Abstract
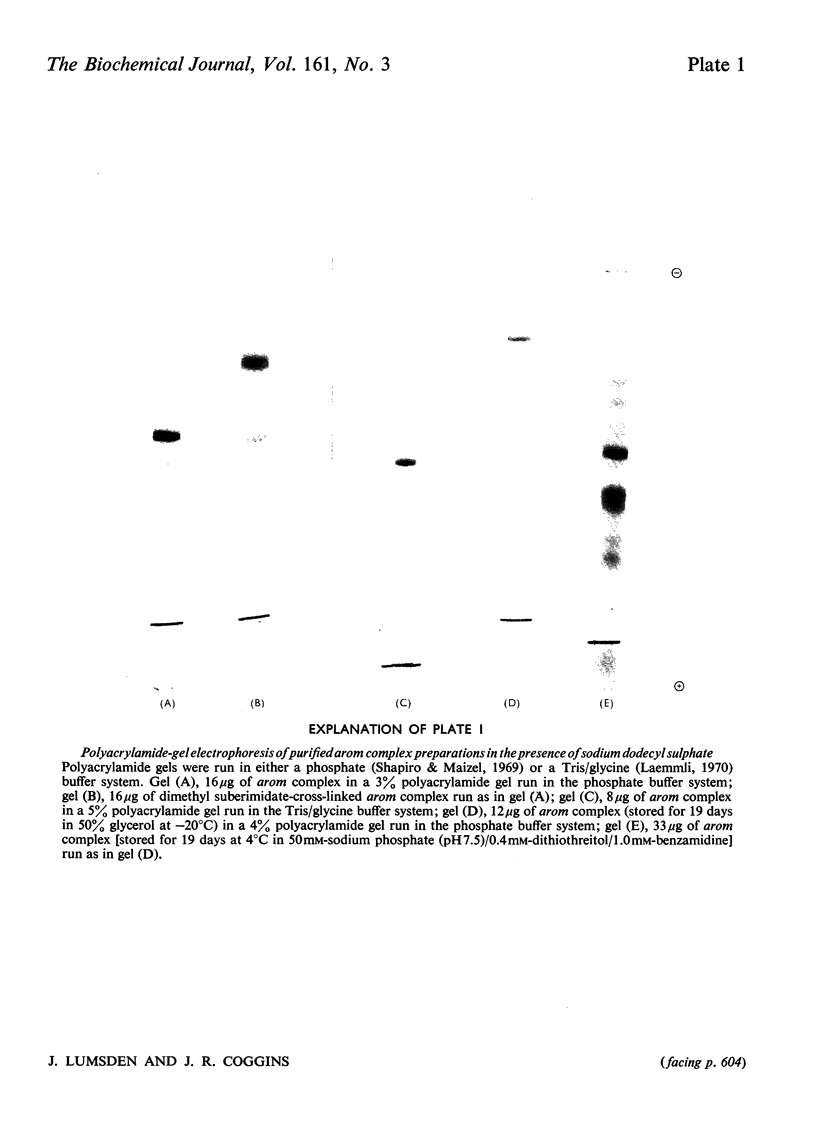
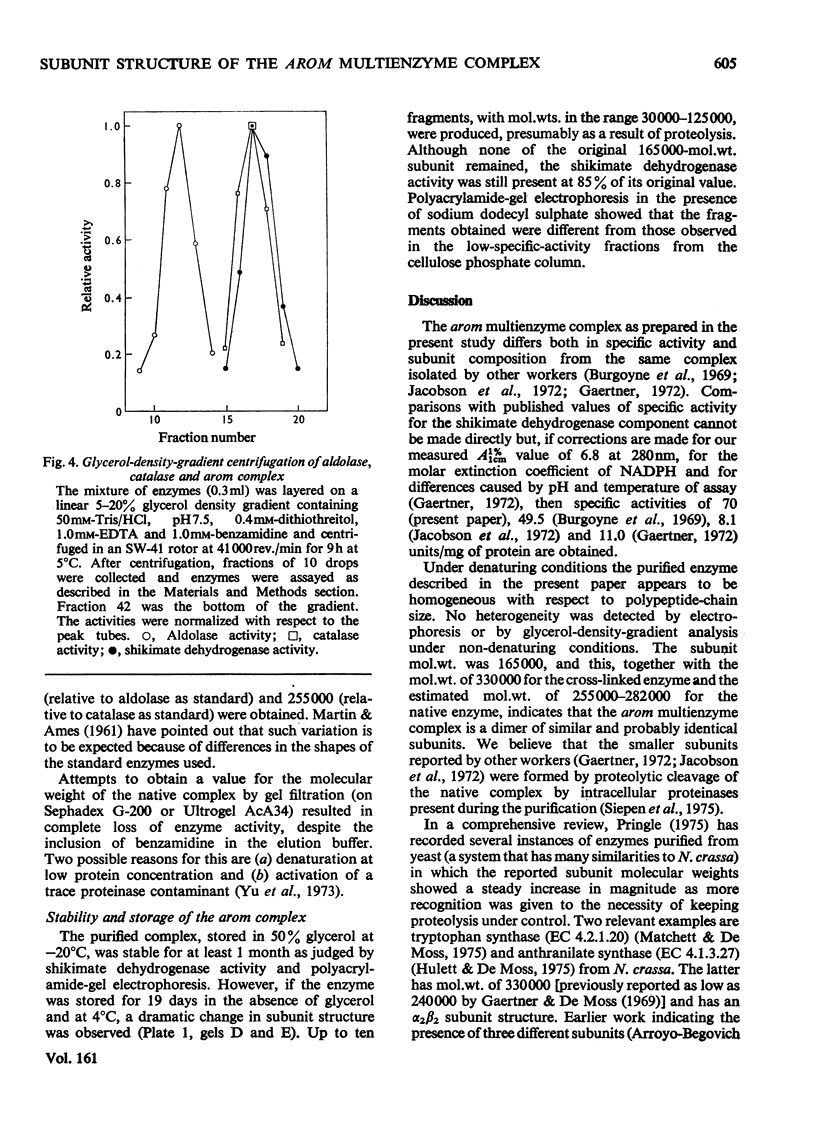
A new procedure for the purification of the arom multienzyme complex from Neurospora crassa is presented. Important factors are the inactivation of proteinases by phenylmethanesulphonyl fluoride and the use of cellulose phosphate as an affinity adsorbent. A homogeneous enzyme, with a specific shikimate dehydrogenase activity of 70 units/mg of protein, is obtained in 25% yield. Polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate, combined with cross-linking studies using dimethyl suberimidate, suggest that the complex is composed of two subunits of molecular weight 165000. Glycerol-density-gradient centrifugation indicates a molecular weight for the intact complex of about 270000. Evidence for the effects of proteolysis, both during the preparation and on storage of the purified complex, is presented, and previous reports in the literature of the occurrence of multiple subunits are discussed in this light.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo-Begovich A., DeMoss J. A. The isolation of the components of the anthranilate synthetase complex from Neurospora crassa. J Biol Chem. 1973 Feb 25;248(4):1262–1267. [PubMed] [Google Scholar]
- Burgoyne L., Case M. E., Giles N. H. Purification and properties of the aromatic (arom) synthetic enzyme aggregate of Neurospora crassa. Biochim Biophys Acta. 1969 Nov 4;191(2):452–462. doi: 10.1016/0005-2744(69)90264-2. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Case M. E., Burgoyne L., Giles N. H. In vivo and in vitro complementation between DHQ synthetase mutants in the arom gene cluster of Neurospora crassa. Genetics. 1969 Nov;63(3):581–588. doi: 10.1093/genetics/63.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Evidence for nonsense mutations in the arom gene cluster of Neurospora crassa. Genetics. 1968 Sep;60(1):49–58. doi: 10.1093/genetics/60.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. W., Gaertner F. H. Phosphocellulose, an affinity chromatographic system for chorismate synthase and the aromatic complex of Neurospora crassa. Biochem Biophys Res Commun. 1975 Nov 3;67(1):170–175. doi: 10.1016/0006-291x(75)90298-3. