Abstract
Objectives: Dietary supplements have gained attention among people with diabetes as an alternative and complementary treatment, yet there is a limited understanding of supplement use and the motivations behind it. Methods: Data from the National Health and Nutrition Examination Survey (NHANES) from the 2009–2020 period were analyzed, including data on 5784 people with diabetes aged 20 years or older. Dietary supplement use was self-reported. Trends in supplement use were examined across three periods: 2009–2012, 2013–2016, and 2017–2020. Statistical analyses were conducted while considering NHANES’s complex survey design to provide nationally representative estimates for the general noninstitutionalized population of the United States. Results: A total of 61.72% of individuals reported using dietary supplements with a notable increase over time. Supplement users were generally older, more likely to be female, better educated, and had superior blood glucose control with lower smoking rates compared to non-users. Common supplements included multivitamins, multimineral supplements, vitamin D, calcium, zinc, vitamin C, and fish oil. Only 44.58% of individuals used dietary supplements based on medical advice, with the rest opting for self-directed usage. The primary specific health reasons for supplement use were to improve bone health and heart health and enhance the immune system. Conclusions: Dietary supplement use is prevalent among people with diabetes, and most diabetic supplement use is self-directed, which reflects a growing trend toward complementary therapies. Healthcare providers are encouraged to inquire about patients’ use of supplements and offer appropriate guidance as an integral component of comprehensive diabetes management.
Keywords: dietary supplement use, diabetes, trends, motivations, NHANES
1. Introduction
Diabetes is a global public health issue with a rising incidence and prevalence. According to the International Diabetes Federation, over 400 million people worldwide have diabetes, and this number is expected to reach 700 million by 2045 [1,2]. Diabetes significantly impacts patients’ health and imposes a substantial burden on society and the economy. Despite advancements in diabetes management, there remains a substantial number of patients whose needs are not fully met through conventional medical approaches alone.
Against this backdrop, dietary supplements have gained attention among people with diabetes as an alternative and complementary treatment [3]. These supplements, including vitamins, minerals, herbal extracts, and other functional food ingredients, are believed to offer benefits such as blood glucose regulation, antioxidant properties, and anti-inflammatory effects [4,5]. However, scientific evidence of their effectiveness and safety remains inconclusive, and the usage patterns, motivations, and influencing factors among patients are not well understood.
To address this gap, we used the latest data from the National Health and Nutrition Examination Survey (NHANES) to (1) describe the overall use of dietary supplements among people with diabetes, (2) compare the characteristics of supplement users and non-users, (3) identify the most commonly used supplements by those managing diabetes, (4) evaluate the efficacy of these supplements by comparing their effects on fasting glucose and HbA1c levels, (5) determine the differences in demographic and health-related characteristics between doctor-advised and self-directed supplement use, and (6) assess the motivations and reasons for supplement use.
This study aims to investigate the current status of dietary supplement use among people with diabetes. By understanding usage patterns, reasons for use, efficacy evaluations, and information sources, we hope to provide insights that can help healthcare professionals better guide patients in the rational use of dietary supplements, thereby improving overall diabetes management outcomes.
2. Materials and Methods
2.1. Study Population
The data for this study come from the NHANES, a nationwide, cross-sectional survey conducted over multiple years in the United States. The NHANES uses a multistage, stratified probability sampling design to collect demographic, dietary, examination, laboratory, and questionnaire data, providing nationally representative estimates for the adult population in the U.S. [6].
NHANES participants provided informed consent to participate in this study, and the survey design was approved by the National Center for Health Statistics Ethics Review Board. This study utilized publicly available anonymized data from the NHANES; therefore, no further institutional review board approval was required.
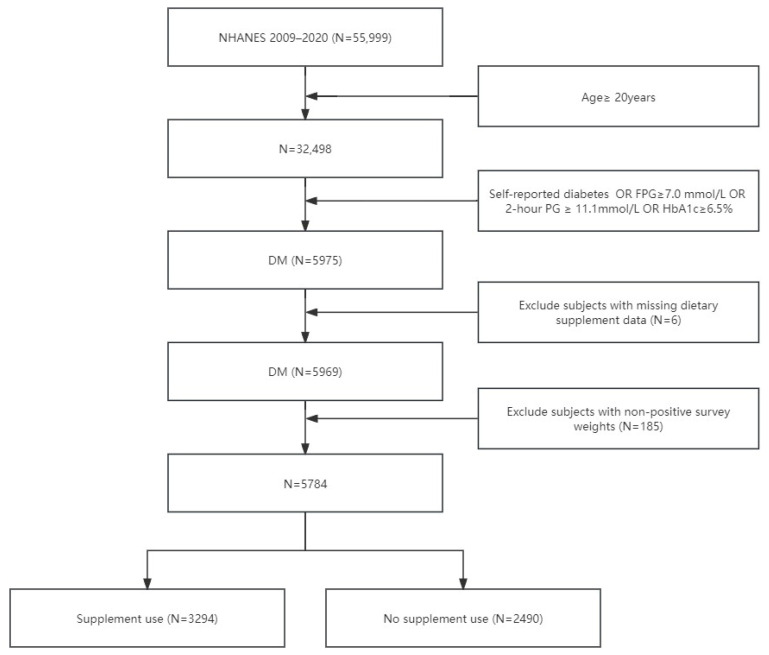
In this analysis, we included people with diabetes from the continuous NHANES in the 2009–2020 period. Only those who were aged 20 years or older at the time of the baseline survey were included. Subjects lacking supplement use data were excluded. As a result, 5784 people were left in our cohort for analysis (Figure 1).
Figure 1.
A flow chart illustrating the inclusion and exclusion of study participants. Abbreviations: DM: Diabetes Mellitus; NHANES: National Health and Nutrition Examination Survey; FPG: Fasting Plasma Glucose; HbA1c: Hemoglobin A1c; 2 h PG: 2 h Postprandial Glucose.
2.2. Definitions of Diabetes and Its Complications
Given the high number of missing values in the laboratory tests, we used self-reported data to confirm diabetes. The subjects were asked whether they had been diagnosed with diabetes by a doctor in the past, and if the answer was yes, they were considered people with diabetes. In addition, based on the ADA’s classification and diagnosis of diabetes, the following factors were used to confirm diabetes: meeting (i) a fasting blood glucose (FPG) ≥ 126 mg/dL (7.0 mmol/L), (ii) a 2 h blood glucose (2 h PG) ≥ 200 mg/dL (11.1 mmol/L) during the oral glucose tolerance test (OGTT), and (iii) HbA1c ≥ 6.5% (48 mmol/mol) [7].
