Abstract
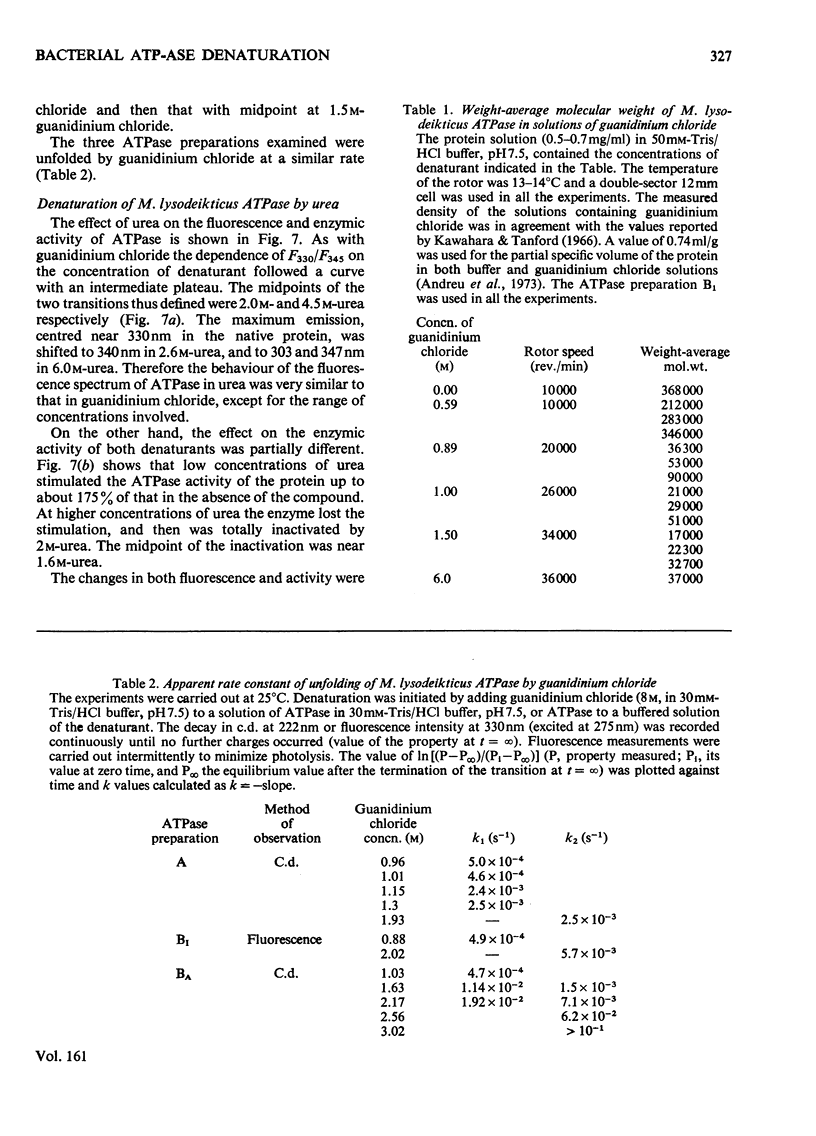
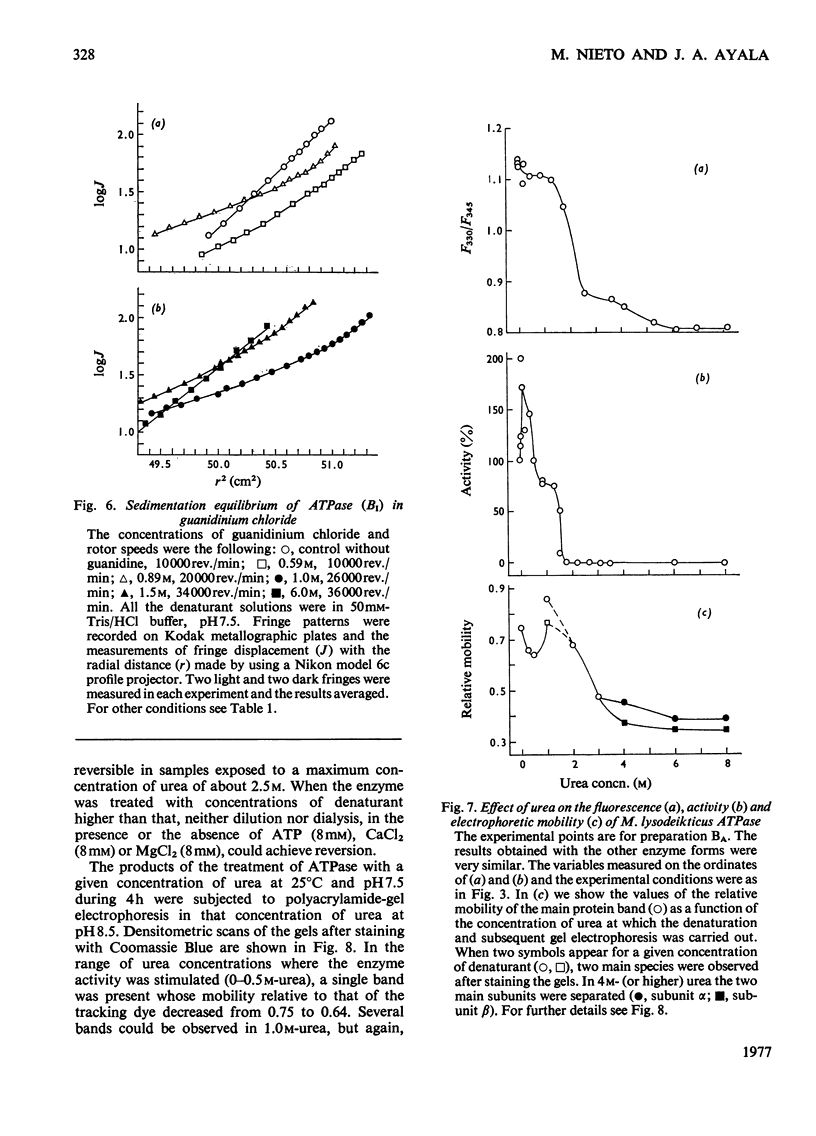
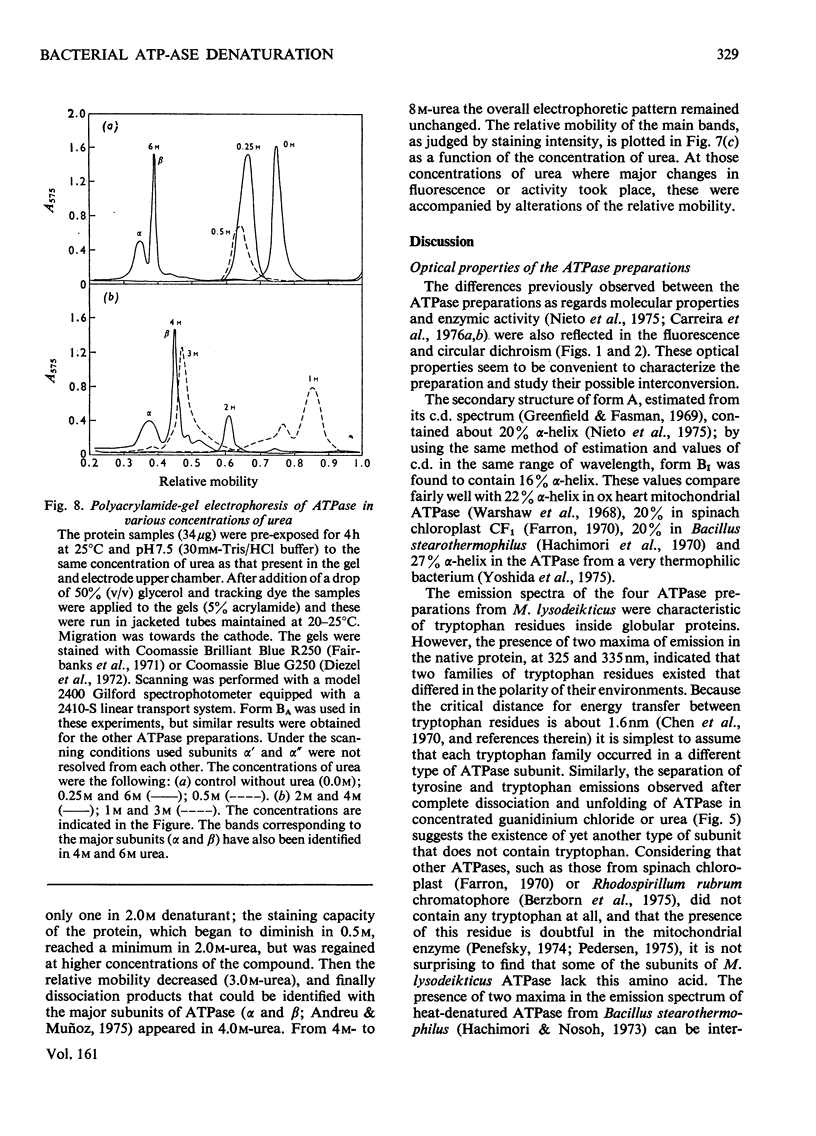
1. The fluorescence and circular dichroism of four homogeneous preparations of ATPase (adenosine triphosphatase) from Micrococcus lysodeikticus differing in molecular structure and enzymic properties were examined at pH 7.5 and 25 degrees. Emission was maximum at 325 and 335 nm and the relative intensities at these wavelengths may be used to characterize the different ATPase preparations. The circular-dichroism spectra exhibited negative extrema at 208 and 220 nm, and the relative value of the molar ellipticity at these wavelengths was also different for each molecular form of the enzyme. 2. The four preparations undergo two consecutive major unfolding transitions in guanidinium chloride (midpoints at 0.94 and 1.5 M denaturant), with concomitant destruction of the quaternary