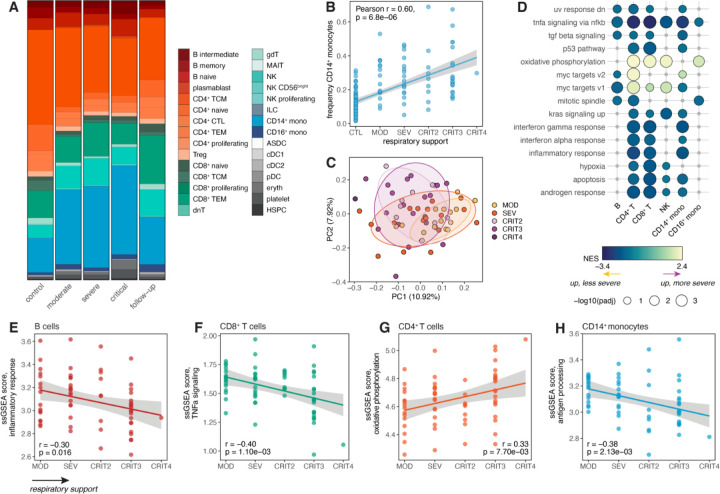
We found that SARS-CoV-2 infection remodels the baseline cell type composition of PBMCs observed in healthy individuals, with the magnitude of disease severity further modifying this effect. The myeloid compartment displayed the most obvious infection- and severity-dependent changes: classical CD14+ monocytes were markedly expanded in all patient groups compared to healthy donors (p < 1 × 10−10 for all comparisons against controls; here, all critical patients [CRIT2 – 4] were considered as a single group), with the greatest expansion seen in severe and critical cases (Fig. 2A). In the follow-up samples, CD14+ monocyte proportions reverted back to frequencies similar to those seen in baseline healthy control donors (Fig. 2A), suggesting that this monocytic expansion is indeed infection-induced. Further, we observed that the frequency of CD14+ monocytes was strongly associated with disease severity, with more severe cases consistently displaying a greater proportion of classical monocytes (Pearson’s r = 0.60, p = 6.8 × 10−6) (Fig. 2B).
Fig. 2. Effects of COVID-19 disease severity on underlying cell type composition and transcriptional signatures in hospitalized patients.
(A) Cell type proportions stratified by disease severity at the time of sample collection. (B) Correlation between respiratory support score at the time of patient sampling and frequency of CD14+ monocytes. (C) PCA decomposition of the CD14+ monocyte expression data in COVID-19 patients colored by respiratory support score. (D) Hallmark enrichments for severity effects in COVID-19 patients across cell types. Colored circles represent pathways with FDR < 0.10; gray circles represent non-significant pathways. Only pathways significant in three or more cell types are shown. (E-H) Correlation between respiratory support score and ssGSEA scores in various cell types for (E) inflammatory response, (F) TNF-α signaling, (G) oxidative phosphorylation, and (H) antigen processing. In (B) and (E-H), p-values and best-fit lines were obtained from linear regression models.
We also detected reductions of CD56bright natural killer (NK) cells (p < 2 × 10−4) and plasmacytoid dendritic cells (pDC) (p < 4 × 10−3) in all severity groups compared to non-infected individuals (Fig. 2A). pDCs are known for their ability to secrete large quantities of type I interferon (IFN) following viral infection16, and NK cells are key facilitators of antiviral immunity, with CD56bright NK cells being efficient producers of IFN-γ, TNF-α, and GM-CSF17. Our observations are in line with previous studies showing reductions in frequencies of both NK cells and pDCs in critical patients compared to healthy controls18–21. Together, this suggests that SARS-CoV-2 infection induces atypical cell type composition that largely resolves after the infection clears, particularly in cell populations known to be important in cytokine production and antiviral immune responses.
Disease severity underlies variation in the transcriptional response to SARS-CoV-2 in hospitalized COVID-19 patients
To tease apart how variation in disease severity influences the transcriptional immune response to SARS-CoV-2 across cell types, we formally modeled the effect of severity on global gene expression estimates among COVID-19 patients sampled during the acute phase of disease (n = 63) within each cell type independently. In these analyses, we defined a set of top-level cell type populations by combining our fine-scale clusters into major groups corresponding to the six main cell types that comprise PBMCs, including CD4+ T cells, CD8+ T cells, B cells, NK cells, CD14+ monocytes, and CD16+ monocytes. Within this broader set of cell populations, we collapsed our single-cell gene expression estimates into pseudobulk estimates per sample, generating six bulk-like gene expression matrices that were used for subsequent modeling. We considered respiratory support score (described above) as a proxy for overall disease severity, and modeled severity score as a numeric variable, which allowed us to capture genes with expression levels linearly correlated with severity.
By far, CD14+ monocytes showed the largest number of genes associated with severity (n = 1,613, 14.8% of the transcriptome; FDR < 0.05), while other cell types had much less prominent effects (< 1.0% severity-associated genes) (Table S2). As expected, severity-associated genes largely overlapped those distinguishing COVID-19 patients from healthy controls (i.e., infection-associated genes, |log2FC| > 0.5, FDR < 0.05) across cell types (gene set overlap: 2.1-fold, p < 1 × 10−10) (Fig. S1B, S1C, Table S3). Principal component analysis (PCA) on the CD14+ monocyte pseudobulk expression data revealed that variation in disease severity had a noticeable impact on the transcriptional response of these cells, reflected in principal component (PC) 1 (10.9% percent variance explained [PVE]) and PC2 (7.9% PVE), which both separated non-critical patients (moderate/severe) from critical patients (Fig. 2C).