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davidson B. E., Blackburn E. H., Dopheide T. A. Chorismate mutase-prephenate dehydratase from Escherichia coli K-12. I. Purification, molecular weight, and amino acid composition. J Biol Chem. 1972 Jul 25;247(14):4441–4446. [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEWSTER J. A. Phosphorylation of shikimic acid by ultrasonic extracts of micro-organisms. Biochem J. 1962 Nov;85:388–393. doi: 10.1042/bj0850388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITONDE M. K., GORDON M. W. A microchemical method for the detection and determination of shikimic acid. J Biol Chem. 1958 Feb;230(2):1043–1050. [PubMed] [Google Scholar]
- Gaertner F. H., DeMoss J. A. Purification and characterization of a multienzyme complex in the tryptophan pathway of Neurospora crassa. J Biol Chem. 1969 May 25;244(10):2716–2725. [PubMed] [Google Scholar]
- Gaertner F. H., Ericson M. C., DeMoss J. A. Catalytic facilitation in vitro by two multienyzme complexes from Neurospora crassa. J Biol Chem. 1970 Feb 10;245(3):595–600. [PubMed] [Google Scholar]
- Gaertner F. H. Purification of two multienzyme complexes in the aromatic-tryptophan pathway of Neurospora crassa. Arch Biochem Biophys. 1972 Jul;151(1):277–284. doi: 10.1016/0003-9861(72)90498-5. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. Kinetic and molecular properties of citraconyl-aldolase. The reversible denaturation and hybridization of the native and modified enzymes. Biochem J. 1974 May;139(2):331–342. doi: 10.1042/bj1390331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. E. Tertiary structure of Escherichia coli beta-D-galactosidase. J Mol Biol. 1969 Dec 28;46(3):441–446. doi: 10.1016/0022-2836(69)90187-9. [DOI] [PubMed] [Google Scholar]
- Gross S R, Fein A. Linkage and Function in Neurospora. Genetics. 1960 Jul;45(7):885–904. doi: 10.1093/genetics/45.7.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. B., Wellner D. Microheterogeneity of L-amino acid oxidase. Separation of multiple components by polyacrylamide gel electrofucusing. J Biol Chem. 1969 Dec 25;244(24):6636–6644. [PubMed] [Google Scholar]
