Abstract
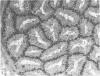
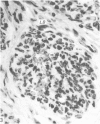
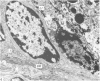
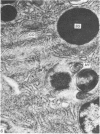
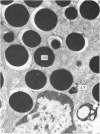
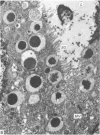
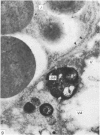
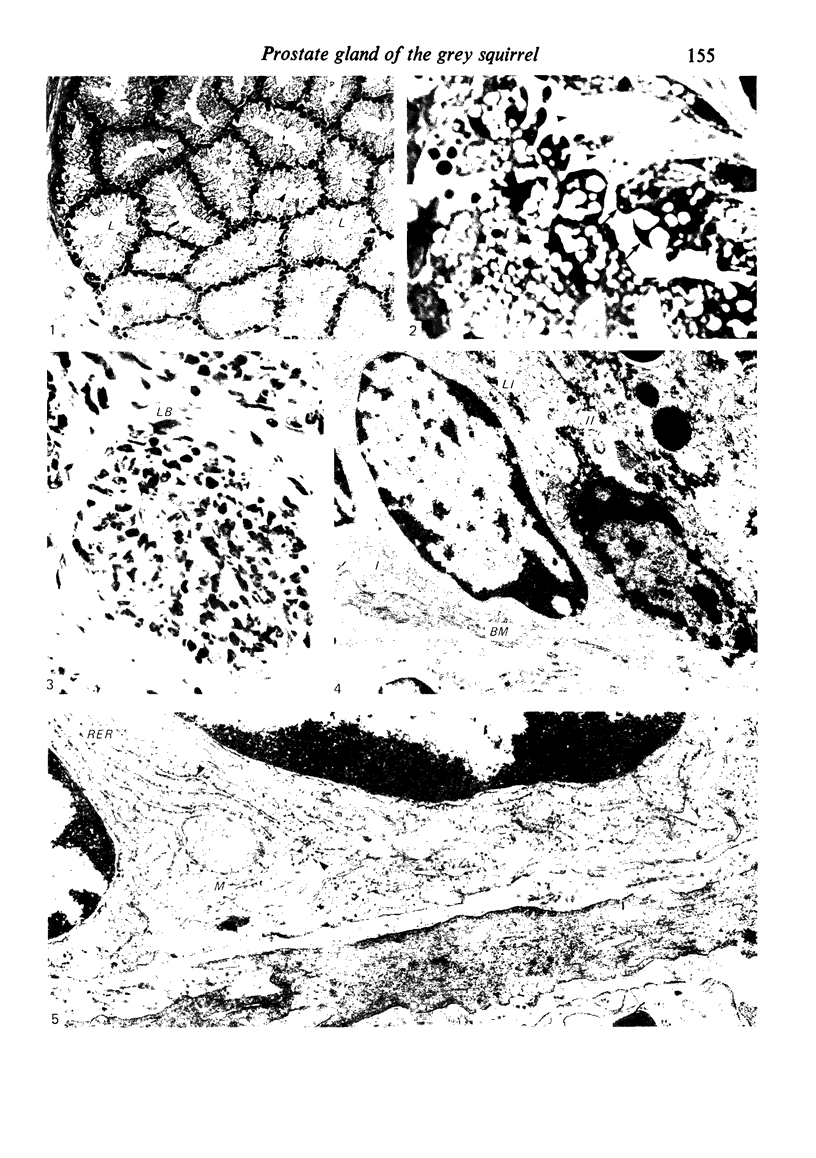
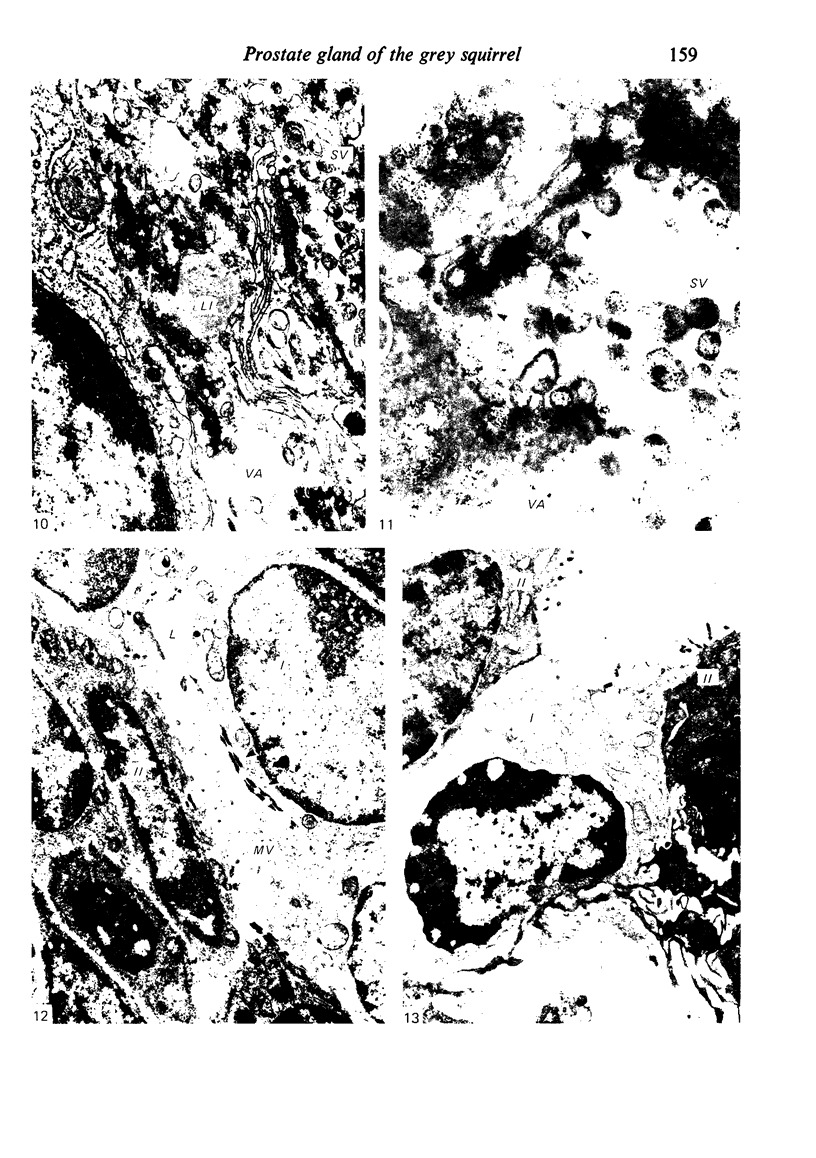
Grey squirrels were obtained from the wild every month for two calendar years. The prostate gland was found to be a single lobed structure, subdivided into many lobules each of which was composed of numerous secretory acini with central lumina. Secretion was active throughout the breeding season (January to June), but the gland became atrophic from July to September, and recovered between October and December. Two 'types' of secretory cells were observed in the secretory epithelium throughout the year in both adults and juveniles. During the secretory period 'Type I' cells were characterised by a large nucleus and abundant granular endoplasmic reticulum, ribosomes and secretory granules. The vacuolated 'Type II' cells were more abundant and possessed a smaller nucleus, more substantial Golgi apparatus and numerous secretory vesicles. In the typically atrophic gland almost all organelles associated with secretory activity disappeared, but both types of cells could still be distinguished by their peculiar nuclei and even by their characteristic light and dark cytoplasm in tissue fixed in glutaraldehyde. Recovery of the prostate gland was preceded by a wave of mitotic activity lasting from October to December. However, secretory activity was not resumed until the following January. The two morphological forms were either two functional phases of a single cell type or