We then performed gene set enrichment analysis for the MSigDB Hallmark pathways22 to define the functional pathways differentiating the transcriptional signatures of COVID-19 patients along the spectrum of disease severity in our cohort (Fig. 2D, Table S4). We identified various immune response pathways significantly associated with severity, including TNF-α signaling via NF-κB in all cell types tested (FDR = 0.03 in CD16+ monocytes and FDR < 2 × 10−3 in other cell types), and IFN-γ response (FDR < 4 × 10−4), IFN-α response (FDR < 0.08), and inflammatory response (FDR < 4 × 10−4) in CD14+ monocytes, CD4+ T cells, and CD8+ T cells (Fig. 2D). All of these enrichments were detected among genes more highly expressed in less severe cases, suggesting that such patients engage stronger proinflammatory and antiviral immune responses compared to those with more severe disease presentations. Importantly, these findings are unlikely to be confounded by potential sampling biases, as sampling time point (i.e., DSO) showed no significant association with respiratory support score (Pearson r = 0.11, p = 0.37) (Fig. S1D). With the exception of TNF-α signaling, these pathway enrichments were cell type-specific, implicating classical monocytes, helper T cells, and cytotoxic T cells as the subsets most influenced by variation in disease severity and morbidity. Only the oxidative phosphorylation pathway was consistently elevated in more severe cases (FDR < 1.5 × 10−3 in CD4+ T cells, CD8+ T cells, NK cells, and CD16+ monocytes), suggesting a rewiring of metabolism in patients who poorly respond to SARS-CoV-2 (Fig. 2D).
To better characterize severity-associated heterogeneity in the transcriptional immune response, we computed single-sample gene set enrichment analysis (ssGSEA) scores capturing the activity of various functional pathways within each sample across cell types (detailed in Methods). Consistent with our enrichment analyses, the level of respiratory support was negatively correlated with ssGSEA inflammatory response scores (Pearson r = −0.30, p = 0.016) (Fig. 2E) and TNF-α signaling scores (Pearson r = −0.40, p = 1.1 × 10−3) (Fig. 2F) in B cells and CD8+ T cells, respectively. Similarly, respiratory support score was also positively associated with oxidative phosphorylation scores in CD4+ T cells (Pearson r = 0.33, p = 7.7 × 10−3) (Fig. 2G). Moreover, we created an antigen processing and presentation score based on the corresponding Biological Process gene set23, given the previously reported finding that SARS-CoV-2 inhibits the major histocompatibility complex (MHC) class I pathway, a pathway that plays a crucial role in antiviral immunity in lung epithelial cells24. Antigen processing scores were negatively correlated with severity in CD14+ monocytes (Pearson r = −0.38, p = 2.1 × 10−3) (Fig. 2H), while no significant association was found in any other cell type that we tested (p > 0.20), indicating that antigen presentation-associated functions are shut down in circulating classical monocytes in severe patients.
Genetic interaction effects shape transcriptional response variation during acute SARS-CoV-2 infection
All individuals were genotyped for 4.19 million single nucleotide polymorphisms (SNPs), allowing us to delineate the role of cis-regulatory genetic and gene-environment interaction effects in the context of SARS-CoV-2 infection in patient-derived cells. To directly measure the contribution of cell type-specific and disease state-specific genetic variation during the course of a viral infection, we mapped cis-eQTL, defined as SNPs located either within or flanking (±100 kilobases, kb) each gene of interest, using the pseudobulk expression estimates for all six major cell types independently in i) healthy controls and ii) COVID-19 patients sampled during acute infection. To increase our power to detect shared and cell type- or disease state-specific effects, we utilized a multivariate adaptive shrinkage framework (mash)25 to leverage information about the underlying correlation structure within our dataset.
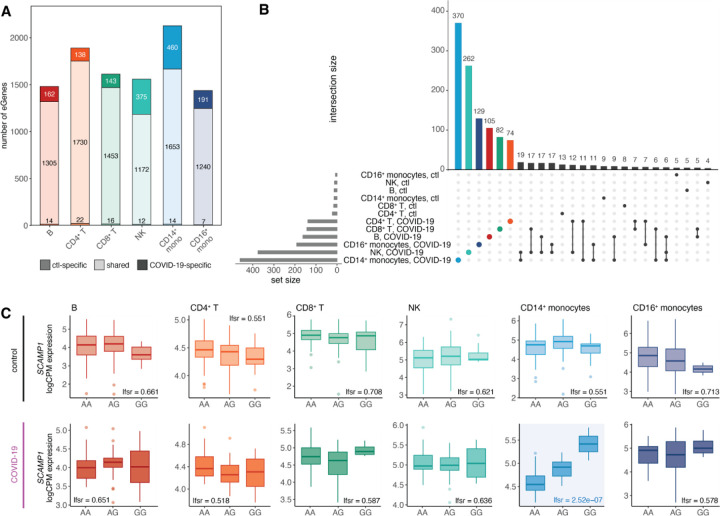
Across cell types and infection conditions, we identified 2,725 genes with at least one significant cis-eQTL [local false sign rate (lfsr) < 0.10 in at least one cell type-condition pair, 35.6% of genes tested; referred to as eGenes] (Fig. 3A, Table S5). B cells (n eGenes = 1,481) and CD16+ monocytes (n eGenes = 1,438) exhibited the fewest genetic effects, while CD14+ monocytes displayed the greatest number (n eGenes = 2,127) (Fig. 3A). Most genetic effects were shared between healthy individuals and COVID-19 patients within a given cell type—84.8% on average, referred to as ‘shared’ eGenes (lfsrCTL < 0.1 and lfsrCOVID < 0.3 or vice versa)—and many of these shared eGenes were also common across cell types, with 59.0% shared across four or more cell types (Fig. S2).
Fig. 3. Cis-regulatory effects are cell type-specific and disease state-specific.
