Abstract
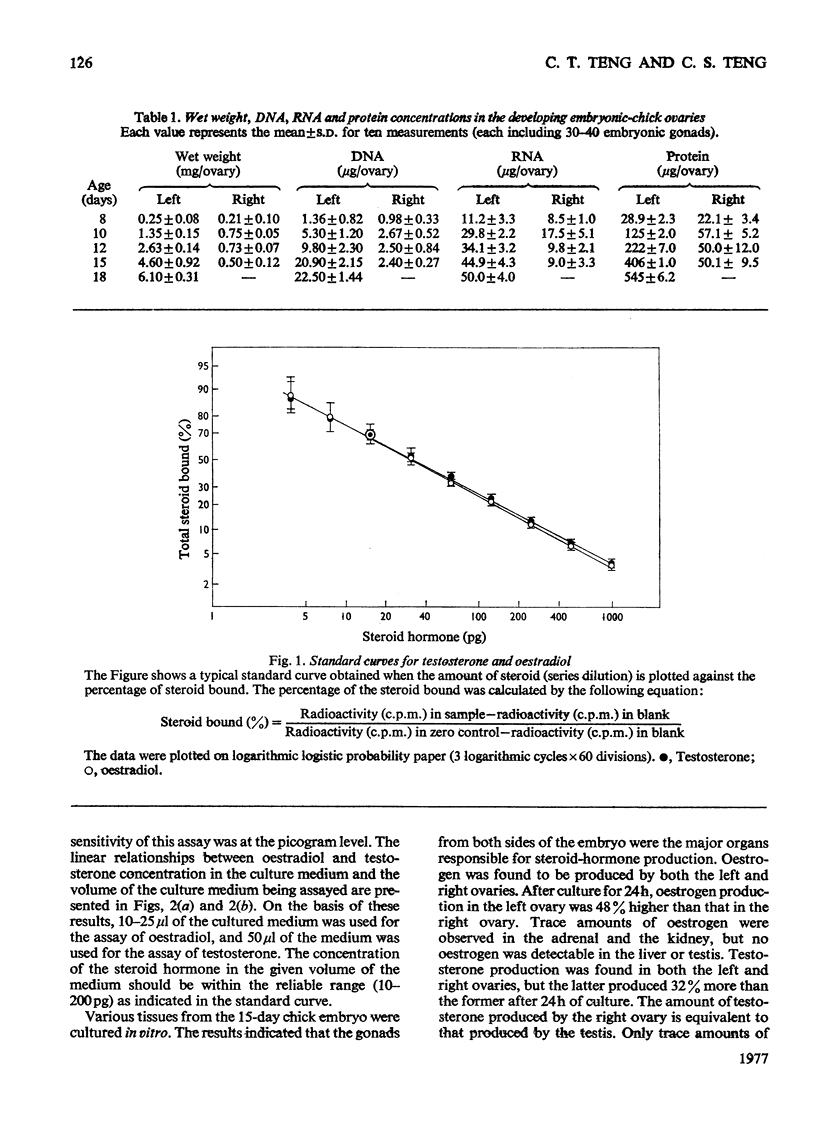
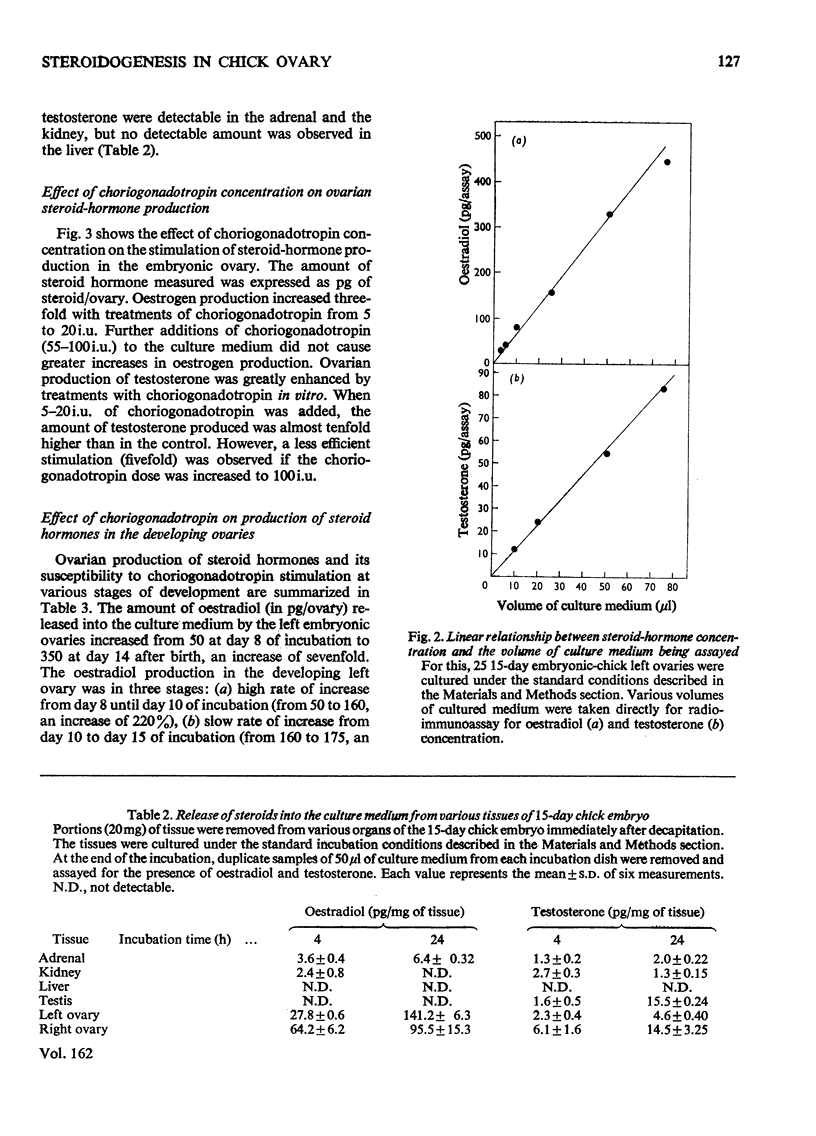
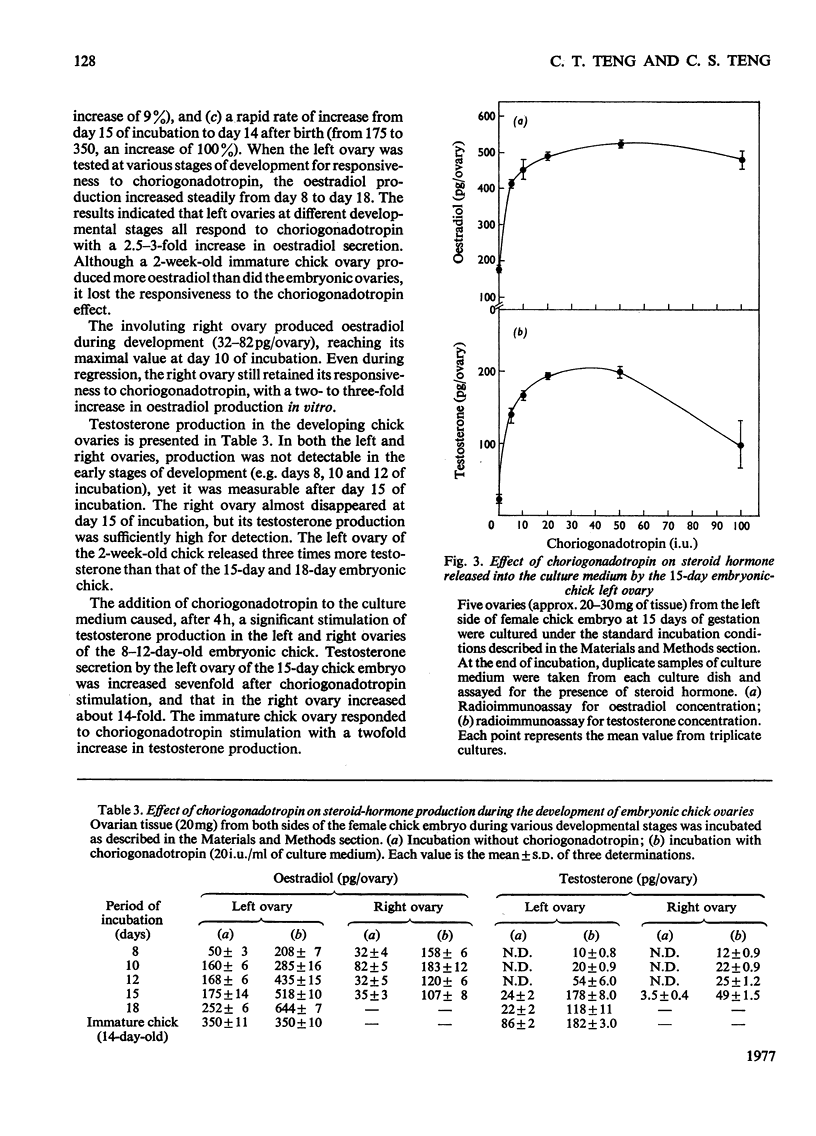
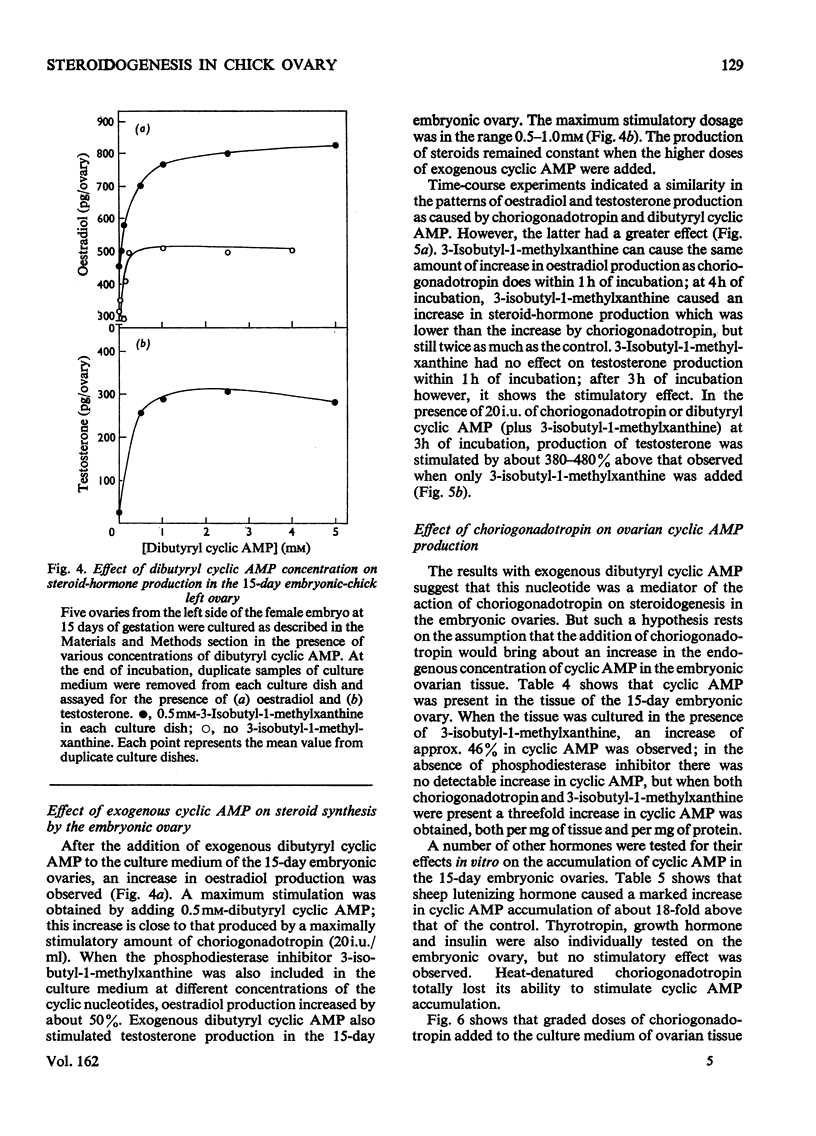
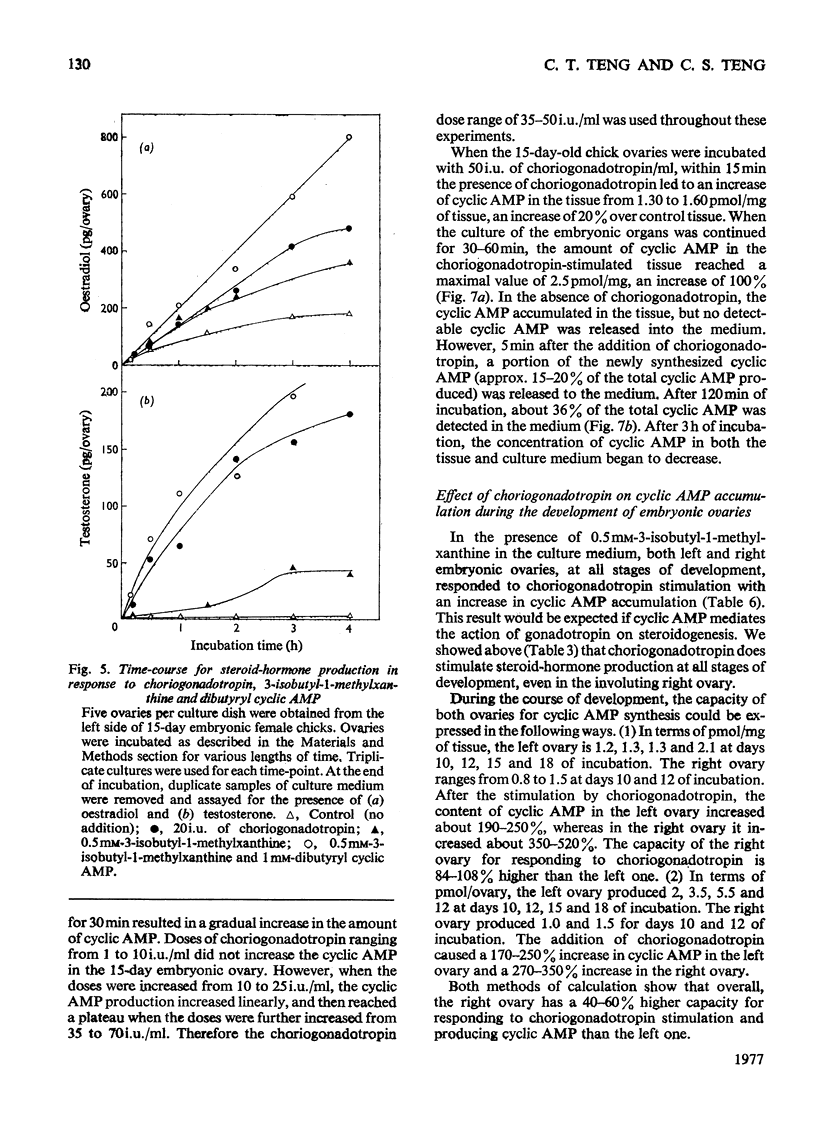
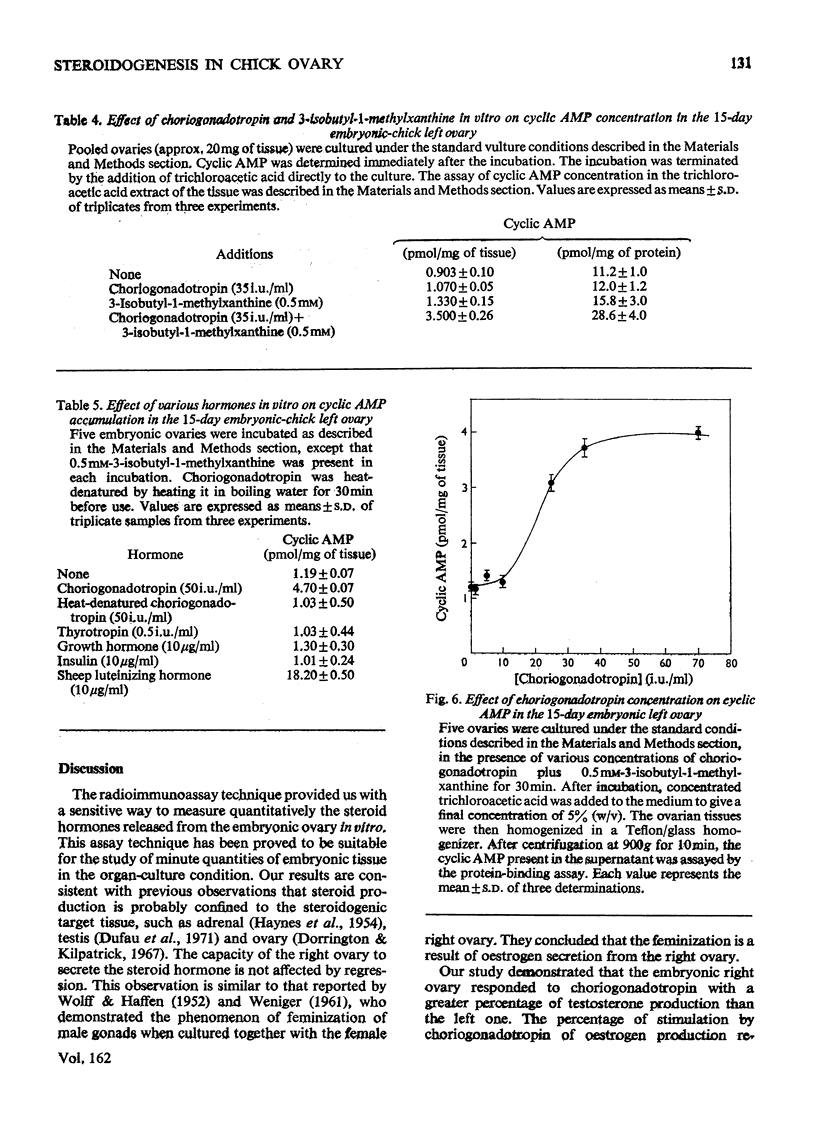
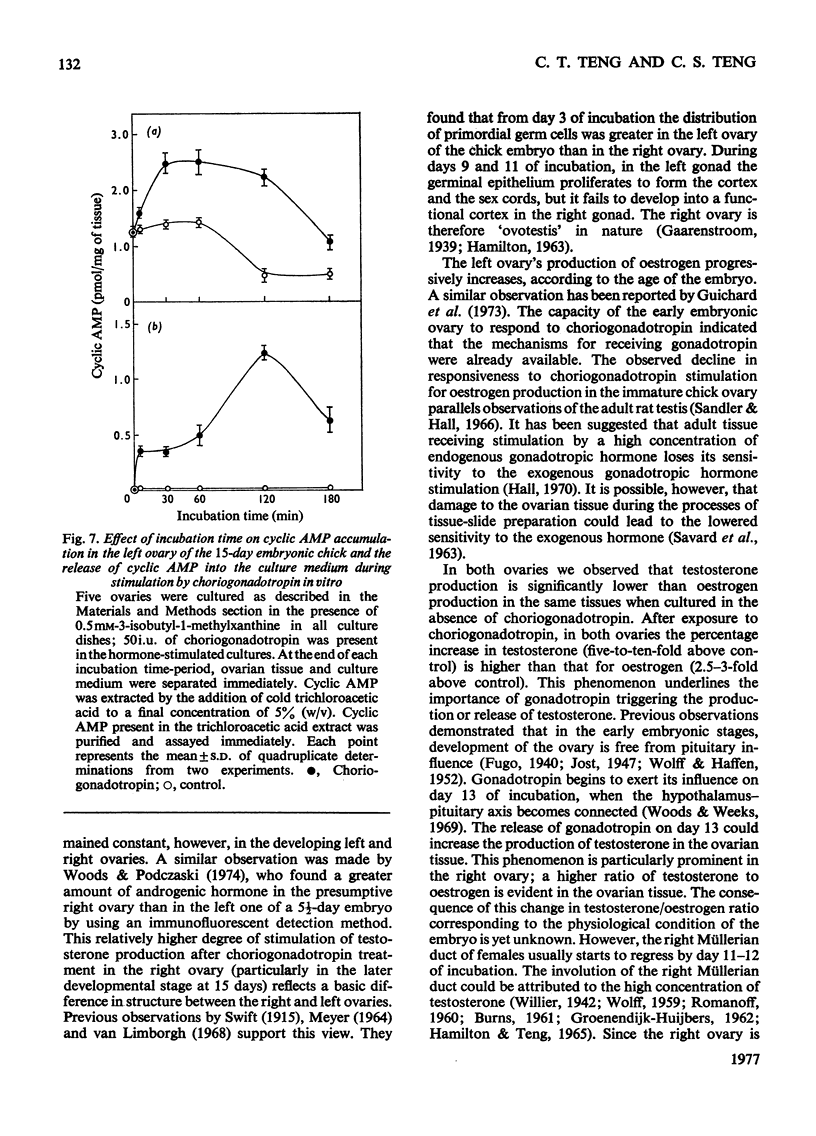
1. We investigated the production of steroid hormones by the ovaries of the developing embryonic chick under conditions of organ culture. Radioimmunoassay techniques were used to measure the amount of steroid hormone released into the culture medium. Stimulation of the production of steroid hormones by choriogonadotropin from the urine of pregnant human was dose-dependent. Oestradio and testosterone production was optimal when 20 i.u. of gonadotropic hormone was present in the culture medium 2. During development, both left and right ovaries responded to gonadotropic hormone stimulation with a 2.5-3-fold increase in oestrogen production. However, the right ovary was twice as efficient in testosterone production as the left one. The presence of dibutyryl cyclic AMP in the culture medium of the embryonic ovaries mimicked the effect of the gonadotropic hormone. 