structure of the protein. A comparatively minor alteration in the ATPase structure also occurred in 0.05-0.2M-guanidine and led to complete inactivation of the enzyme. The inactivation and the first unfolding transition were reversible by dilution of the denaturant; the transition with midpoint at 1.5M-guanidine was irreversible. 3. Similar results were obtained in urea, except that the successive transitions had midpoints at concentrations of denaturant of 0.4, 2.0 and 4.5M. Low concentrations of urea caused a noticeable activation of the enzyme activity and alterations of the electrophoretic mobility of the ATPase. 4. A model is proposed in which one of the major subunits, alpha, is first dissociated and unfolded reversibly by the denaturants, followed by the irreversible unfolding and dissociation of the other major subunit, beta, from subunit delta and/or the components of relative mobility 1.0 in dodecyl sulphate/polyacrylamide-gel electrophoresis (rho).
Full text
PDF

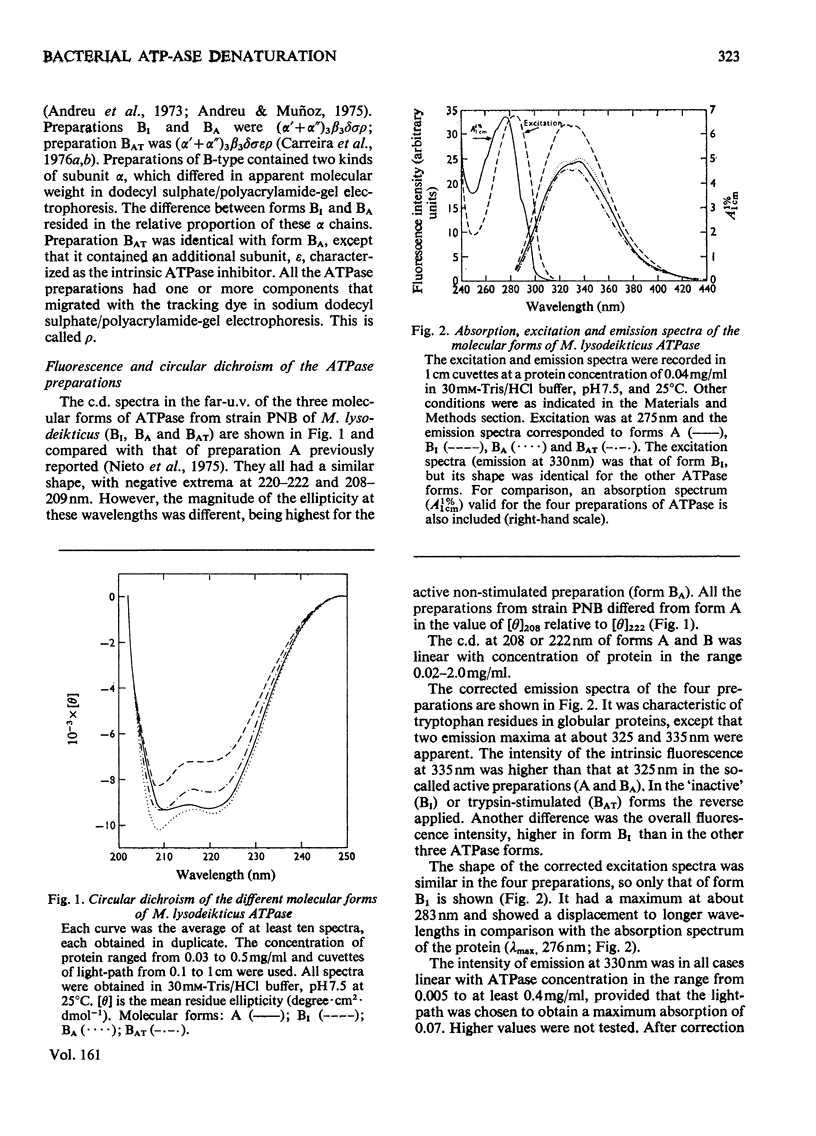
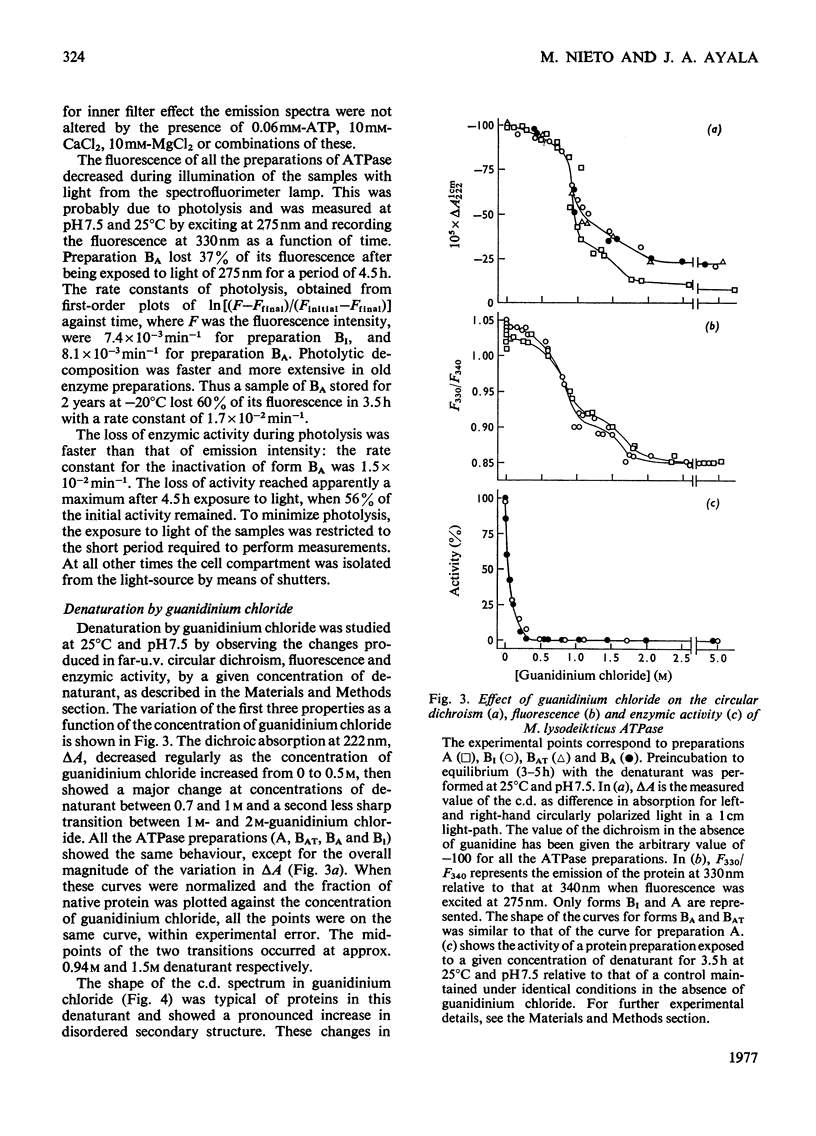
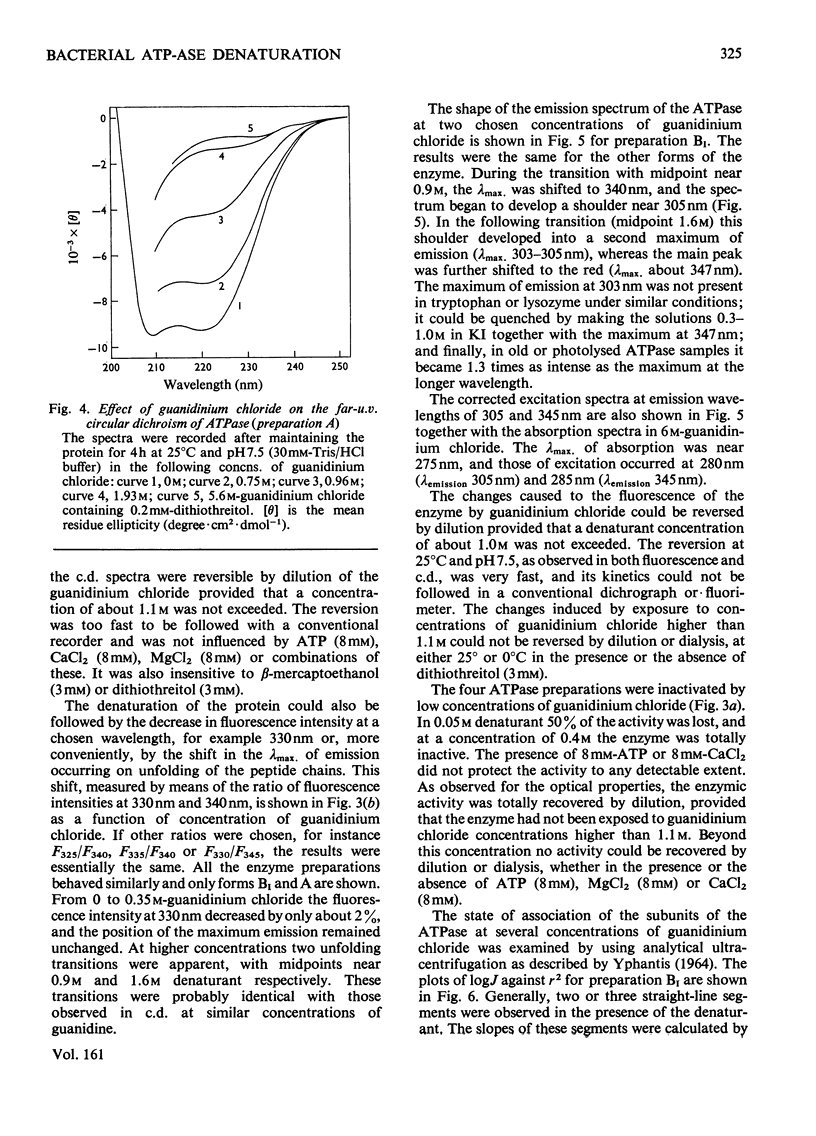
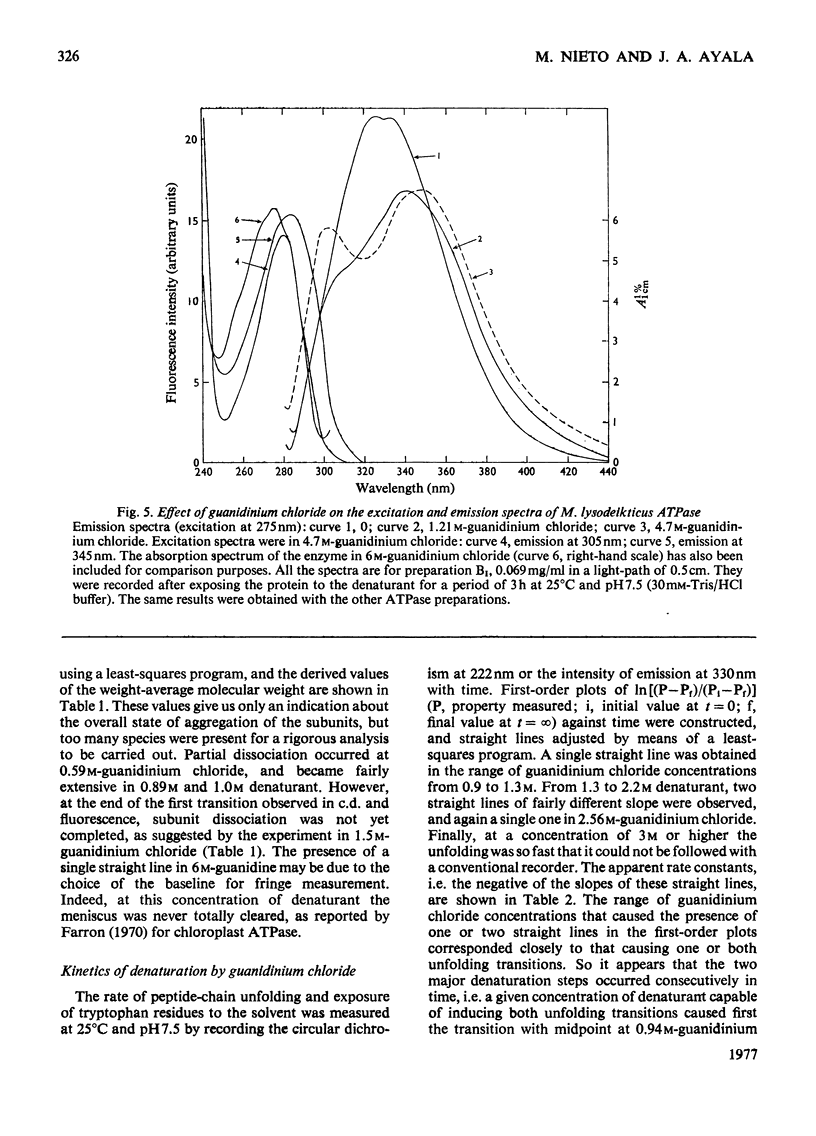


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Albendea J. A., Munõz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Molecular properties of the purified enzyme unstimulated by trypsin. Eur J Biochem. 1973 Sep 3;37(3):505–515. doi: 10.1111/j.1432-1033.1973.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Andreu J. M., Muñoz E. Micrococcus lysodeikticus ATPase. Purification by preparative gel electrophoresis and subunit structure studied by urea and sodium dodecylsulfate gel electrophoresis. Biochim Biophys Acta. 1975 May 15;387(2):228–233. doi: 10.1016/0005-2728(75)90105-x. [DOI] [PubMed] [Google Scholar]