(A) Number of shared and disease state-specific eGenes within each cell type. (B) Significant condition-specific eGene (lfsrCTL < 0.10 and lfsrCOVID > 0.30, lfsrCOVID < 0.10 and lfsrCTL > 0.30) sharing patterns across cell types in healthy controls and COVID-19 patients. Patient-specific eGene sets are highlighted by color per cell type. (C) Example of a patient-specific genetic effect (i.e., SARS-CoV-2 response eQTL) present only in CD14+ monocytes in the gene SCAMP1 (healthy controls, top plots; COVID-19 patients, bottom plots).
In stark contrast, some cell types, particularly CD14+ monocytes and NK cells, displayed a substantial proportion of condition-specific eGenes, where genetic effects were observed exclusively in either control or COVID-19 conditions. Notably, CD14+ monocytes and NK cells displayed the greatest fraction of infection-dependent genetic effects (24.8% in NK cells and 22.3% in CD14+ monocytes), much higher than the average of 11.0% in other cell types. Strikingly, across all cell types, the overwhelming majority of condition-specific genetic effects (86.3–97.0%) were eQTL observed exclusively in COVID-19 patients rather than in healthy individuals, underscoring the virus’s profound impact on the genetic regulation of immune responses (Fig. 3A).
Condition-specific eGenes were highly cell type-specific, with monocytes possessing a particularly large number of COVID-19-specific eGenes (CD14+ monocytes n = 370, CD16+ monocytes n = 129), further highlighting the abundance of SARS-CoV-2 response eQTL in the myeloid lineage (Fig. 3B). One prime example of a monocyte-specific response eQTL is the top cis-eQTL for SCAMP1 (rs6453393), a gene involved in cytokine secretion, vesicular trafficking, and membrane transport26. This variant exhibited a strong genetic effect unique to CD14+ monocytes in COVID-19 patients (lfsr = 2.5 × 10−7), but no significant effect in other cell types or conditions (lfsr > 0.50) (Fig. 3C). These findings highlight the crucial role of genetic factors in shaping the monocyte response to SARS-CoV-2 infection in vivo.
Response eQTL effects are substantially weaker in the innate immune cells of recovered individuals
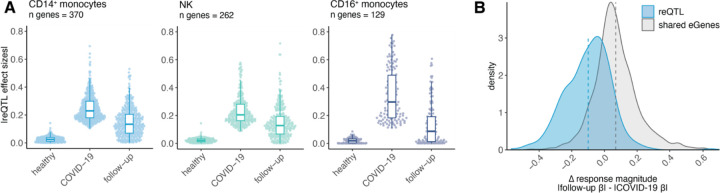
Given the abundance of disease state-specific regulatory variation present in COVID-19 patients and absent in healthy individuals, we hypothesized that these eGenes detected only in patients may represent genetic effects only observed during the active infection state. To test whether these genetic effects disappear as infection resolves, we mapped cis-eQTL in our cohort of recovered COVID-19 patients who were resampled at various time points following primary SARS-CoV-2 infection (DSO > 20 days, n = 39). We then focused on the innate immune cell compartment (i.e., monocytes and NK cells) to determine how disease state-specific regulatory variation may shift in the convalescent period, as these cell types displayed the greatest number of SARS-CoV-2 response-specific genetic effects (‘response eGenes’, n reQTL: 370 in CD14+ monocytes, 262 in NK cells, and 129 in CD16+ monocytes) (Fig. 3B). Among these cell type-specific response eGene sets, effect sizes were significantly higher in acute patients compared to follow-ups (p < 1 × 10−10 in all three cell types). Indeed, for many eGenes, the effect sizes in follow-up individuals reverted back to the magnitude observed in healthy controls (Fig. 4A), an outcome that was seen across cell types. This result held true even after adjusting for sample size differences across disease state groups and when focusing on the 21 individuals with paired acute and follow-up samples (Fig. S3A).
Fig. 4. SARS-CoV-2 response eQTL effects revert to baseline in longitudinal follow-up samples.
(A) Effect sizes for the cell type-specific reQTL gene sets plotted across innate immune cell types in healthy controls, patients, and follow-up samples. All eQTL effect sizes correspond to mash posterior effect sizes. (B) Distribution of the change in eQTL effect sizes between follow-up and patient samples (defined as |follow-up βeQTL| - |COVID-19 patient βeQTL|) for response eQTL (n = 370, blue) and shared eQTL (n = 1,653, gray) in CD14+ monocytes. Dashed lines represent the mean Δ response magnitude for the respective gene sets.
To explicitly measure the extent of reQTL effect size reversion coinciding with recovery, we calculated a paired ΔreQTL metric, defined as the difference in magnitude of a response eGene’s effect size in follow-ups compared to COVID-19 patients (i.e., |follow-up βreQTL| -|COVID-19 patient βreQTL|) specifically in CD14+ monocytes. Here, we considered only the effect size magnitude because the vast majority of response eGenes had effect sizes with concordant signs in the patient and follow-up groups (Fig. S3B). For comparison, we also computed this change in response magnitude for the set of shared eGenes between COVID-19 patients and healthy controls (n = 1,653). The mean ΔreQTL for response eGenes was below zero (mean ΔreQTL = −0.10), substantially lower than that for shared eGenes (mean = 0.07) (Fig. 4B). This value was also significantly lower than expected by chance (p < 0.001), as determined by randomly sampling the same number of genes (n = 370) from shared CD14+ monocyte eGenes 1,000 times (Fig. S3C). These results indicate that infection mediates dynamic genetic effects and plays a significant role in disease state-dependent gene-environment interactions.