two distinct populations of secretory cells. Whichever may be the case the prostate gland of the grey squirrel is unique. No other animal has yet been observed to possess secretory cells capable of passing from one morphological and functional phase to the next. If there are two types of secretory cell within this single lobed structure, the organisation of the grey squirrel prostate gland differs from that in the rat and in man where each lobe contains only one single type of secretory cell.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRANDES D., KIRCHHEIM D., SCOTT W. W. ULTRASTRUCTURE OF THE HUMAN PROSTATE: NORMAL AND NEOPLASTIC. Lab Invest. 1964 Dec;13:1541–1560. [PubMed] [Google Scholar]
- BRANDES D., PORTELA A. The fine structure of the epithelial cells of the mouse prostate. II. Ventral lobe epithelium. J Biophys Biochem Cytol. 1960 Jun;7:511–514. doi: 10.1083/jcb.7.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl E., Kjaerheim A. The ultrastructure of the accessory sex organs of the male rat. II. The post-castration involution of the ventral, lateral and the dorsal prostate. Z Zellforsch Mikrosk Anat. 1973 Nov 5;144(2):167–178. doi: 10.1007/BF00307300. [DOI] [PubMed] [Google Scholar]
- Dahl E., Kjaerheim A., Tveter K. J. The ultrastructure of the accessory sex organs of the male rat. I. Normal structure. Z Zellforsch Mikrosk Anat. 1973 Feb 23;137(3):345–359. doi: 10.1007/BF00307208. [DOI] [PubMed] [Google Scholar]
- FISHER E. R., JEFFREY W. ULTRASTRUCTURE OF HUMAN NORMAL AND NEOPLASTIC PROSTATE; WITH COMMENTS RELATIVE TO PROSTATIC EFFECTS OF HORMONAL STIMULATION IN THE RABBIT. Am J Clin Pathol. 1965 Aug;44:119–134. doi: 10.1093/ajcp/44.2.119. [DOI] [PubMed] [Google Scholar]