Participants who self-reported diabetes were asked to disclose the age at which they were first diagnosed with diabetes mellitus (DM), as well as their current use of medications to manage their blood sugar levels, including diabetic pills and insulin. Diabetic retinopathy (DR) is classified as someone who answered “yes” to the statement “Diabetes affects the eyes/has retinopathy”. Diabetic nephropathy (DN) is defined as a condition in individuals with diabetes characterized by a urinary albumin-to-creatinine ratio exceeding 30 mg/g or an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2. The CKD-EPI (2021) equation was used to estimate the GFR using the following formula [8]: eGFR = 142 × [min(serum creatinine in mg/dL)/κ, 1)]**α × [max(serum creatinine/κ, 1)]** − 1.20 × 0.9938**age × (1.012 if female).
2.3. Definition of Covariates
The baseline survey questionnaire was used to collect covariate information, including age, sex, race/ethnicity, educational level, smoking status, and drinking habits. Additionally, the baseline questionnaire recorded a history of diabetes or heart disease in the family, self-reported health status, and baseline self-reported medical history of hypertension, cardiovascular diseases, chronic obstructive pulmonary disease, and cancer. Height, weight, and blood pressure were measured at the mobile examination center, BMI was calculated using weight (kg)/height (m)2, while blood pressure was obtained by averaging multiple measurements. Data on blood lipids, blood sugar, blood creatinine, and urine albumin-to-creatinine ratio were derived from laboratory tests. Detailed testing methods and specifics can be found on the NHANES’s official website.
2.4. Dietary Supplement Data
The NHANES collected self-reported information on dietary supplement use during in-home interviews conducted by trained interviewers using a computer-assisted interview system. Individuals were asked if they indicated they had taken any dietary supplements in the past 30 days and were asked by the interviewer to view the supplements, if any. Supplement users were asked whether they took the product on their own or under the advice of a doctor. The dietary supplement interview also included questions about the reasons for taking supplements. More specifically, participants were shown a hand card with a list of potential reasons and were asked to select one or more reasons for each supplement. Referring to the previous literature [9], we manually reviewed all dietary supplements in the NHANES dataset and categorized them by name.
2.5. Statistical Analysis
We accounted for the complex survey design factors of the NHANES, including sample weights, clustering, and stratification, as specified in the instructions for utilizing NHANES data. To compare baseline characteristics, we employed the Rao-Scott χ2 test for categorical variables and an analysis of variance adjusted for sampling weights for continuous variables. Adjusted utilization estimates for dietary supplements were calculated based on the NHANES in the 2009–2020 period. A trend analysis was performed by combining data from three 4-year periods: 2009–2012, 2013–2016, and 2017–2020. Logistic regression was employed to assess trends in dietary supplement usage over time. A two-tailed p value of less than 0.05 was considered to be statistically significant; all statistical analyses were performed using SAS 9.4, which allows for the appropriate use of the NHANES weights to project the results of the analysis to the general noninstitutionalized population in the U.S.
3. Results
3.1. Utilization of Dietary Supplements
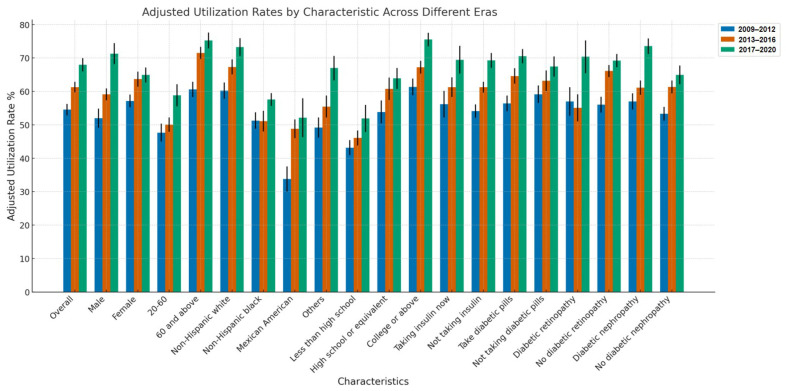
Our study encompassed 5784 people with diabetes, with 3294 reporting the use of dietary supplements, representing 56.95% of the cohort, and an adjusted rate of utilization of 61.72%. A data analysis spanning from 2009 to 2020 revealed a consistent upward trend in dietary supplement usage among individuals with diabetes, with a significant time trend (p < 0.0001). Usage rates increased from 54.53% in the 2009–2012 period to 67.94% in the 2017–2020 period, as detailed in Table 1 and illustrated in Figure 2. This trend was consistently observed across all analyzed demographic and health-related characteristics, highlighting that the widespread integration of supplements into the health management routines of individuals with diabetes is now a reality.
Table 1.
Adjusted utilization rate of supplement use based on selected characteristics in U.S. adults with diabetes *.