Cell state-dependent cis-regulatory effects are prevalent in CD14+ monocytes and can capture clinical features of patient cohorts
We identified several immune and metabolism-related pathways, including TNF-α signaling via NF-κB, oxidative phosphorylation, IFN-γ and IFN-α responses, inflammatory response, and apoptosis, as being strongly associated with disease severity across multiple cell types in COVID-19 patients (Fig. 2D). Given this, we hypothesized that some of the patient-specific genetic effects detected might be driven by heterogeneity in functional cell states within these clinically relevant pathways. To determine whether cell states defined at the single-cell level are dynamically regulated by cis variation, we directly mapped single-cell eQTL in COVID-19 patients using our comprehensive single-cell data.
To measure cell state-dependent cis-regulatory effects, we applied a continuous measure of cell state, which has been shown to capture more state-dependent regulatory variation than discrete classifications. For each pathway, we calculated a numeric score summarizing the activity for each single cell using ssGSEA (see Methods for details). To map continuous state-dependent cis-eQTLs within each cell type, we used a poisson mixed-effects interaction model, a method that has proven successful in identifying state-dependent eQTLs in CD4+ T cells7. This model tests for genotype-cell state interactions by modeling unique molecular identifier (UMI) counts per gene as a function of genotype at the eQTL variant. We controlled for donor- and cell-level covariates, including age, sex, gene expression PCs, genotype PCs, total UMI count, and mitochondrial UMI percentage (illustrated in Fig. 5A).
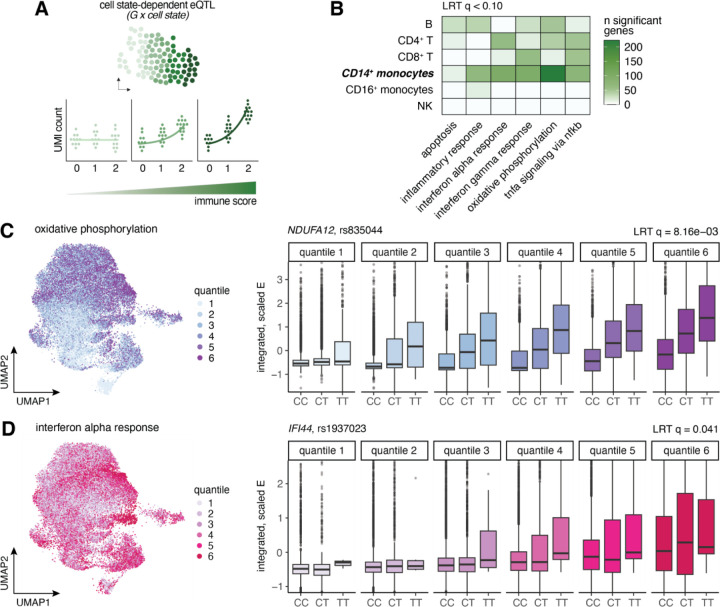
Fig. 5. Cell state-dependent single-cell eQTL are prevalent, particularly in CD14+ monocytes of COVID-19 patients.
(A) Schematic of how cell state-dependent single-cell eQTL were evaluated. For each cell type independently, a poisson mixed effects (PME) model was fit to the UMI counts for each gene, correcting for various biological and technical covariates, to test for the interaction between genotype (0, 1, 2) and various functional cell state scores (represented by the green gradient bar). In this instance, no genetic effect is observed among cells with a low immune score (light green), whereas cells with a high immune score display a substantially larger genotype effect (dark green). (B) Number of significant cell state-dependent eQTL (LRT q-value < 0.10) for each functional cell state tested (x-axis) across cell types. (C-D) UMAP visualizations of all CD14+ monocytes in COVID-19 patients colored by (C) oxidative phosphorylation score quantiles and (D) IFN-α response score quantiles (left), and examples of cell state-dependent eQTL for each of the corresponding functional pathways (right). In these examples, single-cell gene expression estimates (y-axis) are plotted by genotype and binned by cell state score quantiles for each visualization, although we treated cell state as a continuous variable in our models. The quantiles shown directly correspond to the UMAP quantile scale.
For each cell type, we focused on the top gene-SNP pairs identified as eQTLs in COVID-19 patients from the pseudobulk analysis (ranging from 1,395 genes in B cells to 2,084 genes in CD14+ monocytes) to assess cell state-dependent genotype effects. Of the six pathways considered, we detected 1,022 significant cell state-dependent interactions with genotype (likelihood ratio test [LRT] q value < 0.10) across all cell type and cell state combinations, mapping to 468 unique eGenes total (17.2% of tested genes) (Fig. 5B, Table S6). CD14+ monocytes displayed the largest number of cell state-dependent eQTL across pathways (n = 569 eGenes), while other cell types exhibited more modest state-dependent effects (n = 0 – 171 eGenes). In CD14+ monocytes, five of the six pathways were associated with over 50 state-dependent loci, including oxidative phosphorylation (n = 223), IFN-α response (n = 99), IFN-γ response (n = 98), TNF-α signaling via NF-κB (n = 73), and inflammatory response (n = 66) (Fig. 5B).
Oxidative phosphorylation stood out as the functional state most associated with dynamic state-dependent genetic effects, with 223 eGenes detected, corresponding to 10.7% of those tested. One of the top oxidative phosphorylation-dependent variants was rs835044 (LRT q = 8.2 × 10−3), a lead cis-eQTL 2 kb upstream of NDUFA12, a gene encoding the A12 subunit of mitochondrial complex I27, which shows a strong genetic effect in cells with high oxidative phosphorylation scores (quantiles 4 – 6) but virtually no genetic effect in cells with low scores (quantile 1) (Fig. 5C). Loss-of-function variants in NDUFA12 have been linked to a wide array of clinical phenotypes, most frequently a progressive neurodegenerative disorder known as Leigh syndrome27,28, suggesting that variation in A12 subunit levels can have substantial clinical consequences.
