Abstract
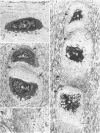
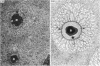
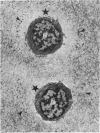
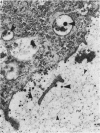
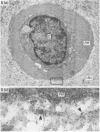
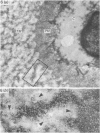
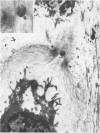
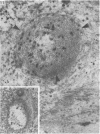
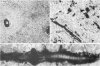
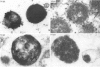
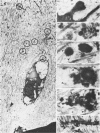
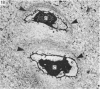
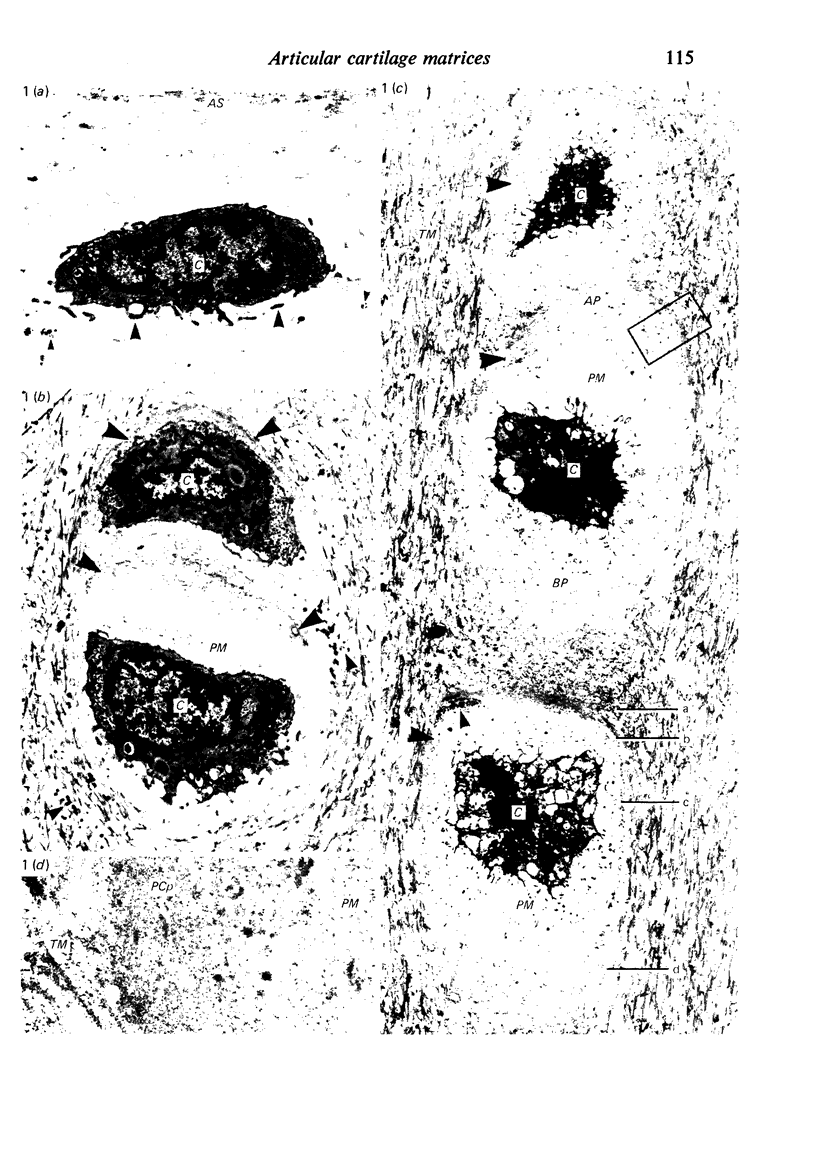
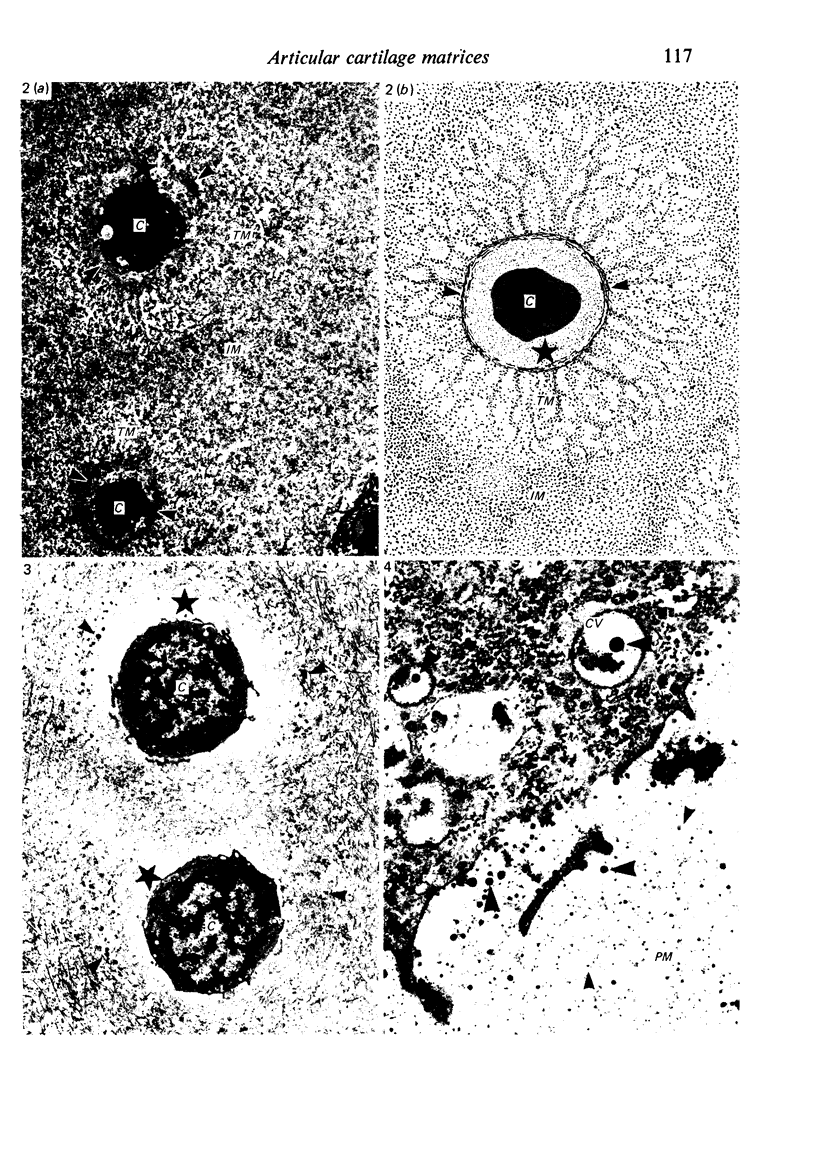
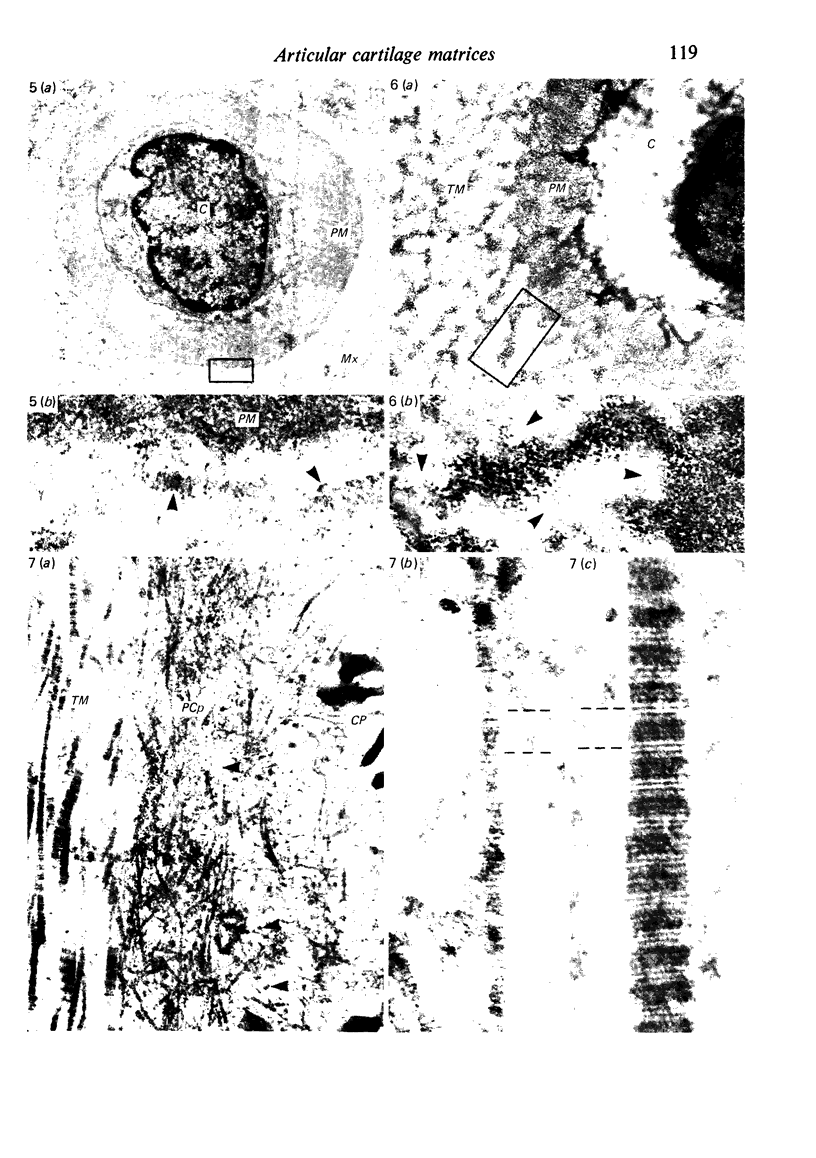
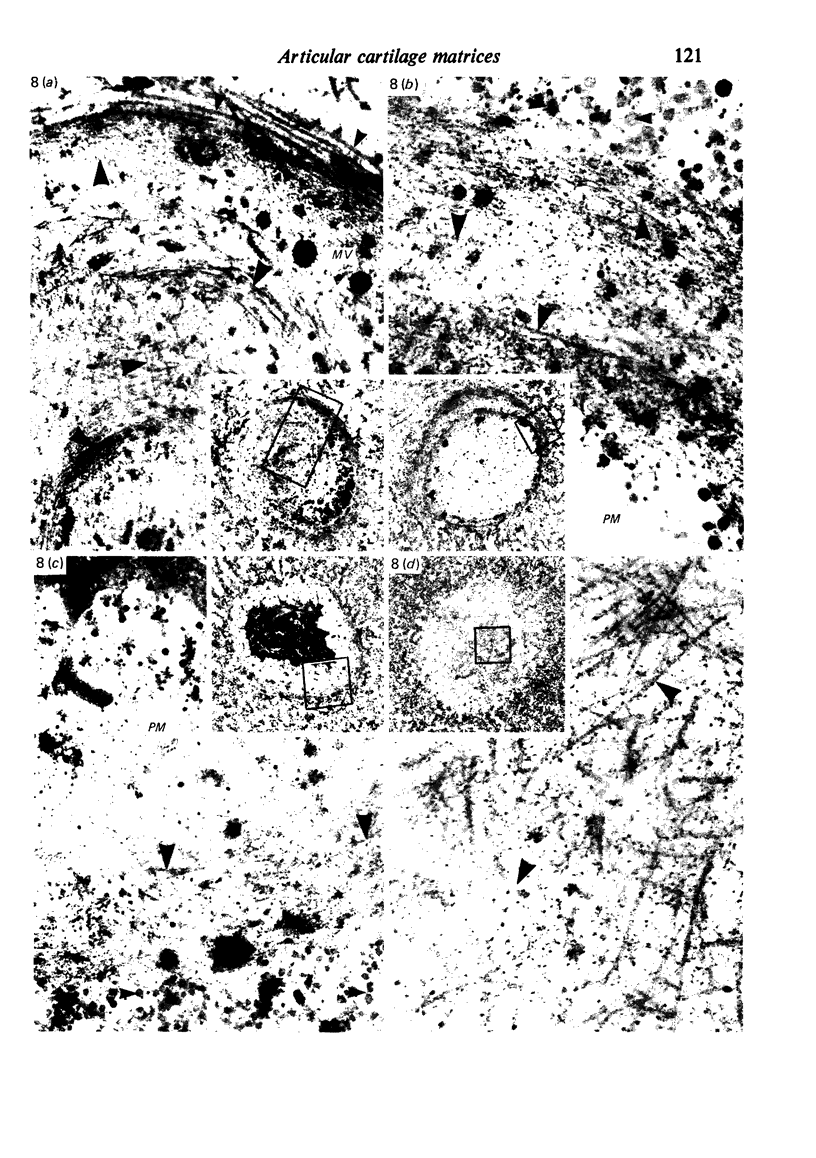
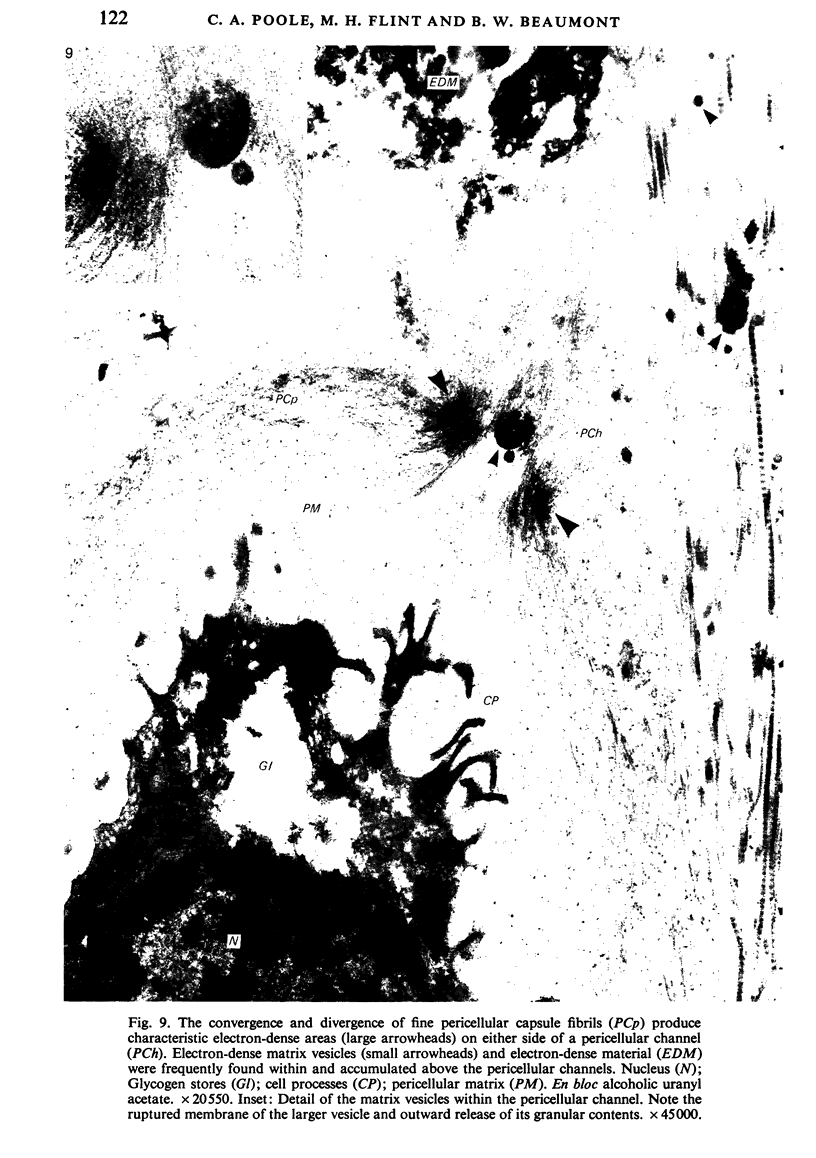
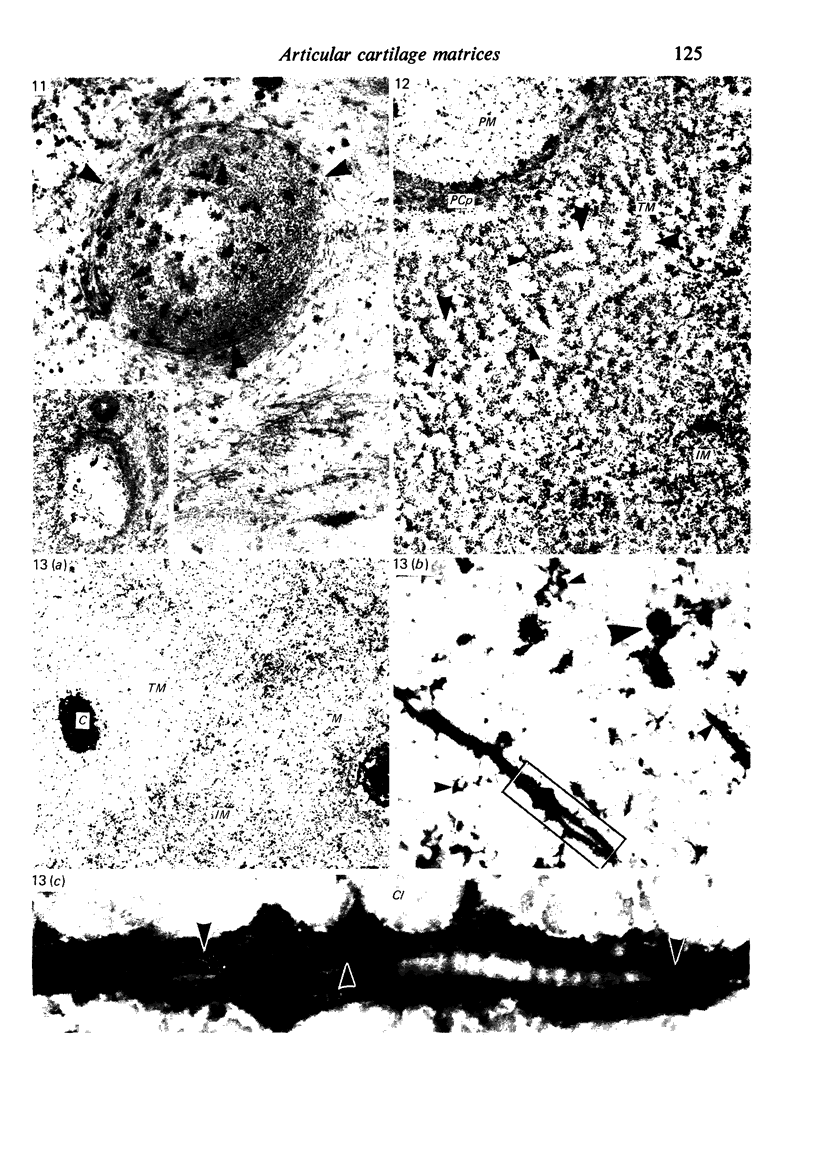
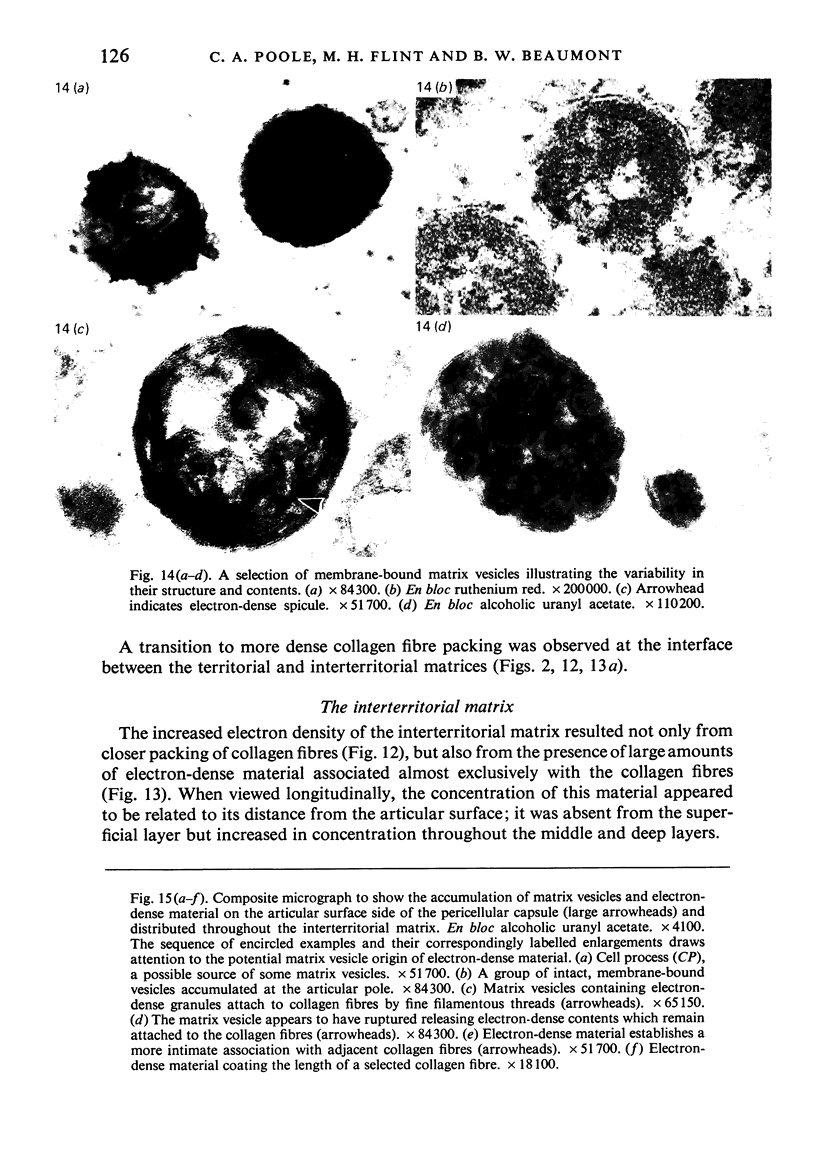
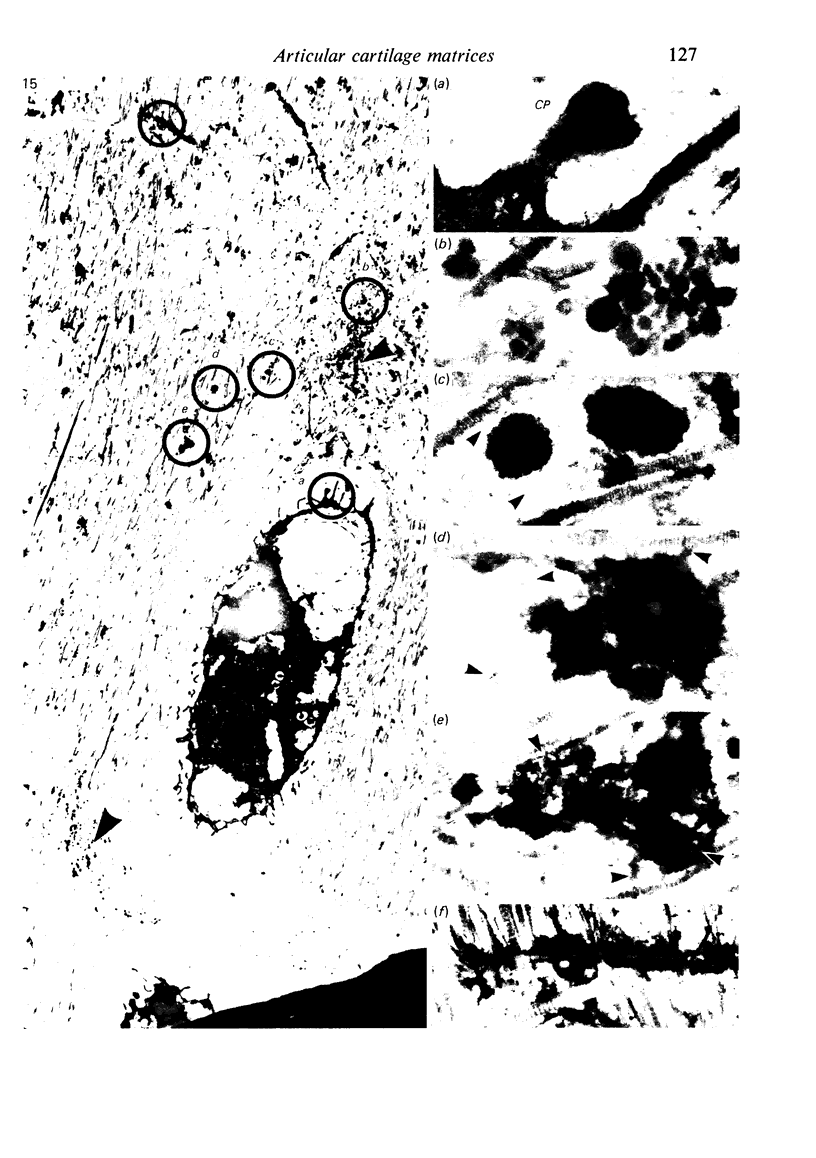
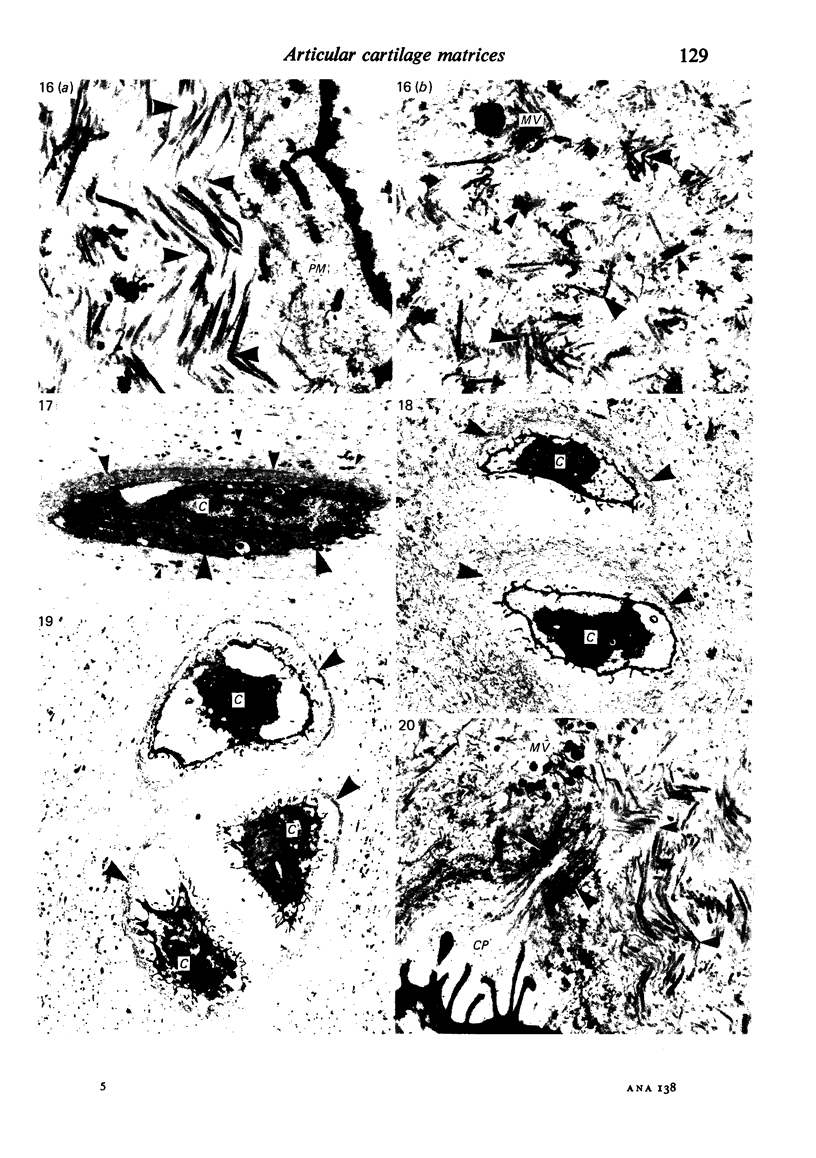
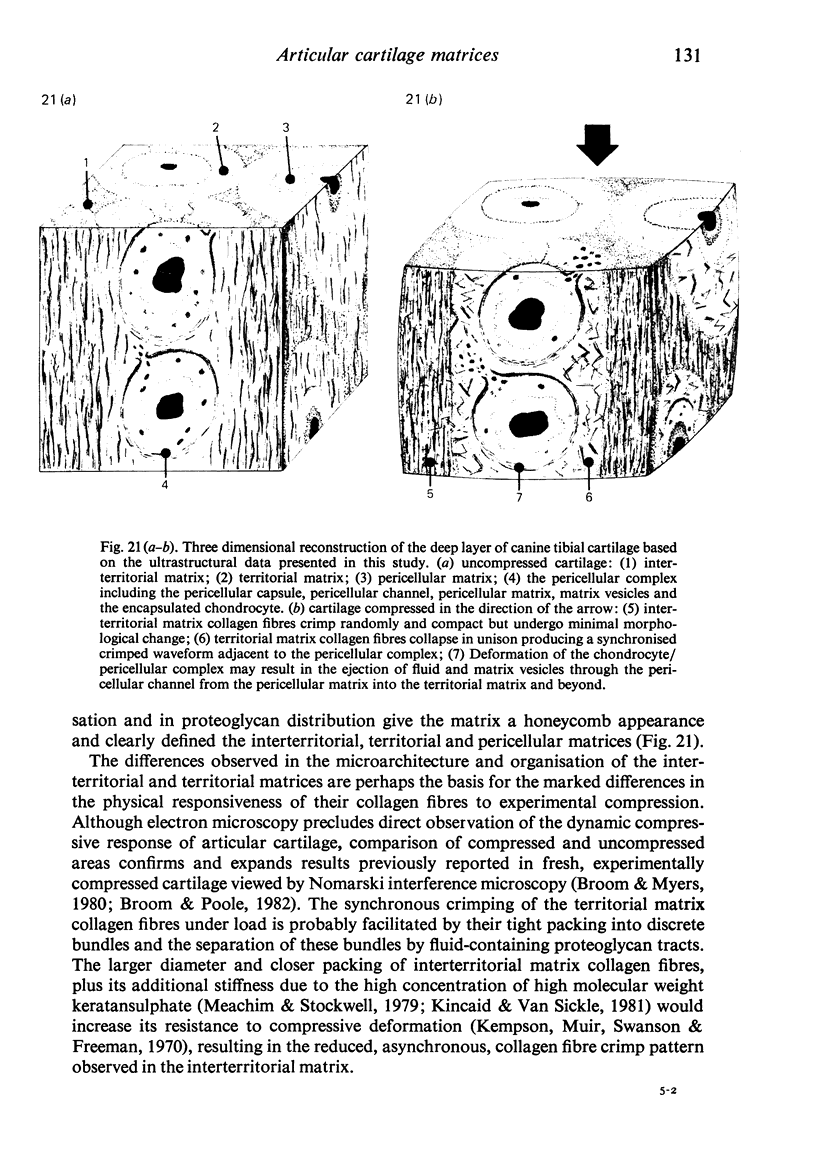
The pericellular, territorial and interterritorial matrices of canine tibial cartilage have been identified ultrastructurally on the basis of their collagen fibre density and organisation, proteoglycan distribution and their structural