| Characteristics | Adjusted Utilization Rate % (SE) | |||
|---|---|---|---|---|
| NHANES 2009–2020 (Total) | NHANES 2009–2012 (era1) | NHANES 2013–2016 (era2) | NHANES 2007–2020 (era3) | |
| Overall | 61.72 (1.04) | 54.53 (1.74) | 61.28 (1.58) | 67.94 (1.99) |
| Sex | ||||
| Male | 64.42 (1.47) | 52.00 (2.87) | 59.13 (1.79) | 71.31 (3.14) |
| Female | 59.24 (1.30) | 57.12 (1.91) | 63.68 (2.23) | 64.93 (2.25) |
| Age groups (years) | ||||
| 20–60 | 52.42 (1.60) | 47.67 (2.71) | 50.05 (2.13) | 58.84 (3.29) |
| 60 and above | 69.77 (1.33) | 60.62 (2.27) | 71.54 (1.79) | 75.27 (2.33) |
| Ethnicity | ||||
| Non-Hispanic white | 67.36 (1.44) | 60.23 (2.44) | 67.34 (2.22) | 73.28 (2.63) |
| Non-Hispanic black | 53.35 (1.46) | 51.26 (2.47) | 51.10 (3.07) | 57.58 (1.91) |
| Mexican American | 45.66 (2.67) | 33.80 (3.75) | 48.82 (2.80) | 52.13 (5.84) |
| Others | 58.64 (2.09) | 49.16 (3.01) | 55.45 (3.28) | 67.01 (3.65) |
| Education | ||||
| Less than high school | 46.61 (1.65) | 43.18 (2.24) | 46.06 (2.23) | 51.94 (4.05) |
| High school or equivalent | 60.31 (1.89) | 53.86 (3.43) | 60.78 (3.33) | 63.89 (3.15) |
| College or above | 68.62 (1.21) | 61.37 (2.51) | 67.21 (1.92) | 75.53 (2.03) |
| Taking insulin now | ||||
| Yes | 63.03 (2.14) | 56.20 (3.97) | 61.27 (2.98) | 69.47 (4.17) |
| No | 61.18 (1.14) | 54.11 (1.97) | 61.26 (1.64) | 69.27 (2.25) |
| Taking diabetic pills to lower blood sugar | ||||
| Yes | 64.66 (1.32) | 56.39 (2.35) | 64.61 (2.32) | 70.55 (2.16) |
| No | 63.76 (1.73) | 59.16 (2.64) | 63.22 (3.05) | 67.48 (3.03) |
| Diabetic retinopathy | ||||
| Yes | 61.75 (2.73) | 56.98 (4.29) | 55.07 (4.07) | 70.37 (4.92) |
| NO | 64.48 (1.18) | 56.01 (2.36) | 66.10 (1.83) | 69.26 (1.99) |
| Diabetic nephropathy | ||||
| Yes | 64.65 (1.37) | 56.99 (2.46) | 61.13 (2.16) | 73.54 (2.29) |
| NO | 60.25 (1.35) | 53.32 (2.07) | 61.35 (1.93) | 64.93 (2.79) |
* All estimates accounted for complex survey designs and sampling weights of NHANES. Abbreviations: SE: standard error.
Figure 2.
Adjusted utilization rate trends by group and era (2009–2020).
3.2. Characteristics of Supplement Users vs. Non-Users
A comparative analysis of the baseline characteristics, as detailed in Table 2, indicates that individuals with diabetes who use dietary supplements are typically older, predominantly female, more educated, and exhibit better glycemic control, evidenced by lower levels of glycated hemoglobin and fasting blood glucose. These individuals also have lower levels of low-density lipoprotein (LDL) cholesterol and total cholesterol, along with reduced smoking rates. Predominantly, these patients are non-Hispanic white and report a better self-assessed health status. Although the prevalence of diabetic retinopathy remains similar between supplement users and non-users, the incidence of diabetic nephropathy is notably higher among supplement users. Furthermore, a higher proportion of individuals in the supplement group report comorbid conditions such as hypertension, cardiovascular diseases, and cancer. While the use of insulin and hypoglycemic drugs shows no significant difference between the two groups, the use of lipid-lowering and antihypertensive medications is more prevalent among those who take dietary supplements.
Table 2.
Characteristics of U.S. adults with diabetes based on NHANES. Overall results and results stratified by supplement use.
| Overall DM Population | Supplement Use | No Supplement Use | p Value * | |
|---|---|---|---|---|
| Participants † | 5784 | 3294 | 2490 | |
| Mean (95% CI) age, years | 59.77 (59.16–60.37) | 62.16 (61.35–62.98) | 55.90 (55.07–56.73) | <0.0001 |
| Women | 2783 (46.38) | 1682 (50.02) | 1101 (44.55) | 0.0059 |
| Mean (95% CI) body mass index | 32.98 (32.66–33.30) | 32.70 (32.29–33.10) | 33.44 (32.94–33.94) | 0.0233 |
| Ethnicity | <0.0001 | |||
| Non-Hispanic white | 1868 (59.04) | 1206 (64.43) | 662 (50.34) | |
| Non-Hispanic black | 1525 (14.19) | 831 (12.27) | 694 (17.30) | |
| Mexican American | 979 (10.12) | 454 (7.48) | 525 (14.37) | |
| Others | 1412 (16.63) | 803 (15.80) | 609 (17.97) | |
| Education | <0.0001 | |||
| Less than high school | 1846 (21.40) | 839 (16.16) | 1007 (29.84) | |
| High school or equivalent | 1347 (26.42) | 753 (25.85) | 594 (27.42) | |
| College or above | 2578 (52.14) | 1695 (57.97) | 883 (42.72) | |
| Alcohol drinking | 3800 (77.13) | 2217 (78.68) | 1583 (74.55) | 0.0069 |
| Smoking status | <0.0001 | |||
| Never smoked | 2995 (50.04) | 1750 (50.36) | 1254 (49.51) | |
| Former smoker | 1861 (34.30) | 1156 (38.26) | 705 (27.91) | |