3. The human choriogonadotropic hormone stimulated cyclic AMP production in the embryonic ovarian tissue. Thyrotropin, growth hormone and insulin had no stimulating effect. 3-Isobutyl-1-methylxanthine potentiated the gonadotropic hormone effect by increasing the concentration of cyclic AMP in the ovarian tissue. 4. The amount of cyclic AMP synthesized in the embryonic ovary was gradually increased (from 1.2 to 6.5 pmol/mg of tissue) when incubated with increasing doses of human choriogonadotropic hormone in vitro. The newly synthesized cyclic AMP reached the maximum concnentration after 30 min of incubation, then decreased at 2 h of incubation. A portion of the newly synthesized cyclic AMP was released into the culture medium. 5. At various developmental stages, both left and right embryonic-chick ovaries responded to stimulation by gonadotropic hormone with an increase in cyclic AMP production. The cyclic AMP concentration in the right ovary was 80% higher than that in the corresponding left ovary.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Carchman R. A., Jaanus S. D., Rubin R. P. The role of adrenocorticotropin and calcium in adenosine cyclic 3', 5'-phosphate production and steroid release from the isolated, perfused cat adrenal gland. Mol Pharmacol. 1971 Sep;7(5):491–499. [PubMed] [Google Scholar]
- Coffino P., Bourne H. R., Tomkins G. M. Mechanism of lymphoma cell death induced by cyclic AMP. Am J Pathol. 1975 Oct;81(1):199–204. [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dorrington J. H., Kilpatrick R. Effect of adenosine 3',5'-(cyclic)-monophosphate on the synthesis of progestational steroids by rabbit ovarian tissue in vitro. Biochem J. 1967 Sep;104(3):725–730. doi: 10.1042/bj1040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington J. H., Kilpatrick R. Effects of pituitary hormones on progestational hormone production by the rabbit ovary in vivo and in vitro. J Endocrinol. 1966 May;35(1):53–63. doi: 10.1677/joe.0.0350053. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Catt K. J., Tsuruhara T. Gonadotrophin stimulation of testosterone production by the rat testis in vitro. Biochim Biophys Acta. 1971 Dec 21;252(3):574–579. doi: 10.1016/0304-4165(71)90161-9. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Watanabe K., Catt K. J. Stimulation of cyclic AMP production by the rat testis during incubation with hCG in vitro. Endocrinology. 1973 Jan;92(1):6–11. doi: 10.1210/endo-92-1-6. [DOI] [PubMed] [Google Scholar]
- GROENENDIJK-HUIJBERS M. M. The cranio-caudal regression of the right Mullerian duct in the chick embryo as studied by castration experiments ad estrogen treatment. Anat Rec. 1962 Jan;142:9–19. doi: 10.1002/ar.1091420103. [DOI] [PubMed] [Google Scholar]
- Galli F. E., Wassermann G. F. Steroid biosynthesis by gonads of 7- and 10-day-old chick embryos. Gen Comp Endocrinol. 1973 Aug;21(1):77–83. doi: 10.1016/0016-6480(73)90157-3. [DOI] [PubMed] [Google Scholar]
- Groenendijk-Huijbers M. M., Burggraaff J. M. Experimental studies on the capability of embryonic and young chick testes to regress embryonic chick oviducts. Anat Anz. 1974;135(1-2):43–46. [PubMed] [Google Scholar]