response to experimentally applied compressive loads. In addition, a discrete pericellular capsule composed of fine, faintly banded fibrils is described which surrounds and encloses the pericellular matrix and chondrocytes of the middle and deep layers but not of the superficial layer. It is suggested that the fine fibrils which comprise this pericellular capsule represent some of the new minor collagen species recently localised in a similar position in hyaline cartilages. The densely compacted cupola which forms the articular pole of the capsule is frequently penetrated by a clearly defined pericellular channel, consistently orientated in the direction of the articular surface. Membrane-bound vesicles are observed in the pericellular matrix, within the lumen of the pericellular channel and accumulated in the territorial matrix immediately beyond the pericellular channel. The constancy of this distribution pattern strongly suggests a flow of material through the pericellular channel from the pericellular matrix to the territorial matrix and beyond, possibly in response to minute pressure gradients generated during compressive deformation of the non-distensible capsule. Furthermore, it is suggested that the random dispersal and subsequent rupture of matrix vesicles may represent a mechanism whereby chondrocytes, with limited mobility, could exercise homeostatic control over the cartilage matrix at some distance from the cell. Chondrocytes in the deeper layers of canine tibial cartilage are each surrounded by three distinct compartments, a pericellular matrix and capsule, a territorial matrix and an interterritorial matrix. The response of each of these concentric compartments to experimental load suggests that they function synergistically to produce an integrated, biological, hydro-elastic suspension system capable of resisting physiological compression.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S., Abedin M. Z., Grundy S. M., Weiss J. B. Isolation and characterisation of an unusual collagen from hyaline cartilage and intervertebral disc. FEBS Lett. 1981 Jan 26;123(2):195–199. doi: 10.1016/0014-5793(81)80286-4. [DOI] [PubMed] [Google Scholar]