| Current smoker | 923 (15.65) | 385 (11.36) | 538 (22.57) | |
| Family history of diabetes or heart attack | 3930 (68.74) | 2280 (69.05) | 1650 (68.25) | 0.6900 |
| Self-reported health | <0.0001 | |||
| Very good to excellent | 1005 (21.14) | 636 (23.18) | 369 (17.84) | |
| Good | 2138 (41.06) | 1270 (42.56) | 868 (38.64) | |
| Poor to fair | 2636 (37.78) | 1386 (34.24) | 1250 (43.50) | |
| Self-reported chronic diseases | ||||
| Hypertension | 3827 (65.60) | 2321 (70.13) | 1506 (58.30) | <0.0001 |
| Cardiovascular diseases | 1457 (24.89) | 896 (26.52) | 561 (22.26) | 0.0090 |
| Chronic obstructive pulmonary diseases | 451 (7.69) | 254 (7.76) | 197 (7.58) | 0.8609 |
| Cancer ‡ | 730 (14.14) | 477 (16.21) | 253 (10.79) | 0.0003 |
| Diabetic retinopathy | 866 (18.61) | 508 (17.96) | 358 (19.76) | 0.3063 |
| Diabetic nephropathy | 2156 (33.44) | 1255 (35.03) | 901 (30.88) | 0.0191 |
| Mean (95% CI) fasting glucose, mmol/L | 8.45 (8.28–8.61) | 8.16 (7.97–8.36) | 8.89 (8.55–9.24) | <0.0001 |
| Mean (95% CI) glycohemoglobin, % | 7.19 (7.12–7.25) | 6.99 (6.92–7.07) | 7.50 (7.39–7.61) | 0.0008 |
| Mean (95% CI) duration of diabetes, years | 6.56 (4.55–8.58) | 7.71 (5.53–9.90) | 4.52 (0.43–8.62) | 0.1781 |
| Taking insulin now | 1237 (23.02) | 728 (23.55) | 509 (22.16) | 0.4392 |
| Taking diabetic pills to lower blood sugar | 3139 (64.75) | 1909 (65.07) | 1230 (64.17) | 0.6783 |
| Mean (95% CI) systolic pressure, mmHg | 128.45 (127.02–129.88) | 128.92 (127.09–130.74) | 127.45 (125.24–129.65) | 0.3367 |
| Mean (95% CI) diastolic pressure, mmHg | 74.91 (73.90–75.91) | 74.49 (73.28–75.70) | 75.81 (74.72–76.90) | 0.0732 |
| Taking prescription for HBP | 3661 (95.66) | 2250 (96.87) | 1411 (93.31) | 0.0037 |
| Mean (95% CI) direct HDL cholesterol, mmol/L | 1.22 (1.21–1.24) | 1.24 (1.22–1.26) | 1.19 (1.16–1.21) | <0.0001 |
| Mean (95% CI) total cholesterol, mmol/L | 4.72 (4.67–4.77) | 4.66 (4.59–4.72) | 4.82 (4.74–4.90) | 0.0006 |
| Mean (95% CI) LDL cholesterol, mmol/L | 2.69 (2.63–2.75) | 2.62 (2.55–2.69) | 2.79 (2.71–2.87) | 0.0003 |
| Mean (95% CI) triglyceride, mmol/L | 1.77 (1.67–1.87) | 1.74 (1.61–1.88) | 1.82 (1.71–1.94) | 0.3281 |
| Taking prescription for cholesterol | 3285 (66.30) | 2090 (69.72) | 1195 (59.95) | <0.0001 |
| Mean (95% CI) eGFR, mL/min/1.73 m2 | 86.30 (85.46–87.15) | 83.90 (82.70–85.10) | 90.18 (89.00–91.37) | <0.0001 |
* The p value represents the comparison between supplement users and those not using supplements. † Totals of 13, 138, 463,5, 5, 15, 389, 1025, 972, 1960, 188, 2718, 1561, 342, 342, 311, 311, 2903, 2821, and 1596 participants had missing information for baseline education level, BMI, alcohol drinking, smoking status, self-reported health, taking insulin, taking diabetic pills, taking prescription for cholesterol, taking prescription for HBP, glycohemoglobin, fasting glucose, duration of diabetes, systolic pressure, diastolic pressure, direct hdl cholesterol, total cholesterol, ldl cholesterol, triglyceride, and diabetic retinopathy, respectively. ‡ Skin cancer was not included. Abbreviations: DM: Diabetes Mellitus; CI: Confidence Interval; HBP: High Blood Pressure; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; eGFR: Estimated Glomerular Filtration Rate.
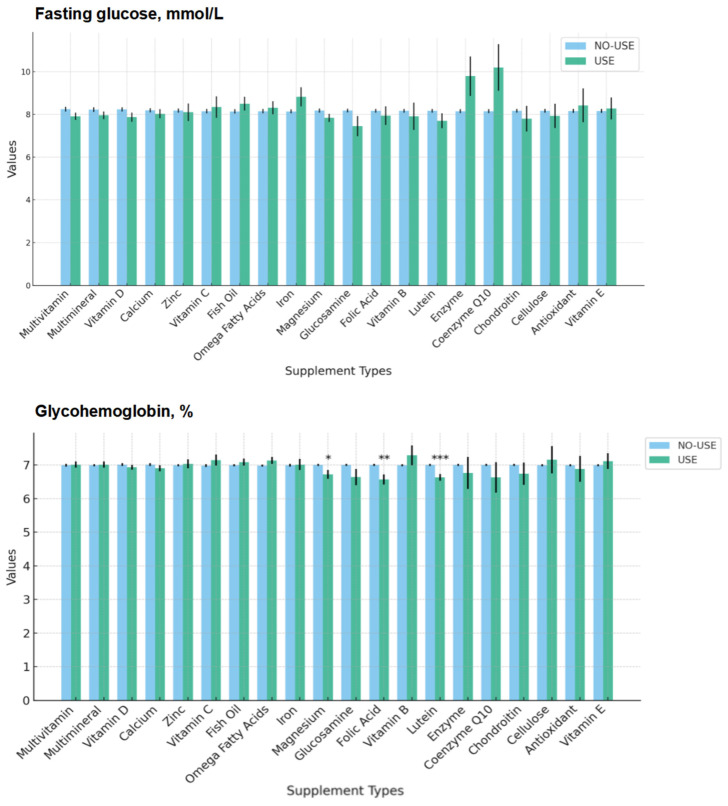
Figure 3 provides a comparison of fasting blood glucose and glycated hemoglobin, categorized according to specific types of dietary supplements (top 20 commonly used supplements). When we grouped patients by specific types of supplements, there were minimal differences in the fasting blood glucose levels between most groups. However, the HbA1c levels were lower in the subjects who took folic acid, lutein, and magnesium.
Figure 3.
Comparison of fasting blood glucose and glycated hemoglobin, categorized according to specific types of dietary supplements (top 20 commonly used supplements).
3.3. Types and Reasons for Dietary Supplement Use
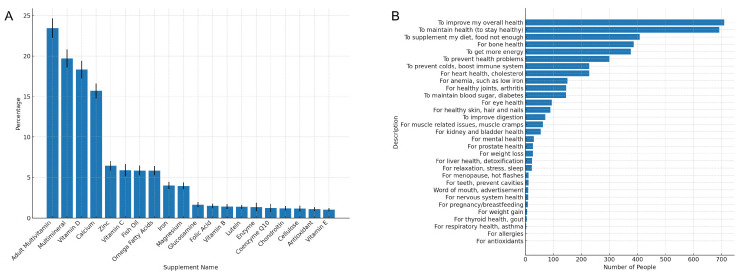
As shown in Figure 4A, the most commonly used supplements included multivitamins, multimineral supplements, vitamin D, calcium, zinc, vitamin C, and fish oil. The primary specific health reasons for using these supplements, as elucidated in Figure 4B, were to improve overall health, maintain wellness, support bone health, augment their diet, boost energy levels, enhance heart health, and strengthen immune function. Notably, controlling blood sugar was not a major motive for supplement use among these patients. Figure S1 presents a ranking of the reasons for using dietary supplements (top 10 commonly used supplements).
