Abstract
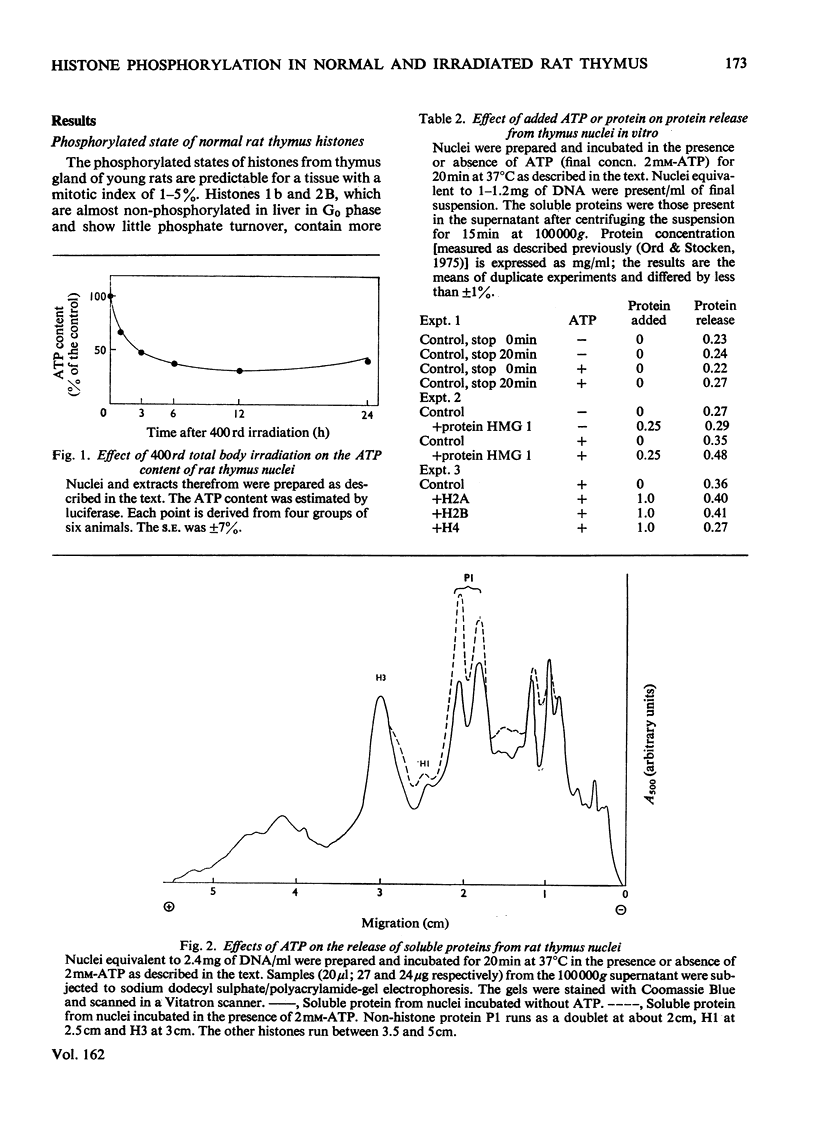
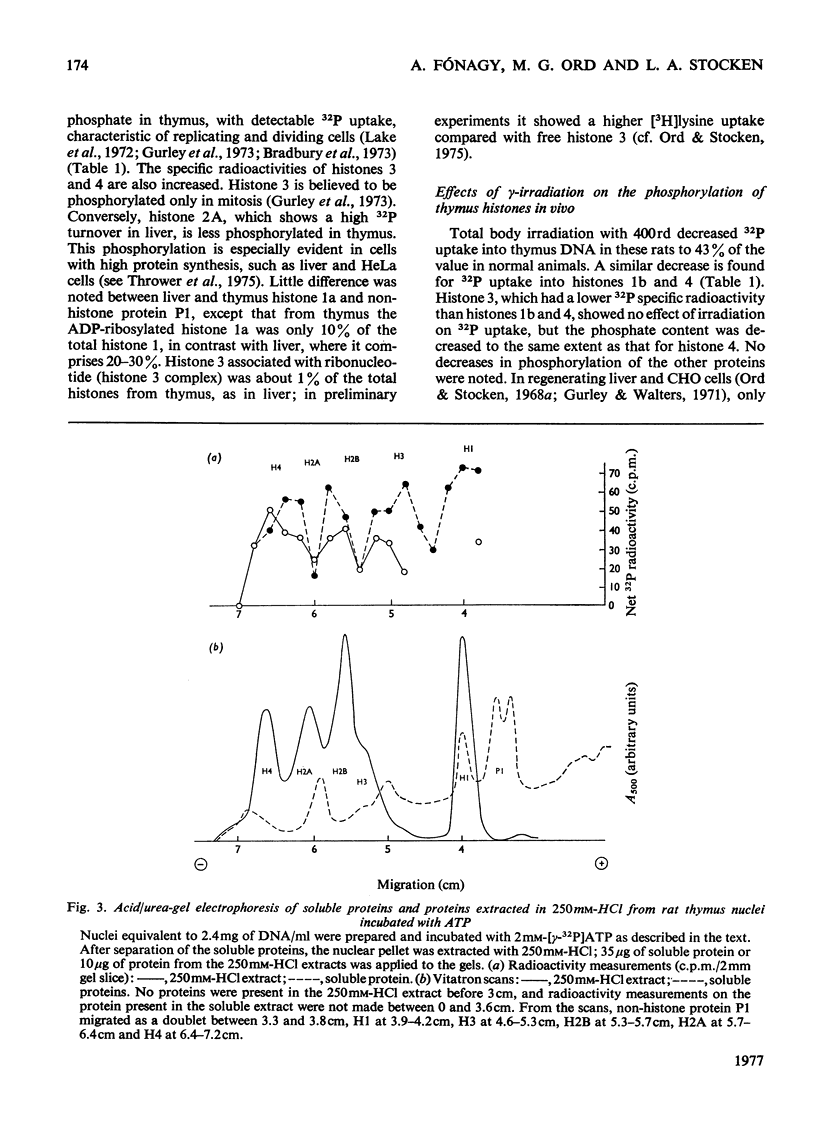
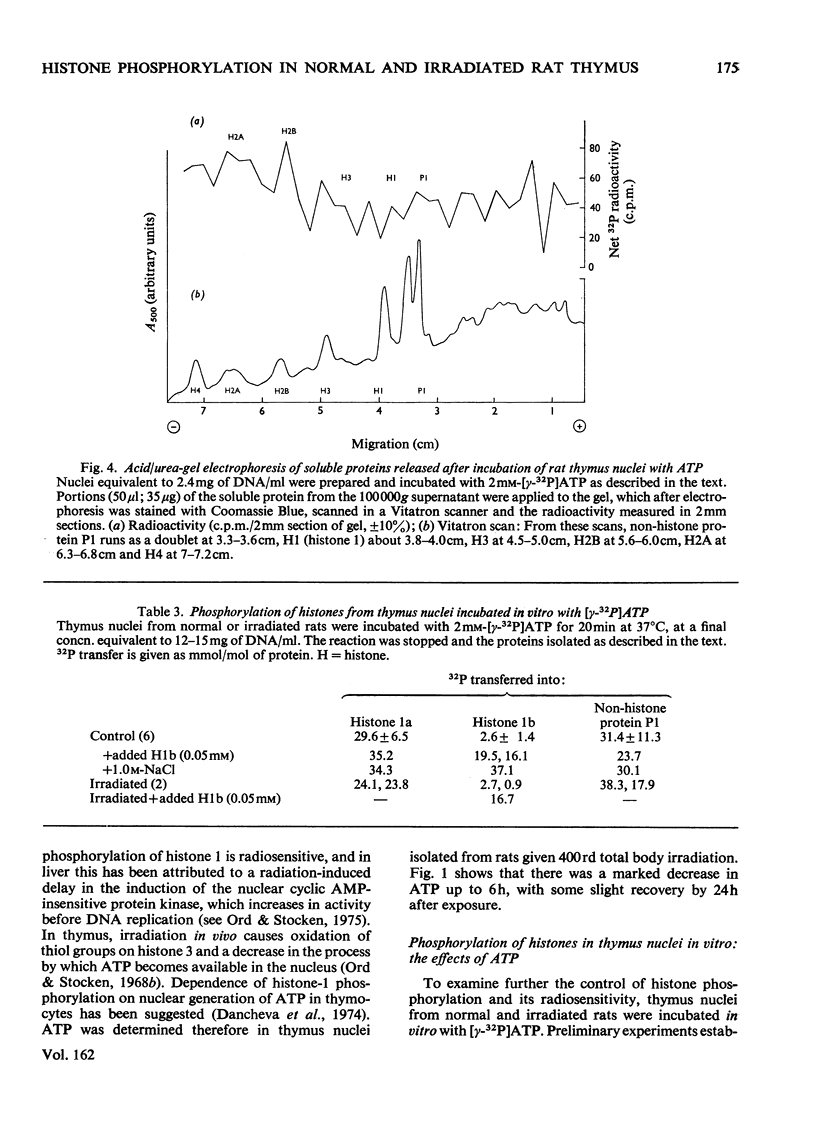
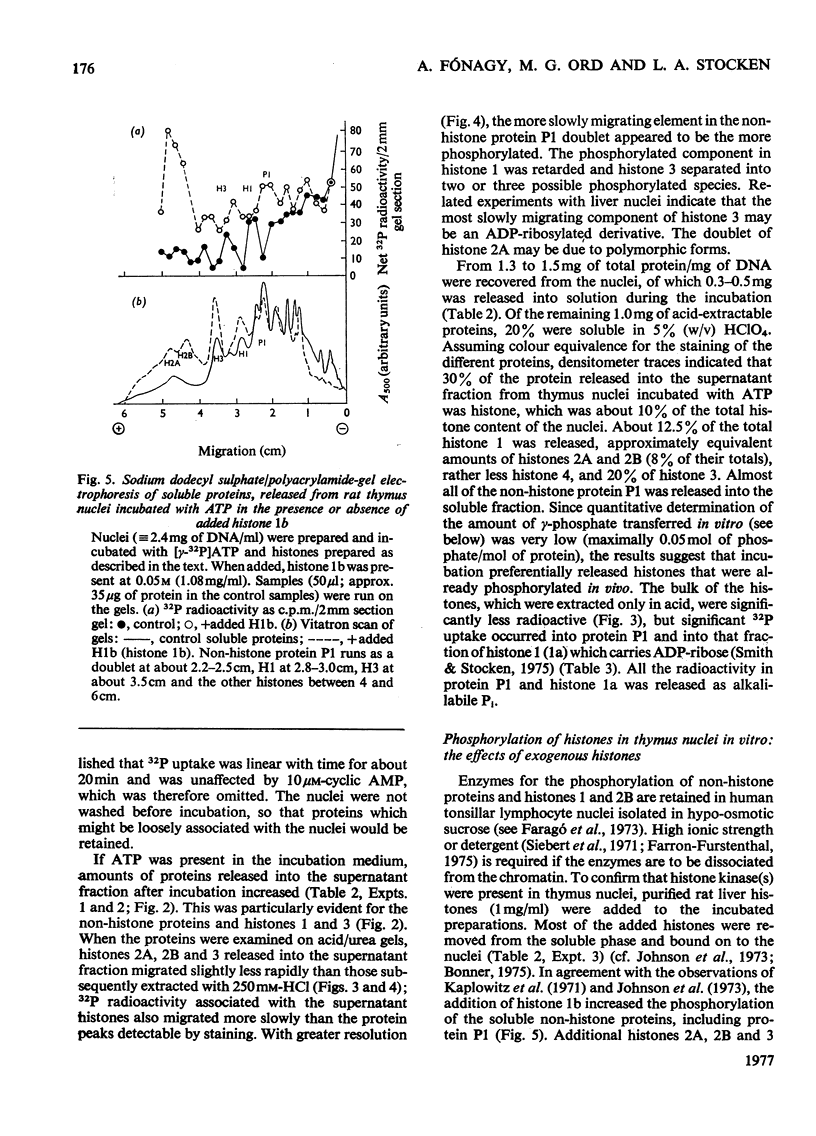
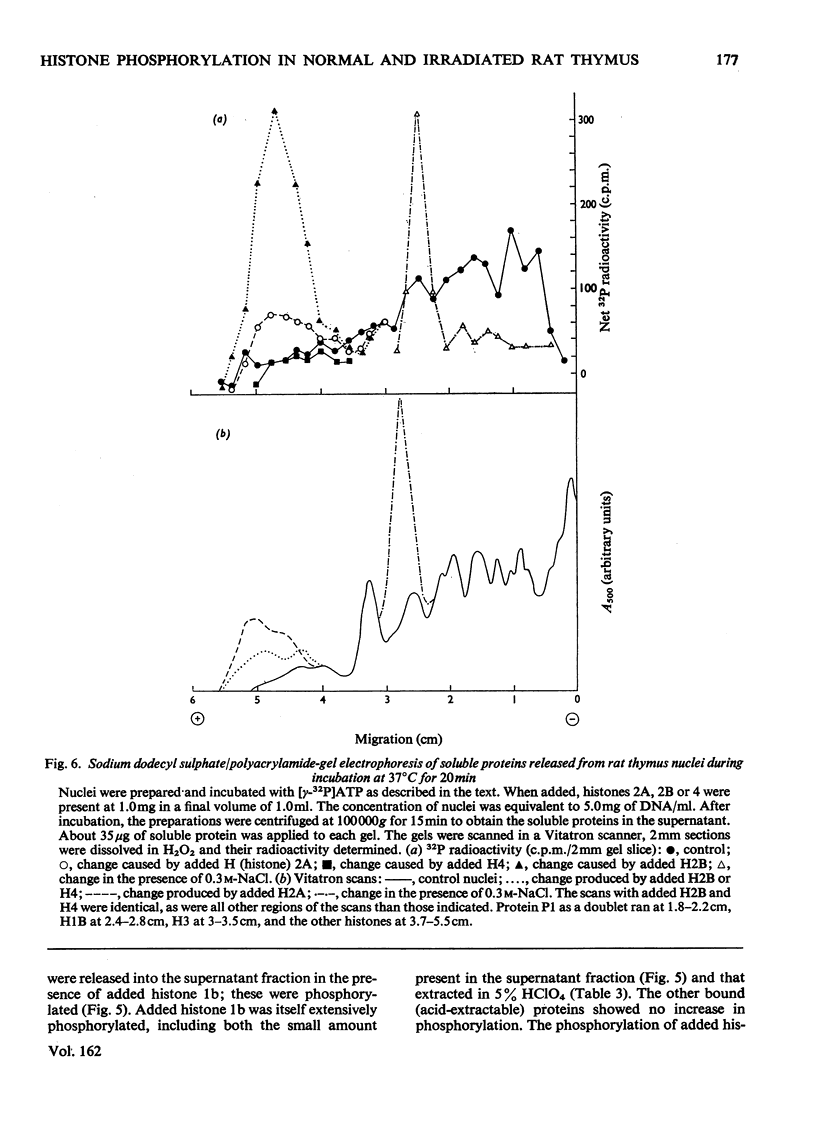
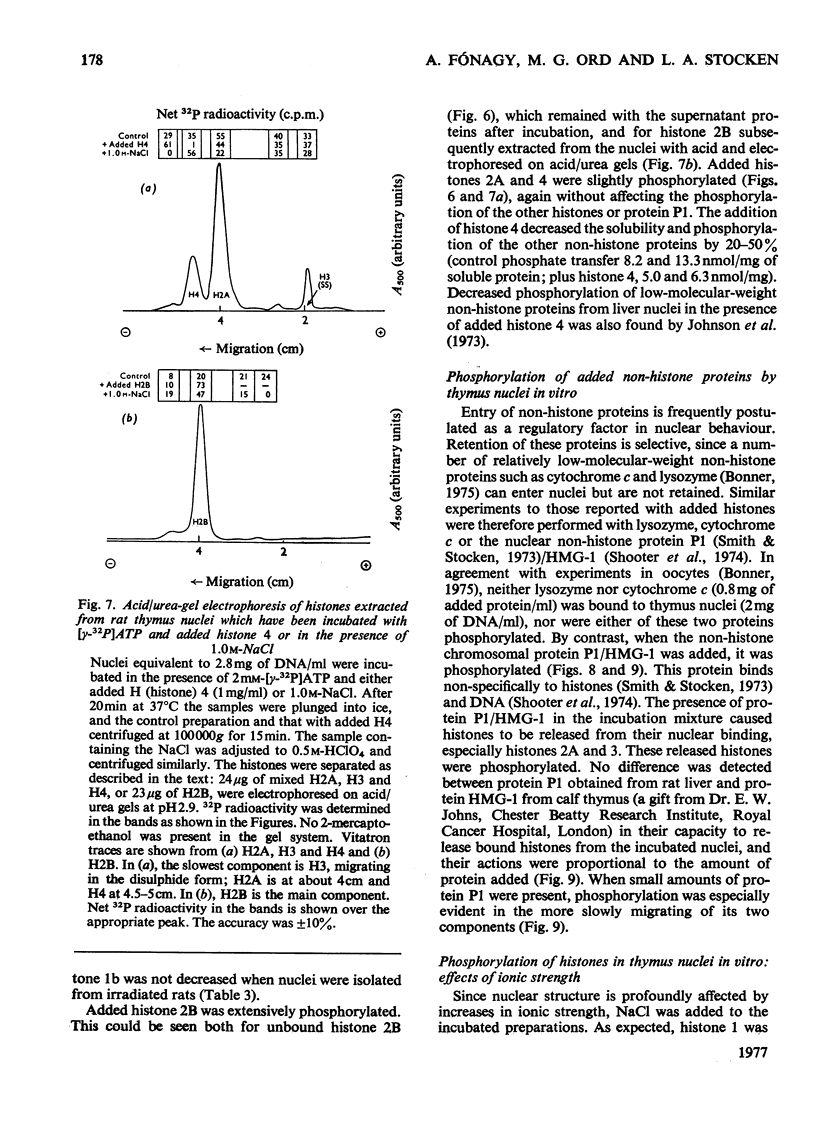
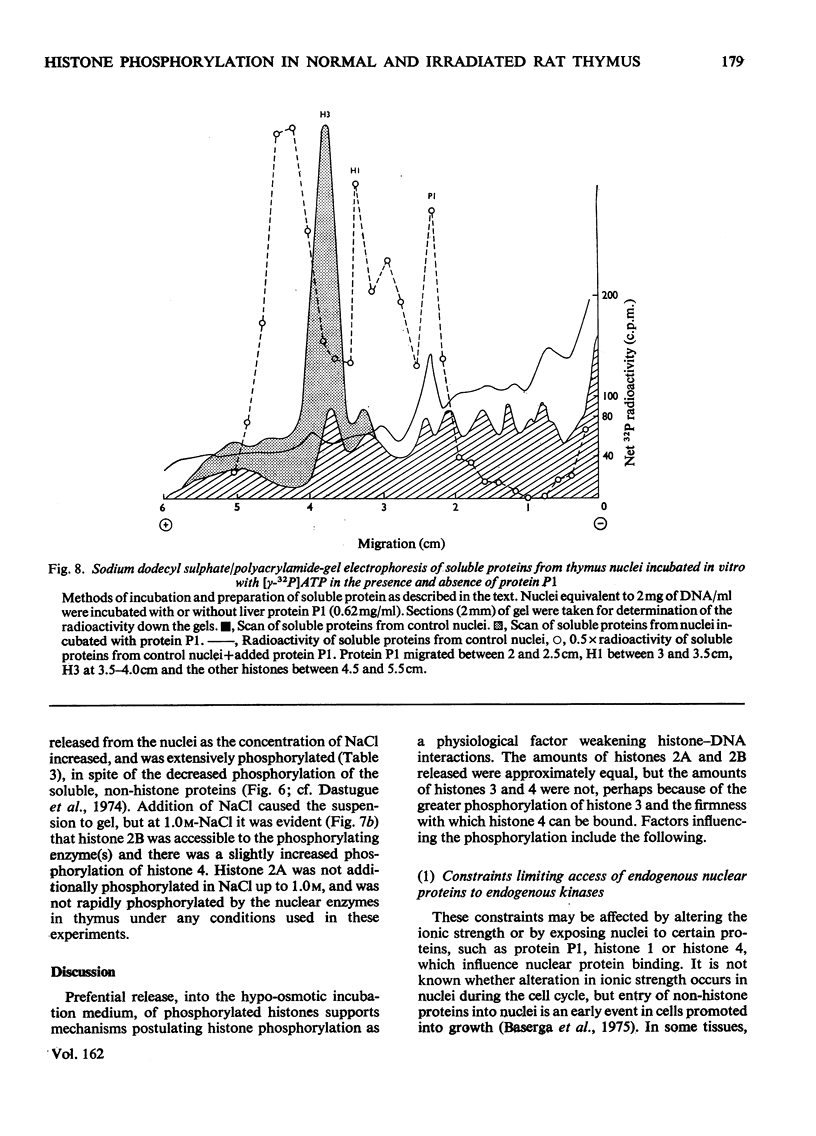
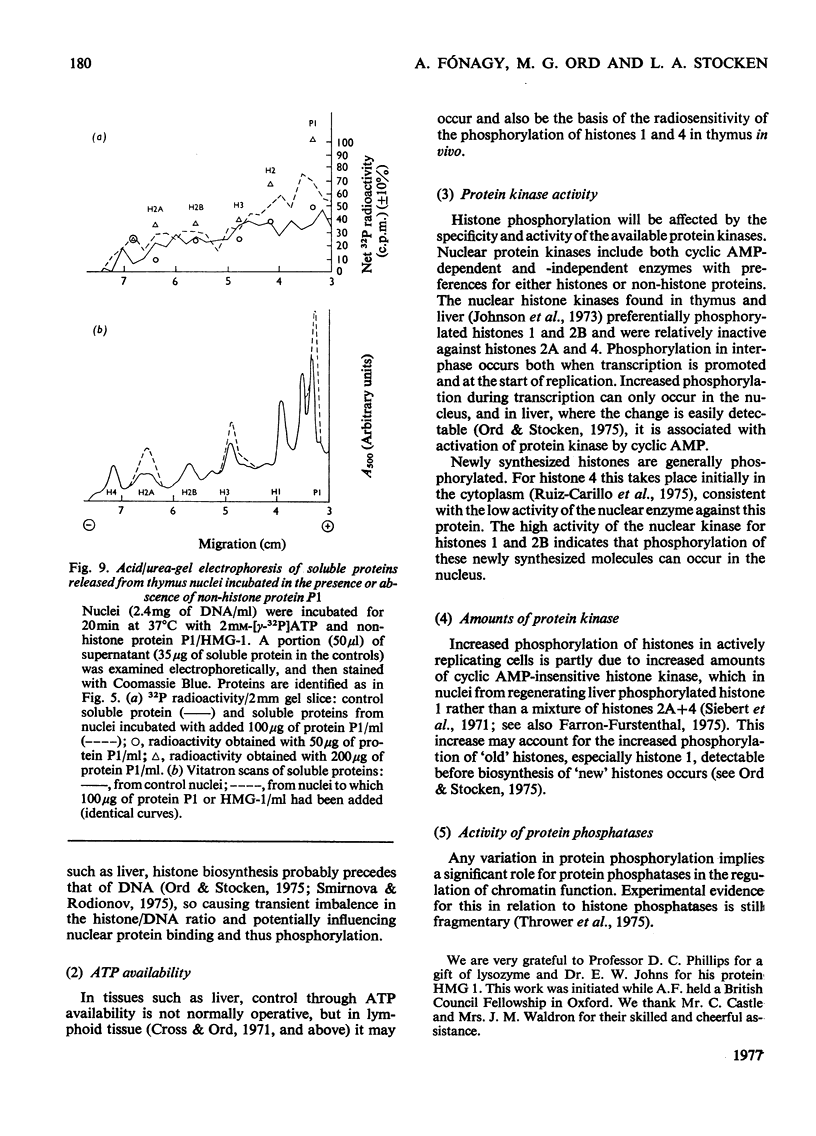
The phosphate content of rat thymus histones was determined. As expected for a replicating tissue, histones 1 and 2B were more phosphorylated and had higher 32P uptakes than did histones from resting liver nuclei; the other histones all showed 32P uptake, but the phosphate content and uptake of histone 2A was about half that for liver histone 2A. When thymus nuclei were incubated in a slightly hypo-osmotic medium, non-histone proteins and phosphorylated histones were released into solution; this