- Ayad S., Abedin M. Z., Weiss J. B., Grundy S. M. Characterisation of another short-chain disulphide-bonded collagen from cartilage, vitreous and intervertebral disc. FEBS Lett. 1982 Mar 22;139(2):300–304. doi: 10.1016/0014-5793(82)80875-2. [DOI] [PubMed] [Google Scholar]
- Bab I., Sela J., Stein H. Transplantation of free perichondrial grafts into rabbit articular cartilage is associated with matrix vesicle calcification. Acta Anat (Basel) 1982;113(1):53–60. doi: 10.1159/000145537. [DOI] [PubMed] [Google Scholar]
- Barland P., Janis R., Sandson J. Immunofluorescent studies of human articular cartilage. Ann Rheum Dis. 1966 Mar;25(2):156–164. doi: 10.1136/ard.25.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom N. D., Myers D. B. A study of the structural response of wet hyaline cartilage to various loading situations. Connect Tissue Res. 1980;7(4):227–237. doi: 10.3109/03008208009152358. [DOI] [PubMed] [Google Scholar]
- Broom N. D., Poole C. A. A functional-morphological study of the tidemark region of articular cartilage maintained in a non-viable physiological condition. J Anat. 1982 Aug;135(Pt 1):65–82. [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Hebda P. A., Morris N. P., Hollister D. W. Human cartilage collagens. Comparison