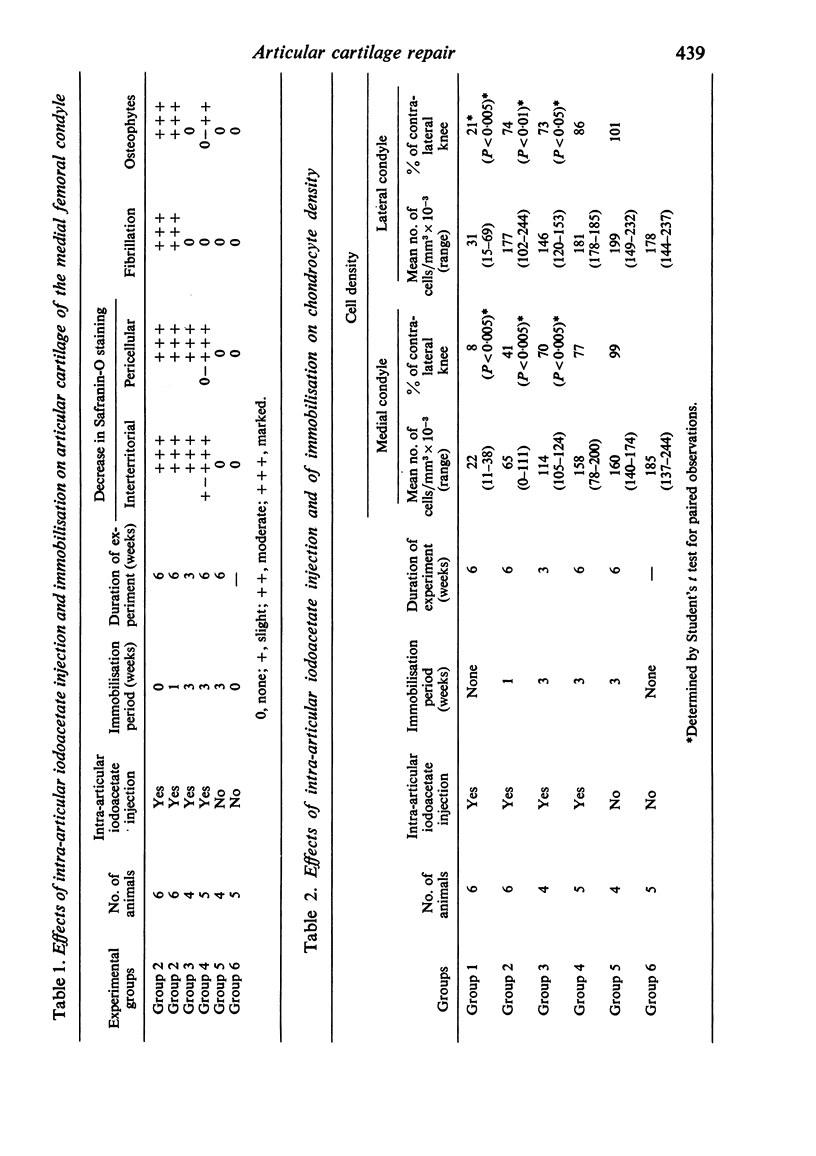
Abstract
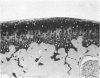
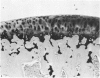
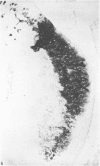
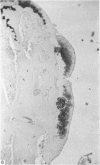
Recent studies have indicated that immobilisation of the lower limb may prevent surface fibrillation and osteophyte formation, and reduce cell depletion, following injection of iodoacetate into the ipsilateral knee of the guinea-pig. The present study shows that temporary immobilisation also facilitates repair of the damaged cartilage during a subsequent period of remobilisation in which the animal is permitted to move 'on all fours'. Thus, in animals killed six weeks after a single intra-articular injection of iodoacetate (0.3 mg in 0.1 ml saline), and in which the injected knee had been immobilised for three weeks, Safranin-O staining of the articular cartilage was more intense, chondrocyte density greater, and osteophytosis much less marked than in animals injected with iodoacetate but killed immediately after the three weeks immobilisation period. By contrast, immobilisation for only one week failed to protect against degenerative changes and osteophytes caused by iodoacetate injection. Immobilisation alone produced no apparent pathological changes in animals which did not receive iodoacetate.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISMAN O. D., FESSEL J. M., SOUTHWICK W. O. EXPERIMENTAL PRODUCTION OF SYNOVITIS AND MARGINAL ARTICULAR EXOSTOSES IN THE KNEE JOINTS OF DOGS. Yale J Biol Med. 1965 Apr;37:409–412. [PMC free article] [PubMed] [Google Scholar]
- Danielsson L., Hernborg J. Clinical and roentgenologic study of knee joints with osteophytes. Clin Orthop Relat Res. 1970 Mar-Apr;69:302–312. doi: 10.1097/00003086-197003000-00035. [DOI] [PubMed] [Google Scholar]
- DePalma A. F., McKeever C. D., Subin D. K. Process of repair of articular cartilage demonstrated by histology and autoradiography with tritiated thymidine. Clin Orthop Relat Res. 1966 Sep-Oct;48:229–242. [PubMed] [Google Scholar]
- Ghadially F. N., Ailsby R. L., Oryschak A. F. Scanning electron microscopy of superficial defects in articular cartilage. Ann Rheum Dis. 1974 Jul;33(4):327–332. doi: 10.1136/ard.33.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadially J. A., Ghadially R., Ghadially F. N. Long-term results of deep defects in articular cartilage. A scanning electron microscope study. Virchows Arch B Cell Pathol. 1977 Oct 27;25(2):125–136. doi: 10.1007/BF02889427. [DOI] [PubMed] [Google Scholar]
- Gilbertson E. M. Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis. 1975 Feb;34(1):12–25. doi: 10.1136/ard.34.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMRE C. J., YEAGER V. L. Influence of denervated muscles on exostoses of rats fed a sweet-pea diet. AMA Arch Pathol. 1958 Feb;65(2):215–227. [PubMed] [Google Scholar]
- HAMRE C. J., YEAGER V. L. Influence of muscle section on exostoses of lathyric rats. AMA Arch Pathol. 1957 Oct;64(4):426–433. [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Moskowitz R. W., Goldberg V. M., Malemud C. J. Metabolic responses of cartilage in experimentally induced osteoarthritis. Ann Rheum Dis. 1981 Dec;40(6):584–592. doi: 10.1136/ard.40.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Immobilization of the knee prevents osteoarthritis after anterior cruciate ligament transection. Arthritis Rheum. 1982 Oct;25(10):1201–1208. doi: 10.1002/art.1780251009. [DOI] [PubMed] [Google Scholar]
- Palmoski M., Perricone E., Brandt K. D. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979 May;22(5):508–517. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- Salter R. B., Simmonds D. F., Malcolm B. W., Rumble E. J., MacMichael D., Clements N. D. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1980 Dec;62(8):1232–1251. [PubMed] [Google Scholar]
- Stockwell R. A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971 Sep;109(Pt 3):411–421. [PMC free article] [PubMed] [Google Scholar]
- Telhag H., Lindberg L. A method for inducing osteoarthritic changes in rabbits' knees. Clin Orthop Relat Res. 1972 Jul-Aug;86:214–223. doi: 10.1097/00003086-197207000-00033. [DOI] [PubMed] [Google Scholar]