was enhanced if ATP was present in the medium. [gamma-32P]ATP was incorporated into non-histone proteins, including protein P1, and into the ADP-ribosylated form of histone 1; negligible 32P was incprporated into the other, bound, histones. Histones 1 and 2B added to the incubation medium were extensively, and histones 2A and 4 slightly, phosphorylated. Histones released by increasing the ionic strength of the medium were phosphorylated. Added lysozyme and cytochrome c were neither bound nor phosphorylated, but added non-histone protein P1 was phosphorylated, causing other histones to be released from the nuclei, especially histones 2A and 3. The released histones were phosphorylated. gamma-Irradiation decreased 32P uptake into the non-ADP-ribosylated histones 1 and 4; phosphorylation of histone 1 in vitro was unaffected. The importance of non-histone proteins, ATP availability and nuclear protein kinases to the control of histone phosphorylation in vivo is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Cross M. E., Ord M. G. Changes in histone phosphorylation and associated early metabolic events in pig lymphocyte cultures transformed by phytohaemagglutinin or 6-N,2'-O-dibutyryladenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Aug;124(1):241–248. doi: 10.1042/bj1240241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastugue B., Tichonicky L., Kruh J. Multiple forms of protein kinase in liver cell. II. Nuclear kinases and cytosol phosvitin kinase. Biochimie. 1974;56(4):491–500. doi: 10.1016/s0300-9084(74)80064-7. [DOI] [PubMed] [Google Scholar]