of cartilage collagens with human type V collagen. J Biol Chem. 1982 Jul 10;257(13):7852–7856. [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Clarke I. C. Articular cartilage: a review and scanning electron microscope study. II. The territorial fibrillar architecture. J Anat. 1974 Nov;118(Pt 2):261–280. [PMC free article] [PubMed] [Google Scholar]
- DAVIES D. V., BARNETT C. H., COCHRANE W., PALFREY A. J. Electron microscopy of articular cartilage in the young adult rabbit. Ann Rheum Dis. 1962 Mar;21:11–22. doi: 10.1136/ard.21.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duance V. C., Shimokomaki M., Bailey A. J. Immunofluorescence localization of type-M collagen in articular cartilage. Biosci Rep. 1982 Apr;2(4):223–227. doi: 10.1007/BF01136720. [DOI] [PubMed] [Google Scholar]
- GHADIALLY F. N., MEACHIM G., COLLINS D. H. EXTRA-CELLULAR LIPID IN THE MATRIX OF HUMAN ARTICULAR CARTILAGE. Ann Rheum Dis. 1965 Mar;24:136–146. doi: 10.1136/ard.24.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially F. N., Lalonde J. M. Intramatrical lipidic debris and calcified bodies in human semilunar cartilages. J Anat. 1981 Jun;132(Pt 4):481–490. [PMC free article] [PubMed] [Google Scholar]