Figure 4.
(A) Commonly used dietary supplements ordered by frequency of use among people with diabetes. (B) Reasons for taking dietary supplements among people with diabetes.
3.4. Doctor-Advised vs. Self-Directed Supplement Use
Only 44.58% of people with diabetes used dietary supplements based on medical advice, with the rest opting for self-directed usage. Those following a doctor’s recommendations were generally older, in poorer health, and more likely to suffer from comorbid conditions such as cancer, hypertension, and heart disease, as outlined in Table 3. Individuals who were taking dietary supplements based on a physician’s advice exhibited a higher incidence of diabetic nephropathy, lower levels of glycated hemoglobin, more frequent use of lipid-lowering medications, and lower total cholesterol levels. Figure S2 shows the most commonly used types of supplements and reasons for dietary supplement use under doctor-advised and self-directed conditions.
Table 3.
Characteristics of adults with diabetes who take dietary supplements, categorized as self-directed versus doctor-advised use.
| Self-Directed Use | Doctor-Advised Use | p Value * | |
|---|---|---|---|
| Participants † | 1579 | 1249 | |
| Mean (95% CI) age, years | 60.40 (59.33–61.46) | 64.60 (63.64–65.57) | <0.0001 |
| Women | 743 (45.56) | 693 (55.27) | <0.0001 |
| Mean (95% CI) body mass index | 32.54 (32.02–33.06) | 32.82 (32.29–33.36) | 0.4065 |
| Ethnicity | 0.2273 | ||
| Non-Hispanic white | 548 (62.43) | 468 (65.87) | |
| Non-Hispanic black | 397 (12.46) | 318 (11.93) | |
| Mexican American | 215 (7.71) | 181 (7.58) | |
| Others | 419 (17.37) | 282 (14.60) | |
| Education | 0.0609 | ||
| Less than high school | 365 (14.80) | 328 (16.03) | |
| High school or equivalent | 349 (23.97) | 295 (28.46) | |
| College or above | 862 (61.22) | 624 (55.50) | |
| Alcohol drinking | 1075 (78.25) | 844 (79.58) | 0.4926 |
| Smoking status | 0.4230 | ||
| Never smoked | 852 (52.87) | 670 (48.98) | |
| Former smoker | 550 (36.39) | 438 (39.23) | |
| Current smoker | 175 (10.72) | 141 (11.77) | |
| Family history of diabetes or heart attack | 1071 (68.57) | 878 (68.69) | 0.9616 |
| Self-reported health | 0.0005 | ||
| Very good to excellent | 365 (26.14) | 201 (19.69) | |
| Good | 661 (45.96) | 459 (41.30) | |
| Poor to fair | 551 (27.88) | 589 (38.99) | |
| Self-reported chronic diseases | |||
| Hypertension | 1020 (63.05) | 942 (77.55) | <0.0001 |
| Cardiovascular diseases | 334 (19.66) | 405 (32.64) | <0.0001 |
| Chronic obstructive pulmonary diseases | 112 (7.65) | 93 (7.50) | 0.9261 |
| Cancer ‡ | 184 (12.95) | 229 (20.78) | <0.0001 |
| Diabetic retinopathy | 205 (18.02) | 225 (17.94) | 0.9772 |
| Diabetic nephropathy | 522 (31.15) | 528 (38.15) | 0.0077 |
| Mean (95% CI) duration of diabetes, years | 6.81 (3.92–9.70) | 9.46 (6.03–12.89) | 0.2589 |
| Mean (95% CI) fasting glucose, mmol/L | 8.27 (8.01–8.53) | 8.01 (7.70–8.31) | 0.1698 |
| Mean (95% CI) glycohemoglobin, % | 7.08 (6.98–7.18) | 6.89 (6.78–7.01) | 0.0082 |
| Taking insulin now | 297 (23.32) | 314 (25.00) | 0.5852 |
| Taking diabetic pills to lower blood sugar | 869 (62.56) | 756 (67.03) | 0.1281 |
| Mean (95% CI) systolic pressure, mmHg | 128.52 (126.59–130.46) | 128.14 (125.32–130.97) | 0.8201 |
| Mean (95% CI) diastolic pressure, mmHg | 75.02 (73.58–76.46) | 72.99 (71.17–74.80) | 0.0960 |
| Taking prescription for HBP | 988 (96.65) | 918 (97.25) | 0.6813 |
| Mean (95% CI) direct HDL cholesterol, mmol/L | 1.24 (1.21–1.28) | 1.25 (1.22–1.28) | 0.8616 |
| Mean (95% CI) total cholesterol, mmol/L | 4.71 (4.63–4.79) | 4.55 (4.44–4.67) | 0.0208 |
| Mean (95% CI) LDL cholesterol, mmol/L | 2.67 (2.58–2.76) | 2.54 (2.43–2.65) | 0.0604 |
| Mean (95% CI) triglyceride, mmol/L | 1.78 (1.57–1.99) | 1.61 (1.51–1.70) | 0.1095 |
| Taking prescription for cholesterol | 928 (64.61) | 867 (75.25) | 0.0029 |
| Mean (95% CI) eGFR, mL/min/1.73 m2 | 87.75 (86.17–89.33) | 79.39 (77.55–81.22) | <0.0001 |
* The p value represents the comparison between supplement users and those not using supplements. † Totals of 5, 59, 185, 2, 5, 2, 196, 395, 83, 1304, 698, 160, 160, 135, 135, 1380, 1345 and 711 participants had missing information for baseline education level, BMI, alcohol drinking, smoking status, self-reported health, taking insulin, taking diabetic pills, glycohemoglobin, fasting glucose, duration of diabetes, systolic pressure, diastolic pressure, direct hdl cholesterol, total cholesterol, ldl cholesterol, triglyceride, and diabetic retinopathy, respectively. ‡ Skin cancer was not included. Abbreviations: DM: Diabetes Mellitus; CI: Confidence Interval; HBP: High Blood Pressure; LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; eGFR: Estimated Glomerular Filtration Rate.