- Herrmann K. M., Poling M. D. The synthesis of 3-deoxyheptulosonic acid 7-phosphate. J Biol Chem. 1975 Sep 10;250(17):6817–6821. [PubMed] [Google Scholar]
- Huang L., Montoya A. L., Nester E. W. Characterization of the functional activities of the subunits of 3-deoxy-D-arabinoheptulosonate 7-phosphate synthetase-chorismate mutase from Bacillus subtilis 168. J Biol Chem. 1974 Jul 25;249(14):4473–4470. [PubMed] [Google Scholar]
- Hucho F., Müllner H., Sund H. Investigation of the symmetry of oligomeric enzymes with bifunctional reagents. Eur J Biochem. 1975 Nov 1;59(1):79–87. doi: 10.1111/j.1432-1033.1975.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Hulett F. M., DeMoss J. A. Subunit structure of anthranilate synthetase from Neurospora crassa. J Biol Chem. 1975 Sep 10;250(17):6648–6652. [PubMed] [Google Scholar]
- Jacobson J. W., Hart B. A., Doy C. H., Giles N. H. Purification and stability of the multienzyme complex encoded in the arom gene cluster of Neurospora crassa. Biochim Biophys Acta. 1972 Nov 10;289(1):1–12. doi: 10.1016/0005-2744(72)90101-5. [DOI] [PubMed] [Google Scholar]
- Kono T., Yourno J. Proteolytic release of a histidinol dehydrogenase fragment from the double enzyme histidinol dehydrogenase-imidazolylacetol-phosphate: L-glutamate aminotransferase. J Biol Chem. 1971 Apr 10;246(7):2203–2206. [PubMed] [Google Scholar]
- LEVIN J. G., SPRINSON D. B. THE ENZYMATIC FORMATION AND ISOLATION OF 3-ENOLPYRUVYLSHIKIMATE 5-PHOSPHATE. J Biol Chem. 1964 Apr;239:1142–1150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARES-GUIA M., SHAW E. STUDIES ON THE ACTIVE CENTER OF TRYPSIN. THE BINDING OF AMIDINES AND GUANIDINES AS MODELS OF THE SUBSTRATE SIDE CHAIN. J Biol Chem. 1965 Apr;240:1579–1585. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MITSUHASHI S., DAVIS B. D. Aromatic biosynthesis. XII. Conversion of 5-dehydroquinic acid to 5-dehydroshikimic acid dy 5-dehydroquinase. Biochim Biophys Acta. 1954 Sep;15(1):54–61. doi: 10.1016/0006-3002(54)90093-1. [DOI] [PubMed] [Google Scholar]