- Guichard A., Cedard L., Haffen K. Aspect comparatif de la synthèse de stéroïdes sexuels par les gonades embryonnaires de poulet à differents stades du développement (étude en culture organotypique à partir de précurseurs radioactifs. Gen Comp Endocrinol. 1973 Feb;20(1):16–28. doi: 10.1016/0016-6480(73)90126-3. [DOI] [PubMed] [Google Scholar]
- HALL P. F., EIK-NES K. B. The action of gonadotropic hormones upon rabbit testis in vitro. Biochim Biophys Acta. 1962 Oct 8;63:411–422. doi: 10.1016/0006-3002(62)90105-1. [DOI] [PubMed] [Google Scholar]
- HAYNES R. C., Jr, SUTHERLAND E. W., RALL T. W. The role of cyclic adenylic acid in hormone action. Recent Prog Horm Res. 1960;16:121–138. [PubMed] [Google Scholar]
- HAYNES R. C., Jr The activation of adrenal phosphorylase by the adrenocorticotropic hormone. J Biol Chem. 1958 Nov;233(5):1220–1222. [PubMed] [Google Scholar]
- HAYNES R., SAVARD K., DORFMAN R. I. The action of adrenocorticotropic hormone on beef adrenal slices. J Biol Chem. 1954 Apr;207(2):925–938. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARSH J. M., SAVARD K. THE ACTIVATION OF LUTEAL PHOSPHORYLASE BY LUTEINIZING HORMONE. J Biol Chem. 1964 Jan;239:1–7. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MEYER D. B. THE MIGRATION OF PRIMORDIAL GERM CELLS IN THE CHICK EMBRYO. Dev Biol. 1964 Aug;10:154–190. doi: 10.1016/0012-1606(64)90009-0. [DOI] [PubMed] [Google Scholar]
- Maraud R., Stoll R., Coulaud H. Action de la greffe testiculaire sur les canaux de Müller de l'embryon de poulet. C R Seances Soc Biol Fil. 1966;160(5):964–966. [PubMed] [Google Scholar]
- Means A. R., MacDougall E., Soderling T. R., Corbin J. D. Testicular adenosine 3':5'-monophosphate-dependent protein kinase. Regulation by follicle-stimulating hormone. J Biol Chem. 1974 Feb 25;249(4):1231–1238. [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. The regulatory role of adenosine-3', 5'-phosphate. Cold Spring Harb Symp Quant Biol. 1961;26:347–354. doi: 10.1101/sqb.1961.026.01.042. [DOI] [PubMed] [Google Scholar]
- SAVARD K., MARSH J. M., HOWELL D. S. PROGESTERONE BIOSYNTHESIS IN LUTEAL TISSUE: ROLE OF NICOTINAMIDE ADENINE DINUCLEOTIDE PHOSPHATE AND NADP-LINKED DEHYDROGENASES. Endocrinology. 1963 Nov;73:554–563. doi: 10.1210/endo-73-5-554. [DOI] [PubMed] [Google Scholar]
- SAVARD K., MARSH J. M., RICE B. F. GONADOTROPINS AND OVARIAN STEROIDOGENESIS. Recent Prog Horm Res. 1965;21:285–365. [PubMed] [Google Scholar]
- Sandler R., Hall P. F. Stimulation in vitro by adenosine-3',5'-cyclic monophosphate of steroidogenesis in rat testis. Endocrinology. 1966 Sep;79(3):647–649. doi: 10.1210/endo-79-3-647. [DOI] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Studies on sex-organ development. Isolation and characterization of an oestrogen receptor from chick Müllerian duct. Biochem J. 1975 Aug;150(2):183–190. doi: 10.1042/bj1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Studies on sex-organ development. Ontogeny of cytoplasmic oestrogen receptor in chick Müllerian duct. Biochem J. 1975 Aug;150(2):191–194. doi: 10.1042/bj1500191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T. Study on sex-organ development. Oestrogen-receptor translocation in the developing chick Müllerian duct. Biochem J. 