- Faragó A., Antoni F., Takáts A., Fábián F. Adenosine 3':5'-monophosphate-dependent and independent histone kinases isolated from human tonsillar lymphocytes. Biochim Biophys Acta. 1973 Feb 28;297(2):517–526. [PubMed] [Google Scholar]
- Farron-Furstenthal F. Protein kinases in hepatoma, and adult and fetal liver of the rat. I. Subcellular distribution. Biochem Biophys Res Commun. 1975 Nov 3;67(1):307–314. doi: 10.1016/0006-291x(75)90317-4. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A. Response of histone turnover and phosphorylation to X irradiation. Biochemistry. 1971 Apr 27;10(9):1588–1593. doi: 10.1021/bi00785a013. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., Walters R. A., Tobey R. A. Histone phosphorylation in late interphase and mitosis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):744–750. doi: 10.1016/0006-291x(73)91307-7. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Vidali G., Littau V. C., Allfrey V. G. Modulation by exogenous histones of phosphorylation of non-histone nuclear proteins in isolated rat liver nuclei. J Biol Chem. 1973 Nov 10;248(21):7595–7600. [PubMed] [Google Scholar]
- Kaplowitz P. B., Platz R. D., Kleinsmith L. J. Nuclear phosphoproteins. 3. Increase in phosphory- lation during histone-phosphoprotein interaction. Biochim Biophys Acta. 1971 Mar 23;229(3):739–748. [PubMed] [Google Scholar]
- Lake R. S., Goidl J. A., Salzman N. P. F1-histone modification at metaphase in Chinese hamster cells. Exp Cell Res. 1972 Jul;73(1):113–121. doi: 10.1016/0014-4827(72)90108-5. [DOI] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A., Thrower S. Histone phosphorylations and their disparate roles in interphase. Subcell Biochem. 