Many cell state-dependent eQTL were also found for the IFN-α and IFN-γ response pathways, with 4.0% and 3.9% of tested eGenes showing state-dependent genetic variation, respectively. One such variant was rs1937023, a lead cis-eQTL upstream of IFI44, an interferon-stimulated gene encoding interferon-induced protein 44, which only displays a genetic effect in cells with high IFN-α response scores (LRT q = 0.041) (Fig. 5D). Experimental knockout of IFI44 in mammalian airway epithelial cells led to increased respiratory syncytial virus (RSV) titers29, suggesting that variation in IFI44 levels can have functional repercussions specifically in the context of viral infection.
Cis-genetic signals colocalize with COVID-19 disease severity risk loci exclusively in COVID-19 patients
Genome-wide association studies (GWAS) provide a means to link regions of the genome with particular traits of interest, giving us the ability to uncover associations with complex disease phenotypes. Cis-genetic effects that colocalize with GWAS signals are strongly enriched for causal drivers of variation in disease susceptibility across individuals30. To evaluate whether our response eQTL may mechanistically underlie any known COVID-19 GWAS risk loci, we performed colocalization analysis using GWAS results derived from the COVID-19 Host Genetics Initiative11, a consortium that has conducted the largest COVID-19 GWAS to date13. We integrated our eQTL mapping data in healthy controls, COVID-19 patients, and follow-ups across cell types with two GWAS meta-analyses for COVID-19 disease severity phenotypes: critical illness (A2, very severe respiratory-confirmed COVID-19 versus population) and hospitalization (B2, hospitalized versus population)11 to test for common etiological genetic signals.
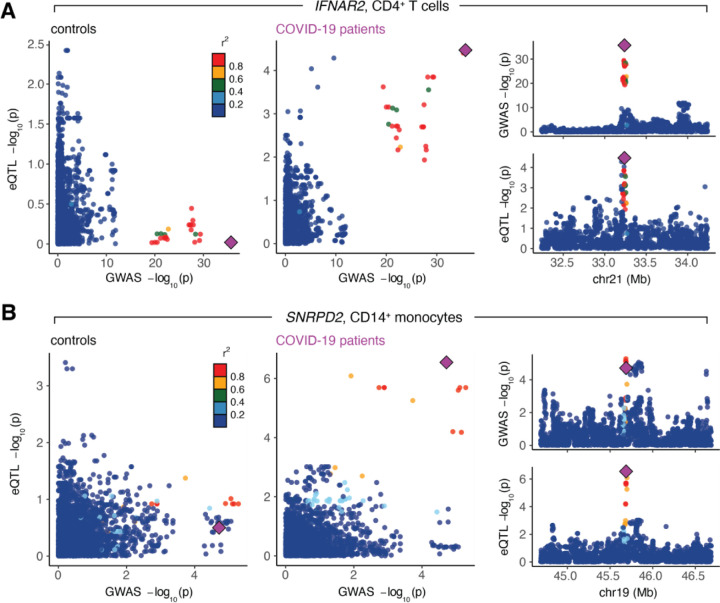
Across cell types and disease states, we detected 19 signals across 6 unique eGenes that significantly colocalized (posterior probability of colocalization [PP4] > 0.80) with critical illness or hospitalization GWAS risk loci (defined as GWAS SNP meta p-value < 1 × 10−4) (Table S7). Of these eGenes, 50% (3 out of 6) colocalized with eQTL exclusively found in COVID-19 patients: IFNAR2 in CD4+ T cells (critical illness and hospitalization GWAS), JAK1 in CD16+ monocytes (hospitalization GWAS only), and SNRPD2 in CD14+ monocytes (hospitalization GWAS only). Notably, two of these genes, JAK1 and IFNAR2, are key canonical mediators of the immune response, both playing critical roles in cytokine signal transduction and interferon response pathways31,32. The lead SNP driving the colocalization signal for IFNAR2 in CD4+ T cells of patients, rs9636867 (PP4A2 = 0.84, PP4B2 = 0.84) (Fig. 6A, right), has previously been shown to colocalize for severe COVID-19 outcomes in whole blood and CD4+ T cells of COVID-19 patients and was estimated to be causal33,34. This colocalization signature was noticeably absent in healthy controls (Fig. 6A, left) and in follow-ups (Fig. S4A). Similarly, the lead SNP driving the eQTL signal in SNRPD2, rs7246757, colocalized in CD14+ monocytes of acute COVID-19 patients (PP4B2 = 0.87) (Fig. 6B, right), and the gene itself has been implicated as a protein-protein interaction network hub gene associated with SARS-CoV-2 infection35. Again, this colocalization signature was entirely absent in control (Fig. 6B, left) and follow-up samples (Fig. S4B), indicating that variation in severe COVID-19 outcomes may, in part, be due to cis-regulatory variants that exert their effects in disease-specific and cell type-specific manners.
Fig. 6. Colocalization signals for COVID-19 disease severity phenotypes are specific to COVID-19 patients.
(A) The lead SNP for IFNAR2, rs9636867, colocalizes in CD4+ T cells for hospitalization due to severe COVID-19 in patients (right) but not controls (left). (B) The lead SNP for SNRPD2, rs7246757, colocalizes in CD14+ monocytes for hospitalization due to severe COVID-19 in patients (right) but not controls (left). For both (A) and (B), the larger plots on the left show the correlation between GWAS p-values (x-axis) and eQTL p-values (y-axis) in controls and patients. The smaller plots on the right show Manhattan plots for the GWAS signal (top) and the eQTL signal in the COVID-19 patients (bottom). The lead SNP is depicted as a purple diamond.