- Berzborn R. J., Johansson B. C., Baltscheffsky M. Immunological and fluorescence studies with the coupling factor ATPase from Rhodospirillum rubrum. Biochim Biophys Acta. 1975 Sep 8;396(3):360–370. doi: 10.1016/0005-2728(75)90142-5. [DOI] [PubMed] [Google Scholar]
- Carreira J., Andreu J. M., Nieto M., Muñoz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. ISolation of two forms of the enzyme complex and correlation between ezymatic stability, latency and activity. Mol Cell Biochem. 1976 Feb 16;10(2):67–76. doi: 10.1007/BF01742200. [DOI] [PubMed] [Google Scholar]
- Carreira J., Muńoz E., Andreu J. M., Nieto M. Micrococcus lysodeikticus membrane ATPase. Effect of trypsin on stimulation of a purified form of the enzyme and idenfification of its natural inhibitor. Biochim Biophys Acta. 1976 Jun 4;436(1):183–189. doi: 10.1016/0005-2736(76)90229-7. [DOI] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Farron F. Isolation and properties of a chloroplast coupling factor and heat-activated adenosine triphosphatase. Biochemistry. 1970 Sep 15;9(19):3823–3828. doi: 10.1021/bi00821a023. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Hachimori A., Muramatsu N., Noso Y. Studies on an ATPase of thermophilic bacteria. I. Purification and properties. Biochim Biophys Acta. 1970 Jun 10;206(3):426–437. doi: 10.1016/0005-2744(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Tanford C. Viscosity and density of aqueous solutions of urea and guanidine hydrochloride. J Biol Chem. 1966 Jul 10;241(13):3228–3232. [PubMed] [Google Scholar]
- Kozlov I. A., Mikelsaar H. N. On the subunit structure of soluble mitochondrial ATPase. FEBS Lett. 1974 Jul 15;43(2):212–214. doi: 10.1016/0014-5793(74)81002-1. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Nieto M., Muñoz E., Carreira J., Andreu J. M. Conformational and molecular responses to pH variation of the purified membrane adenosine triphosphatase of Micrococcus lysodeikticus. Biochim Biophys Acta. 1975 Dec 16;413(3):394–414. doi: 10.1016/0005-2736(75)90123-6. [DOI] [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]
- Vogel G., Steinhart R. ATPase of Escherichia coli: purification, dissociation, and reconstitution of the active complex from the isolated subunits. Biochemistry. 1976 Jan 13;15(1):208–216. doi: 10.1021/bi00646a032. [DOI] [PubMed] [Google Scholar]
- Warshaw J. B., Lam K. W., Nagy B., Sanadi D. R. Studies on oxidative phosphorylation. XV. Latent adenosine 5'-triphosphatase activity of factor A. Arch Biochem Biophys. 1968 Feb;123(2):385–396. doi: 10.1016/0003-9861(68)90149-5. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. A highly stable adenosine triphosphatase from a thermophillie bacterium. Purification, properties, and reconstitution. J Biol Chem. 1975 Oct 10;250(19):7910–7916. [PubMed] [Google Scholar]