1976 Jun;4(2):147–156. [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Variations in the phosphate content and thiołdisulphide ratio of histones during the cell cycle. Studies with regenerating rat liver and sea urchins. Biochem J. 1968 Apr;107(3):403–410. doi: 10.1042/bj1070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The heterogeneity of histones. I. A quantitative analysis of calf histones in very long polyacrylamide gels. Biochemistry. 1969 Oct;8(10):3972–3979. doi: 10.1021/bi00838a013. [DOI] [PubMed] [Google Scholar]
- Peter E., Candido M., Dixon G. H. Acetylation of trout testis histones in vivo. Site of the modification in histone IIb 1 . J Biol Chem. 1972 Jun 25;247(12):3868–3873. [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Shooter K. V., Goodwin G. H., Johns E. W. Interactions of a purified non-histone chromosomal protein with DNA and histone. Eur J Biochem. 1974 Sep 1;47(2):263–270. doi: 10.1111/j.1432-1033.1974.tb03690.x. [DOI] [PubMed] [Google Scholar]
- Siebert G., Ord M. G., Stocken L. A. Histone phosphokinase activity in nuclear and cytoplasmic cell fractions from normal and regenerating rat livers. Biochem J. 1971 May;122(5):721–725. doi: 10.1042/bj1220721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. Chemical and metabolic properties of adenosine diphosphate ribose derivatives of nuclear proteins. Biochem J. 1975 Jun;147(3):523–529. doi: 10.1042/bj1470523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Stocken L. A. The characterization of a non-histone protein isolated from histone F1 preparations. Biochem J. 1973 Apr;131(4):859–861. doi: 10.1042/bj1310859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]