4. Discussion
The findings of this study underscore the growing reliance on dietary supplements among patients with diabetes, particularly as a complementary strategy to conventional diabetes management. Our analysis, based on data from the NHANES in the 2009–2020 period, reveals that a significant proportion of people with diabetes, specifically 61.72%, use dietary supplements, and this trend has been increasing over the years. This rise in dietary supplement use aligns with the global trend of patients seeking alternative therapies to manage chronic conditions like diabetes [10,11,12]. Moreover, the similarity in trends across different baseline characteristics such as age, gender, and education level suggests that the increase in dietary supplement use is a widespread phenomenon among people with diabetes. This trend has also been observed in other studies [13,14], which have suggested that the growing popularity of dietary supplements may be driven by increased awareness of the potential health benefits of these products and the rise of integrative medicine. This widespread adoption could also be attributed to the perceived benefits of dietary supplements, including improved antioxidant properties and anti-inflammatory effects [15,16]. However, it is important to note that while the popularity of these supplements is growing, the scientific evidence supporting their efficacy and safety remains mixed [17,18,19,20]. Healthcare professionals should be aware of this trend and guide their patients accordingly, ensuring that any supplement use complements rather than complicates their diabetes management plan.
Our study also highlights important demographic distinctions between supplement users and non-users. People with diabetes who use dietary supplements tend to be older and more educated and are more likely to be female and white. According to the previous literature, it is estimated that 57.6% of Americans consume dietary supplements, with higher proportions being observed among older adults and women [12]. These characteristics are similar to those of our study’s population. People with diabetes who use dietary supplements also exhibit better blood glucose control and report better overall health, with a lower prevalence of smoking. This demographic pattern suggests that certain groups may be more inclined to adopt supplementary treatments, possibly due to greater health awareness or access to health information [21,22].
We found that people with diabetes who used dietary supplements had better glycemic control, reflected in lower fasting blood glucose and HbA1c levels, even though there were no significant differences in insulin and oral hypoglycemic agent use between those who used supplements and those who did not. When we grouped subjects by specific types of supplements, there were minimal differences in fasting blood glucose levels between most groups. However, the HbA1c levels were lower in subjects who took folic acid, lutein, and magnesium. Consistent with previous studies [23,24,25], supplementation with lutein, folic acid, and magnesium is thought to have potential benefits for insulin resistance and glycemic control. Studies indicate that folic acid can enhance endothelial function and reduce inflammation levels, both of which are crucial for improving insulin signaling [26,27]. Folic acid indirectly increases insulin sensitivity by lowering levels of homocysteine, an amino acid associated with insulin resistance [28]. Lutein, an antioxidant, has been shown to reduce oxidative stress and inflammation, key drivers of insulin resistance [29]. Additionally, lutein can improve the function of adipocytes, thereby aiding in the enhancement of insulin sensitivity [30]. Magnesium supplementation directly boosts the activity of insulin receptors, thus improving insulin sensitivity and glycemic control [31]. However, due to the cross-sectional design of our study, it is difficult to establish a causal relationship. Future prospective studies are needed to further identify and confirm supplements that may have hypoglycemic effects.
The 20 most commonly used supplements among people with diabetes, as identified in our study, include multivitamins, multimineral supplements, vitamin D, calcium, zinc, vitamin C, fish oil, and others. These supplements are popular not only for their potential to support overall health but also for their specific benefits related to diabetes management, such as improved bone health and heart health [32,33,34,35,36]. The use of omega fatty acids, iron, magnesium, and glucosamine further indicates a focus on managing the broader health challenges associated with diabetes.
Interestingly, only 44.58% of people with diabetes actually use dietary supplements under the advice of doctors, and most individuals use dietary supplements by themselves. The characteristics of those who take dietary supplements based on a doctor’s recommendation differ from those who take them independently. The former group tends to be older, is more likely to be female, and reports poorer health with a higher prevalence of serious conditions such as cancer, hypertension, and heart disease. This suggests that doctors may be recommending supplements to patients with more complex health needs, possibly as a way to mitigate the effects of these comorbidities or to enhance the overall treatment plan. Finally, our study identifies the primary reasons for dietary supplement use among patients with diabetes as improving overall health, maintaining wellness, supporting bone health, augmenting their diet, boosting energy levels, enhancing heart health, and strengthening immune function. These motivations appear closely linked to the common complications of diabetes, such as cardiovascular disease, bone demineralization, and weakened immunity. For example, cardiovascular issues, a frequent complication of diabetes, may explain why many patients prioritize heart health when selecting supplements [37]. Similarly, the focus on immune function could be attributed to the increased susceptibility to infections observed in diabetic populations [38]. Moreover, the diverse range of reasons for supplementation may also reflect varying patient perceptions about disease management and health optimization. While some patients may focus on addressing immediate health challenges, such as fatigue or immune support, others may be motivated by preventative strategies, aiming to reduce the long-term impact of diabetes-related complications. These patterns underscore the importance of personalized patient education.
This study offers several insights for clinical practice and public health policy. Firstly, healthcare providers should be aware of the high prevalence of supplement use among people with diabetes and routinely inquire about their use during clinical consultations. This is particularly crucial given the potential interactions between supplements and prescription medications or their impact on disease progression. Clinicians should also provide evidence-based guidance on the use of dietary supplements, helping patients make informed decisions about their health. Secondly, public health initiatives should aim to enhance awareness of the potential risks and benefits of dietary supplements, especially among populations more likely to use these products without medical supervision. Lastly, future research should focus on understanding the motivations behind supplement use among patients with diabetes and explore the long-term health outcomes associated with their use. Large-scale randomized controlled trials are needed to ascertain the efficacy and safety of commonly used supplements in these populations and identify any potential risks associated with their use.
Strengths and Limitations
One of the major strengths of our study is the use of data from the NHANES, which provides a large, nationally representative sample of the U.S. population. This allows our findings to be generalized to a broader sample of people with diabetes, enhancing the external validity of the results. Furthermore, our study spans a decade (2009–2020), enabling us to capture trends over time and observe changes in dietary supplement use patterns.