- Kardos T. B., Hubbard M. J. Are matrix vesicles apoptotic bodies? Prog Clin Biol Res. 1982;101:45–60. [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Kincaid S. A., Van Sickle D. C. Regional histochemical and thickness variations of adult canine articular cartilage. Am J Vet Res. 1981 Mar;42(3):428–432. [PubMed] [Google Scholar]
- Lane J. M., Weiss C. Review of articular cartilage collagen research. Arthritis Rheum. 1975 Nov-Dec;18(6):553–562. doi: 10.1002/art.1780180605. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Lust G., Pronsky W., Sherman D. M. Biochemical and ultrastructural observations in normal and degenerative canine articular cartilage. Am J Vet Res. 1972 Dec;33(12):2429–2440. [PubMed] [Google Scholar]
- Lust G., Sherman D. M. Metabolic and ultrastructural studies on articular cartilage of developing canine hip joints. Cornell Vet. 1973 Jan;63(1):94–105. [PubMed] [Google Scholar]
- Mason R. M. Recent advances in the biochemistry of hyaluronic acid in cartilage. Prog Clin Biol Res. 1981;54:87–112. [PubMed] [Google Scholar]
- Rabinovitch A. L., Anderson H. C. Biogenesis of matrix vesicles in cartilage growth plates. Fed Proc. 1976 Feb;35(2):112–116. [PubMed] [Google Scholar]
- Reese C. A., Wiedemann H., Kühn K., Mayne R. Characterization of a highly soluble collagenous molecule isolated from chicken hyaline cartilage. Biochemistry. 1982 Mar 2;21(5):826–830. doi: 10.1021/bi00534a002. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S., Hartmann D. J., Herbage D., Payen-Meyran C., Ville G. Biochemical properties and immunolocalization of minor collagens in foetal calf cartilage. FEBS Lett. 1982 Sep 20;146(2):343–347. doi: 10.1016/0014-5793(82)80949-6. [DOI] [PubMed] [Google Scholar]
- Roth V., Mow V. C. The intrinsic tensile behavior of the matrix of bovine articular cartilage and its variation with age. J Bone Joint Surg Am. 1980 Oct;62(7):1102–1117. [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of two further collagenous fractions from articular cartilage. Biosci Rep. 1981 Jul;1(7):561–570. doi: 10.1007/BF01116305. [DOI] [PubMed] [Google Scholar]
- Silberberg R. Ultrastructure of articular cartilage in health and disease. Clin Orthop Relat Res. 1968 Mar-Apr;57:233–257. [PubMed] [Google Scholar]
- Slavkin H. C., Croissant R. D., Bringas P., Matosian P., Wilson P., Mino W., Guenther H. Matrix vesicle heterogeneity: possible morphogenetic functions for matrix vesicles. Fed Proc. 1976 Feb;35(2):127–134. [PubMed] [Google Scholar]
- Stein H., Bab I. A., Sela J. The occurrence of hydroxyapatite crystals in extracellular matrix vesicles after surgical manipulation of the rabbit knee joint. Cell Tissue Res. 1981;214(3):449–454. doi: 10.1007/BF00233487. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Observations on the acid glycosaminoglycan (mucopolysaccharide) content of the matrix of aging cartilage. Ann Rheum Dis. 1965 Jul;24(4):341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Rosenberg L., Helfet A. J. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg Am. 1968 Jun;50(4):663–674. doi: 10.2106/00004623-196850040-00002. [DOI] [PubMed] [Google Scholar]
- Wiltberger H., Lust G. Ultrastructure of canine articular cartilage: comparison of normal and degenerative (osteoarthritic) hip joints. Am J Vet Res. 1975 Jun;36(6):727–740. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]