- GUNN S. A., GOULD T. C. A correlative anatomical and functional study of the dorsolateral prostate of the rat. Anat Rec. 1957 May;128(1):41–53. doi: 10.1002/ar.1091280105. [DOI] [PubMed] [Google Scholar]
- Helminen H. J., Ericsson J. L. Ultrastructural studies on prostatic involution in the rat changes in the secretory pathways. J Ultrastruct Res. 1972 Jul;40(1):152–166. doi: 10.1016/s0022-5320(72)80029-7. [DOI] [PubMed] [Google Scholar]
- MOSSMAN H. W., HOFFMAN R. A., KIRKPATRICK C. M. The accessory genital glands of male gray and fox squirrels correlated with age and reproductive cycles. Am J Anat. 1955 Sep;97(2):257–301. doi: 10.1002/aja.1000970204. [DOI] [PubMed] [Google Scholar]
- Nicander L., Plöen L., Larsson M. Specific apocrine secretion in the anterior lobe of the prostate gland of rabbits. Cell Tissue Res. 1974;151(1):69–77. doi: 10.1007/BF00222035. [DOI] [PubMed] [Google Scholar]
- SCHRODT G. R. The fine structure of the lateral lobe of the rat prostate gland. Comparison with the dorsal and other lobes. J Ultrastruct Res. 1961 Oct;5:485–496. doi: 10.1016/s0022-5320(61)80022-1. [DOI] [PubMed] [Google Scholar]
- SEAMAN A. R., WINELL M. The ultrafine structure of the normal prostate gland of the dog. Acta Anat (Basel) 1962;51:1–28. doi: 10.1159/000141937. [DOI] [PubMed] [Google Scholar]
- Siwela A. A., Tam W. H. Metabolism of androgens by the active and inactive prostate gland, and the seasonal changes in systemic androgen levels in the grey squirrel (Sciurus carolinensis Gmelin). J Endocrinol. 1981 Mar;88(3):381–392. doi: 10.1677/joe.0.0880381. [DOI] [PubMed] [Google Scholar]
- Whaley W. G., Dauwalder M., Kephart J. E. Golgi apparatus: influence on cell surfaces. Science. 1972 Feb 11;175(4022):596–599. doi: 10.1126/science.175.4022.596. [DOI] [PubMed] [Google Scholar]