1976 Jan 15;154(1):1–9. doi: 10.1042/bj1540001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Limborgh J. Le premier indice de la différenciation sexuelle des gonades chez l'embryon de poulet. Arch Anat Microsc Morphol Exp. 1968 Jan-Mar;57(1):79–90. [PubMed] [Google Scholar]
- WOLF E., HAFFEN K. Action féminisante de la gonade droite de l'embryon femelle de canard en culture in vitro. C R Seances Soc Biol Fil. 1952 Nov;146(21-22):1772–1774. [PubMed] [Google Scholar]
- Weniger J. P., Zeis A. Biosynthèse d'oestrogènes par les ébauches gonadiques de poulet. Gen Comp Endocrinol. 1971 Apr;16(2):391–395. doi: 10.1016/0016-6480(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Woods J. E., Podczaski E. S. Androgen synthesis in the gonads of the chick embryo. Gen Comp Endocrinol. 1974 Dec;24(4):413–423. doi: 10.1016/0016-6480(74)90155-5. [DOI] [PubMed] [Google Scholar]
- Woods J. E., Weeks R. L. Ontogenesis of the pituitary-gonadal axis in the chick embryo. Gen Comp Endocrinol. 1969 Oct;13(2):242–254. doi: 10.1016/0016-6480(69)90246-9. [DOI] [PubMed] [Google Scholar]