Discussion
Prior studies have leveraged in vitro pathogen challenges and immune stimulations to probe gene regulatory variation in cells, reporting hundreds of response eQTL in different infection contexts1–5,36,37. This experimental approach involves the isolation and culture of primary immune cells from healthy donors, which are then subsequently challenged in laboratory settings. Unlike previous immune response eQTL studies, here we measure genetic effects directly in cells derived from patients responding to a pathogen, revealing considerable context specificity in genetic regulation that is arguably more relevant to disease associations than that measured in controlled in vitro systems. We show that cell type-specific, disease state-specific, and cell state-dependent genetic variation is abundant, affecting 25.6% of all genes tested across cell types and disease states and is particularly common in CD14+ monocytes and NK cells. Further, we establish that single cells can harbor distinct genetic effects that are dependent on their underlying immunological or metabolic functional states and that, in certain cases, these continuous states are associated with clinical features of patients. More broadly, genetic interaction effects likely play a role in dynamically modulating immune responses throughout the course of an infection and may also contribute to differential disease outcomes, especially considering the fact that monocytes, and more generally cells in the myeloid compartment, are susceptible to immune dysregulation following SARS-CoV-2 infection38,39.
Of particular clinical interest is biological variation in the interferon response, a critical antiviral pathway induced upon the detection of viral pattern recognition receptors. This response involves the induction of IFNs, a group of cytokines that directly inhibit viral replication and activate bystander immune cells, such as dendritic cells and monocytes40. Variation in the timing and magnitude of the IFN response across individuals is well-documented, particularly in the context of SARS-CoV-2 infection41–44. Multiple studies have linked this variation with differences in COVID-19 severity and disease progression, revealing a dual role for IFNs in the clinical course of COVID-1945,46. In the blood, the upregulation of type I IFNs and IFN-stimulated genes (ISGs) shortly after initial infection is associated with protection21, but their delayed induction is a hallmark of severe disease47–49. Sustained IFN signaling has also been shown to inhibit the development of appropriate antibody responses, ultimately leading to increased disease pathology and severity44. We also observe a relationship between IFN signaling and severity, with milder COVID-19 cases displaying elevated expression of IFN-α and IFN-γ response genes specifically in T cells and CD14+ monocytes.
Of note, we detect 224 eGenes (47.8% of all state-dependent eGenes identified) across cell types with expression levels simultaneously dependent on both underlying genetic variation and the magnitude of the IFN response itself, revealing it to be one of the pathways most associated with cell state-dependent genetic interaction effects. This finding only adds to the complexity of how dynamic immune response variation is connected to variation in molecular traits, here through an interaction with host genetics, which may ultimately have downstream effects on disease phenotypes. Indeed, we find that IFN response scores calculated at the single-cell level correlate with patient severity. Together, our results argue that gene-environment interactions are abundant and likely play a direct role in the clinical setting.
While we identify only a handful of colocalizing eQTL, of the eGenes that colocalize with COVID-19 disease severity phenotypes, half are detected only in COVID-19 patients, indicating that SARS-CoV-2 infection is necessary to induce these signals. Similar disease state-dependent colocalization has been described previously, with the variant rs8176719 colocalizing only in T effector memory cells 16 hours post-anti-CD3/CD28 stimulation for both severity and susceptibility COVID-19 GWAS at the RALGDS2 locus33. In the same study, the intronic risk variant in IFNAR2, rs9636867, the same lead SNP-eGene pair for which we identify a patient-specific colocalization signal in CD4+ T cells, colocalized with severe COVID-19 disease only for symptomatic individuals who were SARS-CoV-2+ in CD4+ T cells33. A different COVID-19-associated intronic risk variant in IFNAR2, rs13050728, has also been shown to increase IFNAR2 expression in classical monocytes specifically in COVID-19 patients compared to healthy controls in an independent study14.
These findings highlight the role that context specificity plays in the genetic regulation of disease associated-traits and stress the importance of measuring molecular phenotypes in pertinent environmental conditions and cell types. They also raise the question of how gene-environment interactions may contribute to the problem of missing heritability, the phenomenon in which only a small fraction of overall trait heritability is explained by trait-associated variants50,51. Although trait-associated loci are enriched for eQTL52, only ~40% of GWAS variants colocalize with eQTL in relevant tissues, which drops to ~20% for autoimmune trait GWAS53,54. More recently, trait mapping studies have been extended to incorporate a larger array of quantitative traits, including alternative splicing55, chromatin accessibility, and histone modification levels37. The inclusion of alternative regulatory mechanisms has significantly increased the number of colocalizing loci and heritability estimates of GWAS phenotypes, yet a large proportion of heritability remains unexplained, potentially due to context-specific gene-environment interactions.
Although we have described how gene-environment interactions can shape immune responses in one specific viral infection setting, it is necessary to define how such effects contribute to a wider range of disease states and environmental contexts to better understand the genetic and environmental underpinnings of immune response variation across individuals. As the number of patient cohorts with single-cell phenotyping and genotyping data rise, it will be important to extend this framework to other single-cell eQTL mapping studies to measure the full extent of cell state-dependent regulatory heterogeneity.
Supplementary Material
Acknowledgements:
We thank all study participants and the clinical research teams for their contributions. We thank members of the Barreiro lab for their constructive comments and feedback. This work was completed in part with resources provided by the University of Chicago Research Computing Center. Support from Calcul Québec and Compute Canada is additionally acknowledged. We thank the University of Chicago Genomics Facility (RRID: SCR_019196) for their assistance with sequencing. Figures 1A and 5A were created with BioRender.com.