- Matchett W. H., DeMoss J. A. The subunit structure of tryptophan synthase from Neurospora crassa. J Biol Chem. 1975 Apr 25;250(8):2941–2946. [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- Rines H. W., Case M. E., Giles N. H. Mutants in the arom gene cluster of Neurospora crassa specific for biosynthetic dehydroquinase. Genetics. 1969 Apr;61(4):789–800. doi: 10.1093/genetics/61.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Shapiro A. L., Maizel J. V., Jr Molecular weight estimation of polypeptides by SDS-polyacrylamide gel electrophoresis: further data concerning resolving power and general considerations. Anal Biochem. 1969 Jun;29(3):505–514. doi: 10.1016/0003-2697(69)90335-2. [DOI] [PubMed] [Google Scholar]
- Siepen D., Yu P. H., Kula M. R. Proteolytic enzymes of Neurospora crassa. Purification and some properties of five intracellular proteinases. Eur J Biochem. 1975 Aug 1;56(1):271–281. doi: 10.1111/j.1432-1033.1975.tb02230.x. [DOI] [PubMed] [Google Scholar]
- Véron M., Falcoz-Kelly F., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972 Aug 4;28(4):520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Gaertner F. H. Coordinate activation of a multienzyme complex by the first substrate. Evidence for a novel regulatory mechanism in the polyaromatic pathway of Neurospora crassa. Arch Biochem Biophys. 1976 Feb;172(2):476–489. doi: 10.1016/0003-9861(76)90101-6. [DOI] [PubMed] [Google Scholar]
- Welch G. R., Gaertner F. H. Influence of an aggregated multienzyme system on transient time: kinetic evidence for compartmentation by an aromatic-amino-acid synthesizing complex of Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4218–4222. doi: 10.1073/pnas.72.11.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourno J., Kohno T., Roth J. R. Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes. Nature. 1970 Nov 28;228(5274):820–824. doi: 10.1038/228820a0. [DOI] [PubMed] [Google Scholar]
- Yu P. H., Kula M. R., Tsai H. Studies on the apparent instability of Neurospora tryptophan synthase. Evidence for protease. Eur J Biochem. 1973 Jan 3;32(1):129–135. doi: 10.1111/j.1432-1033.1973.tb02588.x. [DOI] [PubMed] [Google Scholar]
- Yu P. H., Siepen D., Kula M. R., Tsai H. On the stoichiometry and reversibility of interaction between Neurospora protease I and its inhibitor. FEBS Lett. 1974 Jun 1;42(2):227–230. doi: 10.1016/0014-5793(74)80791-x. [DOI] [PubMed] [Google Scholar]