However, our study also has limitations that should be acknowledged. First, the cross-sectional design of the NHANES prevents us from drawing causal inferences about the relationship between dietary supplement use and health outcomes. Longitudinal studies would be necessary to determine whether the observed supplement use leads to improved health outcomes over time. Second, our reliance on self-reported data for both diabetes status and dietary supplement use introduces the possibility of recall bias or misreporting. This could lead to inaccuracies in the estimation of supplement use prevalence and the identification of patients with diabetes. Additionally, while we categorized dietary supplements based on available data, we were unable to account for variations in the quality, dosage, and formulation of these supplements, which could influence their effectiveness and safety. Future research should consider these factors, particularly in relation to the potential interactions between dietary supplements and prescribed diabetes medications. Furthermore, this study only included participants diagnosed with diabetes, and thus, it is not appropriate to infer that individuals with diabetes are increasingly relying on supplements compared to those without diabetes.
5. Conclusions
Our analysis of NHANES data from 2009 to 2020 indicates an increasing trend in dietary supplement use among individuals with diabetes. This rise reflects a proactive approach by patients to enhance health outcomes and manage their condition more effectively. However, it also prompts important questions about how these supplements are integrated into conventional treatment regimens. Dietary supplement users are generally older, better educated, and exhibit superior glycemic control, indicating targeted use among those with greater health literacy. The most commonly used supplements include multivitamins, multimineral supplements, vitamin D, calcium, zinc, vitamin C, and fish oil, which are chosen for their general and diabetes-specific health benefits. Supplements such as folic acid and magnesium are beneficial for glycemic control, although further research is required to confirm these effects. Significantly, only 44.58% of supplement use is doctor-advised, with the majority being self-directed. This underlines the importance of healthcare providers playing an active role in guiding supplement use to ensure it is safe and effective. Healthcare providers should play a crucial role in advising patients on the appropriate use of dietary supplements, ensuring their use is grounded in sound scientific evidence and tailored to the individual’s overall health status and treatment goals. Further research is necessary to provide clearer guidance on the efficacy and safety of dietary supplements in the context of diabetes management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16234021/s1. Figure S1. Reasons for using dietary supplements (top 10 commonly used supplements). Figure S2. Most commonly used types and reasons for dietary supplement use under doctor-advised and self-directed conditions.
Author Contributions
Conceptualization: Y.J., X.C., Z.C., S.H. and Y.Y.; methodology: S.H.; software: X.C., Z.C. and S.H.; validation: Y.J., Z.C. and S.H.; formal analysis: Z.C. and S.H.; writing—original draft preparation: Y.J., X.C., Z.C., S.H. and Y.Y.; writing—review and editing: Y.J., X.C., Z.C., S.H. and Y.Y.; supervision: Y.Y.; project administration: Y.Y.; funding acquisition: Y.J. and X.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the research relying on publicly used, de-identified secondary data.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data that support the findings of this study are available from NHANES [https://www.cdc.gov/nchs/nhanes], accessed on 1 June 2024. Furthermore, the cleaned datasets that were analyzed in the current study are also available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the projects from the Natural Science Foundation of Hubei Province (no. 2023AFB901), the National Natural Science Foundation of China (no. 82000927), and the Natural Science Foundation of Hubei Province (no. 2020CFB208).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xie J., Wang M., Long Z., Ning H., Li J., Cao Y., Liao Y., Liu G., Wang F., Pan A. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: Systematic analysis of the Global Burden of Disease Study 2019. BMJ-Br. Med. J. 2022;379:e072385. doi: 10.1136/bmj-2022-072385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinajero M.G., Malik V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. North Am. 2021;50:337–355. doi: 10.1016/j.ecl.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Grossman L.D., Roscoe R., Shack A.R., Diabetes Canada Clinical Practice Guidelines Expert Committee Complementary and Alternative Medicine for Diabetes. Can. J. Diabetes. 2018;42:S154–S161. doi: 10.1016/j.jcjd.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Behrouz V., Dastkhosh A., Sohrab G. Overview of dietary supplements on patients with type 2 diabetes. Diabetes Metab. Syndr.-Clin. Res. Rev. 2020;14:325–334. doi: 10.1016/j.dsx.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Liu X., Zeng X., Liu W., Lu Y., Cheng J., Chen Y. An Overview of Dietary Supplements on Obesity and Type 2 Diabetes: Efficacy and Mechanisms. Curr. Drug Metab. 2021;22:415–440. doi: 10.2174/1389200222666210406110450. [DOI] [PubMed] [Google Scholar]
- 6.Kropp M., Golubnitschaja O., Mazurakova A., Koklesova L., Sargheini N., Vo T.-T.K.S., de Clerck E., Polivka J., Jr., Potuznik P., Polivka J., et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. Epma J. 2023;14:21–42. doi: 10.1007/s13167-023-00314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 8.Inker L.A., Eneanya N.D., Coresh J., Tighiouart H., Wang D., Sang Y., Crews D.C., Doria A., Estrella M.M., Froissart M., et al. New creatinine-and cystatin C–based equations to estimate GFR without race. N. Engl. J. Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assadourian J.N., Peterson E.D., Gupta A., Navar A.M. Use of Dietary Supplements Among People with Atherosclerotic Cardiovascular Disease in the United States: A Population-Based Analysis From NHANES. J. Am. Heart Assoc. 2024;13:e033748. doi: 10.1161/JAHA.123.033748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantor E.D., Rehm C.D., Du M., White E., Giovannucci E.L. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA-J. Am. Med. Assoc. 2016;316:1464–1474. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannon B.A., Fairfield W.D., Adams B., Kyle T., Crow M., Thomas D.M. Use and abuse of dietary supplements in persons with diabetes. Nutr. Diabetes. 2020;10:14. doi: 10.1038/s41387-020-0117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra S., Stierman B., Gahche J.J., Potischman N. Dietary Supplement Use Among Adults: United States, 2017–2018. NCHS Data Brief. 2021;399:1–8. [PubMed] [Google Scholar]