Funding:
This work is supported by grant R01-GM134376 and R35-GM152227 to L.B.B. and by Canadian Institutes of Health Research (CIHR) grants VR2-173203 and 178344 to D.E.K. and A.F. We also acknowledge the support from the UChicago DDRCC, Center for Interdisciplinary Study of Inflammatory Intestinal Disorders (C-IID) (NIDDK P30 DK042086). H.E.R. was supported by a Ruth L. Kirschstein National Research Service Award (NHLBI F31-HL156419). E.B.R. was a recipient of a COVID-19 Excellence Scholarship from the Université de Montréal. T.N. is supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists (22KJ1190, 22J30004). J.B.R.’s research group is supported by the Canadian Institutes of Health Research (CIHR: 365825; 409511, 100558, 169303), the McGill Interdisciplinary Initiative in Infection and Immunity (MI4), the Lady Davis Institute of the Jewish General Hospital, the Jewish General Hospital Foundation, the Canadian Foundation for Innovation, the NIH Foundation, Genome Québec, the Public Health Agency of Canada, McGill University, Cancer Research UK [grant umber C18281/A29019] and the Fonds de Recherche Québec Santé (FRQS). J.B.R. is supported by a FRQS Mérite Clinical Research Scholarship. TwinsUK is funded by the Welcome Trust, Medical Research Council, European Union, the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility and Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London. These funding agencies had no role in the design, implementation, or interpretation of this study. The Biobanque Québécoise de la COVID-19 (BQC19) is supported by the FRQS Génome Québec and the Public Health Agency of Canada.
Footnotes
Disclosures/competing interests: J.B.R.’s institution has received investigator-initiated grant funding from Roche, Eli Lilly, GlaxoSmithKline, and Biogen for projects unrelated to this research. J.B.R. is the CEO of, and holds shares in, 5 Prime Sciences (www.5primesciences.com), which provides research services for biotech, pharma, and venture capital companies to enable genetics-based drug development.
Data and materials availability:
Further information and requests for resources should be directed to Luis B. Barreiro (lbarreiro@uchicago.edu). Raw data contained in BQC19, including whole genome sequencing files, are stored on SecureData4Health (https://www.sd4health.ca/), and are accessible via BQC19’s access procedures. To access these files, a data access request must be submitted. Instructions on how to submit this request are available at https://www.bqc19.ca/en/access-data. Researchers from both academia and private entities are eligible to apply, and the research project must be approved by a research ethics board. A Data Access Committee will then review the application, and the data will be made available to the applicant upon approval.
References
- 1.Lee M. N. et al. Common Genetic Variants Modulate Pathogen-Sensing Responses in Human Dendritic Cells. Science 343, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nédélec Y. et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell 167, 657–669.e21 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Quach H. et al. Genetic Adaptation and Neandertal Admixture Shaped the Immune System of Human Populations. Cell 167, 643–656.e17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randolph H. E. et al. Genetic ancestry effects on the response to viral infection are pervasive but cell type specific. Science 374, 1127–1133 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aquino Y.. et al. Dissecting human popul [Google Scholar]
- 6.van der Wijst M. et al. The single-cell eQTLGen consortium. eLife 9, e52155 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan A. et al. Single-cell eQTL models reveal dynamic T cell state dependence of disease loci. Nature 1–9 (2022) doi: 10.1038/s41586-022-04713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A. et al. Dynamic regulatory elements in single-cell multimodal data implicate key immune cell states enriched for autoimmune disease heritability. Nat Genet 1–11 (2023) doi: 10.1038/s41588-023-01577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.-X. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 26, 1200–1204 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Zhou F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niemi M. E. K. et al. Mapping the human genetic architecture of COVID-19. Nature 600, 472–477 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Severe Covid-19 GWAS Group. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. New England Journal of Medicine 383, 1522–1534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.A second update on mapping the human genetic architecture of COVID-19. Nature 621, E7–E26 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edahiro R. et al. Single-cell analyses and host genetics highlight the role of innate immune cells in COVID-19 severity. Nat Genet 1–15 (2023) doi: 10.1038/s41588-023-01375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzgerald-Bocarsly P., Dai J. & Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 19, 3–19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poli A. et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 126, 458–465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van der Sluis R. M., Holm C. K. & Jakobsen M. R. Plasmacytoid dendritic cells during COVID-19: Ally or adversary? Cell Rep 40, 111148 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zafarani A. et al. Natural killer cells in COVID-19: from infection, to vaccination and therapy. Future Virol 10.2217/fvl-2022-0040 doi: 10.2217/fvl-2022-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rébillard R.-M. et al. Identification of SARS-CoV-2–specific immune alterations in acutely ill patients. J Clin Invest 131, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadjadj J. et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberzon A. et al. The Molecular Signatures Database Hallmark Gene Set Collection. cels 1, 417–425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo J.-S. et al. SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nat Commun 12, 6602 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbut S. M., Wang G., Carbonetto P. & Stephens M. Flexible statistical methods for estimating and testing effects in genomic studies with multiple conditions. Nat Genet 51, 187–195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Chacón R., Achiriloaie M., Janz R., Albanesi J. P. & Südhof T. C. SCAMP1 Function in Endocytosis*. Journal of Biological Chemistry 275, 12752–12756 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Torraco A. et al. Novel NDUFA12 variants are associated with isolated complex I defect and variable clinical manifestation. Hum Mutat 42, 699–710 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Magrinelli F. et al. Biallelic Loss-of-Function NDUFA12 Variants Cause a Wide Phenotypic Spectrum from Leigh/Leigh-Like Syndrome to Isolated Optic Atrophy. Movement Disorders Clinical Practice 9, 218–228 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse D. C. et al. Interferon-Induced Protein 44 and Interferon-Induced Protein 44-Like Restrict Replication of Respiratory Syncytial Virus. J Virol 94, e00297–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hormozdiari F. et al. Colocalization of GWAS and eQTL Signals Detects Target Genes. Am J Hum Genet 99, 1245–1260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Platanias L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5, 375–386 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Shemesh M., Lochte S., Piehler J. & Schreiber G. IFNAR1 and IFNAR2 play distinct roles in initiating type I interferon-induced JAK-STAT signaling and activating STATs. Sci Signal 14, eabe4627 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Willett J. D. S. et al. Colocalization of expression transcripts with COVID-19 outcomes is rare across cell states, cell types and organs. Hum. Genet. 142, 1461–1476 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Antonio M. et al. SARS-CoV-2 susceptibility and COVID-19 disease severity are associated with genetic variants affecting gene expression in a variety of tissues. Cell Reports 37, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosharaf M. P. et al. Computational identification of host genomic biomarkers highlighting their functions, pathways and regulators that influence SARS-CoV-2 infections and drug repurposing. Sci Rep 12, 4279 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairfax B. P. et al. Innate Immune Activity Conditions the Effect of Regulatory Variants upon Monocyte Gene Expression. Science 343, 1246949 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aracena K. A. et al. Epigenetic variation impacts individual differences in the transcriptional response to influenza infection. Nat Genet 56, 408–419 (2024). [DOI] [PubMed] [Google Scholar]
- 38.Schulte-Schrepping J. et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 182, 1419–1440.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knoll R., Schultze J. L. & Schulte-Schrepping J. Monocytes and Macrophages in COVID-19. Frontiers in Immunology 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNab F., Mayer-Barber K., Sher A., Wack A. & O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol 15, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C. et al. Time-resolved Systems Immunology Reveals a Late Juncture Linked to Fatal COVID-19. Cell 0, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernardes J. P. et al. Longitudinal multi-omics analyses identify responses of megakaryocytes, erythroid cells and plasmablasts as hallmarks of severe COVID-19 trajectories. Immunity 0, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galani I.-E. et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nature Immunology 1–9 (2020) doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 44.Brunet-Ratnasingham E. et al. Sustained IFN signaling is associated with delayed development of SARS-CoV-2-specific immunity. Nat Commun 15, 4177 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson Akusjärvi S. & Zanoni I. Yin and yang of interferons: lessons from the coronavirus disease 2019 (COVID-19) pandemic. Current Opinion in Immunology 87, 102423 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zanoni I. Interfering with SARS-CoV-2: are interferons friends or foes in COVID-19? Current Opinion in Virology (2021) doi: 10.1016/j.coviro.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou S. et al. A Neanderthal OAS1 isoform protects individuals of European ancestry against COVID-19 susceptibility and severity. Nature Medicine 1–9 (2021) doi: 10.1038/s41591-021-01281-1. [DOI] [PubMed] [Google Scholar]
- 48.Blanco-Melo D. et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 181, 1036–1045.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucas C. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuk O., Hechter E., Sunyaev S. R. & Lander E. S. The mystery of missing heritability: Genetic interactions create phantom heritability. Proceedings of the National Academy of Sciences 109, 1193–1198 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manolio T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicolae D. L. et al. Trait-Associated SNPs Are More Likely to Be eQTLs: Annotation to Enhance Discovery from GWAS. PLOS Genetics 6, e1000888 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chun S. et al. Limited statistical evidence for shared genetic effects of eQTLs and autoimmune-disease-associated loci in three major immune-cell types. Nat Genet 49, 600–605 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu Z. et al. The impact of cell type and context-dependent regulatory variants on human immune traits. Genome Biology 22, 122 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tremblay K. et al. The Biobanque québécoise de la COVID-19 (BQC19)—A cohort to prospectively study the clinical and biological determinants of COVID-19 clinical trajectories. PLOS ONE 16, e0245031 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auton A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das S. et al. Next-generation genotype imputation service and methods. Nat Genet 48, 1284–1287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taliun D. et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature 590, 290–299 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun 8, 14049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heaton H. et al. Souporcell: robust clustering of single-cell RNA-seq data by genotype without reference genotypes. Nature Methods 17, 615–620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stuart T. et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hafemeister C. & Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biology 20, 296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ritchie M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47–e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hänzelmann S., Castelo R. & Guinney J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 14, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storey J. D. & Tibshirani R. Statistical significance for genomewide studies. PNAS 100, 9440–9445 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korotkevich G. et al. Fast gene set enrichment analysis. 060012 Preprint at 10.1101/060012 (2021). [DOI] [Google Scholar]
- 69.Shabalin A. A. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics 28, 1353–1358 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng X. et al. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 28, 3326–3328 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baglama J., Reichel L. & Lewis B. W. irlba: Fast Truncated Singular Value Decomposition and Principal Components Analysis for Large Dense and Sparse Matrices. (2022). [Google Scholar]
- 73.Bates D., Mächler M., Bolker B. & Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67, 1–48 (2015). [Google Scholar]
- 74.Storey J. D., Bass A. J., Dabney A., Robinson D. & Warnes G. qvalue: Q-value estimation for false discovery rate control. Bioconductor version: Release (3.16) 10.18129/B9.bioc.qvalue (2023). [DOI] [Google Scholar]
- 75.Wang G., Sarkar A., Carbonetto P. & Stephens M. A Simple New Approach to Variable Selection in Regression, with Application to Genetic Fine Mapping. Journal of the Royal Statistical Society Series B: Statistical Methodology 82, 1273–1300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu B., Gloudemans M. J., Rao A. S., Ingelsson E. & Montgomery S. B. Abundant associations with gene expression complicate GWAS follow-up. Nat Genet 51, 768–769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Further information and requests for resources should be directed to Luis B. Barreiro (lbarreiro@uchicago.edu). Raw data contained in BQC19, including whole genome sequencing files, are stored on SecureData4Health (https://www.sd4health.ca/), and are accessible via BQC19’s access procedures. To access these files, a data access request must be submitted. Instructions on how to submit this request are available at https://www.bqc19.ca/en/access-data. Researchers from both academia and private entities are eligible to apply, and the research project must be approved by a research ethics board. A Data Access Committee will then review the application, and the data will be made available to the applicant upon approval.