- 13.Li J., Li X.L., Gathirua-Mwangi W., Song Y.Q. Prevalence and trends in dietary supplement use among US adults with diabetes: The National Health and Nutrition Examination Surveys, 1999–2014. BMJ Open Diabetes Res. Care. 2020;8:e000925. doi: 10.1136/bmjdrc-2019-000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahche J.J., Bailey R.L., Potischman N., Dwyer J.T. Dietary Supplement Use Was Very High among Older Adults in the United States in 2011–2014. J. Nutr. 2017;147:1968–1976. doi: 10.3945/jn.117.255984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zittermann A., Trummer C., Theiler-Schwetz V., Lerchbaum E., Maerz W., Pilz S. Vitamin D and Cardiovascular Disease: An Updated Narrative Review. Int. J. Mol. Sci. 2021;22:2896. doi: 10.3390/ijms22062896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqi S.M., Sun C., Wu X., Shah I., Mehmood A. The Correlation between Dietary Selenium Intake and Type 2 Diabetes: A Cross-Sectional Population-Based Study on North Chinese Adults. Biomed Res. Int. 2020;2020:8058463. doi: 10.1155/2020/8058463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua R., Lam C.S., Chu N., Yang A., Chow E., Cheung Y.T. Association between dietary supplement use and mortality among US adults with diabetes: A longitudinal cohort study. Nutr. Metab. 2023;20:33. doi: 10.1186/s12986-023-00753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mursu J., Robien K., Harnack L.J., Park K., Jacobs D.R., Jr. Dietary Supplements and Mortality Rate in Older Women The Iowa Women’s Health Study. Arch. Intern. Med. 2011;171:1625–1633. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satia J.A., Littman A., Slatore C.G., Galanko J.A., White E. Long-term Use of β-Carotene, Retinol, Lycopene, and Lutein Supplements and Lung Cancer Risk: Results From the VITamins And Lifestyle (VITAL) Study. Am. J. Epidemiol. 2009;169:815–828. doi: 10.1093/aje/kwn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortmann S.P., Burda B.U., Senger C.A., Lin J.S., Whitlock E.P. Vitamin and Mineral Supplements in the Primary Prevention of Cardiovascular Disease and Cancer: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Ann. Intern. Med. 2013;159:824–834. doi: 10.7326/0003-4819-159-12-201312170-00729. [DOI] [PubMed] [Google Scholar]
- 21.Gong W., Liu A., Yao Y., Ma Y., Ding C., Song C., Yuan F., Zhang Y., Feng G., Chen Z., et al. Nutrient Supplement Use among the Chinese Population: A Cross-Sectional Study of the 2010–2012 China Nutrition and Health Surveillance. Nutrients. 2018;10:1733. doi: 10.3390/nu10111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnett A.J., Livingstone K.M., Woods J.L., McNaughton S.A. Dietary Supplement Use among Australian Adults: Findings from the 2011–2012 National Nutrition and Physical Activity Survey. Nutrients. 2017;9:1248. doi: 10.3390/nu9111248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J.V., Schooling C.M., Zhao J.X. The effects of folate supplementation on glucose metabolism and risk of type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Ann. Epidemiol. 2018;28:249–257.1. doi: 10.1016/j.annepidem.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Hajizadeh-Sharafabad F., Tarighat-Esfanjani A., Ghoreishi Z., Sarreshtedari M. Lutein supplementation combined with a low-calorie diet in middle-aged obese individuals: Effects on anthropometric indices, body composition and metabolic parameters. Br. J. Nutr. 2021;126:1028–1039. doi: 10.1017/S0007114520004997. [DOI] [PubMed] [Google Scholar]
- 25.El-said N.H., Sadik N.A., Mohammed N. Magnesium in type 2 diabetes mellitus and its correlation with glycemic control. Int. J. Res. Med. Sci. 2015;3:1958–1963. doi: 10.18203/2320-6012.ijrms20150308. [DOI] [Google Scholar]
- 26.Solini A., Santini E., Ferrannini E. Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int. J. Obes. 2006;30:1197–1202. doi: 10.1038/sj.ijo.0803265. [DOI] [PubMed] [Google Scholar]
- 27.Stanhewicz A.E., Kenney W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr. Rev. 2017;75:61–70. doi: 10.1093/nutrit/nuw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayden M.R., Tyagi S.C. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutr. J. 2004;3:4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahn Y.J., Kim H. Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants. 2021;10:1448. doi: 10.3390/antiox10091448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gopal S.S., Eligar S.M., Vallikannan B., Ponesakki G. Inhibitory efficacy of lutein on adipogenesis is associated with blockage of early phase regulators of adipocyte differentiation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2021;1866:158812. doi: 10.1016/j.bbalip.2020.158812. [DOI] [PubMed] [Google Scholar]
- 31.Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: Focusing on the processes of insulin secretion and signaling. Int. J. Mol. Sci. 2019;20:1351. doi: 10.3390/ijms20061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace I.R., Wallace H.J., McKinley M.C., Bell P.M., Hunter S.J. Vitamin D and insulin resistance. Clin. Endocrinol. 2016;84:159–171. doi: 10.1111/cen.12760. [DOI] [PubMed] [Google Scholar]
- 33.Yedjou C.G., Grigsby J., Mbemi A., Nelson D., Mildort B., Latinwo L., Tchounwou P.B. The Management of Diabetes Mellitus Using Medicinal Plants and Vitamins. Int. J. Mol. Sci. 2023;24:9085. doi: 10.3390/ijms24109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn C., Kang J.H., Jeung E.B. Calcium homeostasis in diabetes mellitus. J. Vet. Sci. 2017;18:261–266. doi: 10.4142/jvs.2017.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gembillo G., Visconti L., Giuffrida A.E., Labbozzetta V., Peritore L., Lipari A., Calabrese V., Piccoli G.B., Torreggiani M., Siligato R., et al. Role of Zinc in Diabetic Kidney Disease. Nutrients. 2022;14:1353. doi: 10.3390/nu14071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egalini F., Guardamagna O., Gaggero G., Varaldo E., Giannone B., Beccuti G., Benso A., Broglio F. The effects of omega 3 and omega 6 fatty acids on glucose metabolism: An updated review. Nutrients. 2023;15:2672. doi: 10.3390/nu15122672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah A., Isath A., Aronow W.S. Cardiovascular complications of diabetes. Expert Rev. Endocrinol. Metab. 2022;17:383–388. doi: 10.1080/17446651.2022.2099838. [DOI] [PubMed] [Google Scholar]
- 38.Cockram C.S., Wong B.C. Textbook of Diabetes. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2017. Diabetes and infections; pp. 799–818. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from NHANES [https://www.cdc.gov/nchs/nhanes], accessed on 1 June 2024. Furthermore, the cleaned datasets that were analyzed in the current study are also available from the corresponding author upon reasonable request.