Abstract
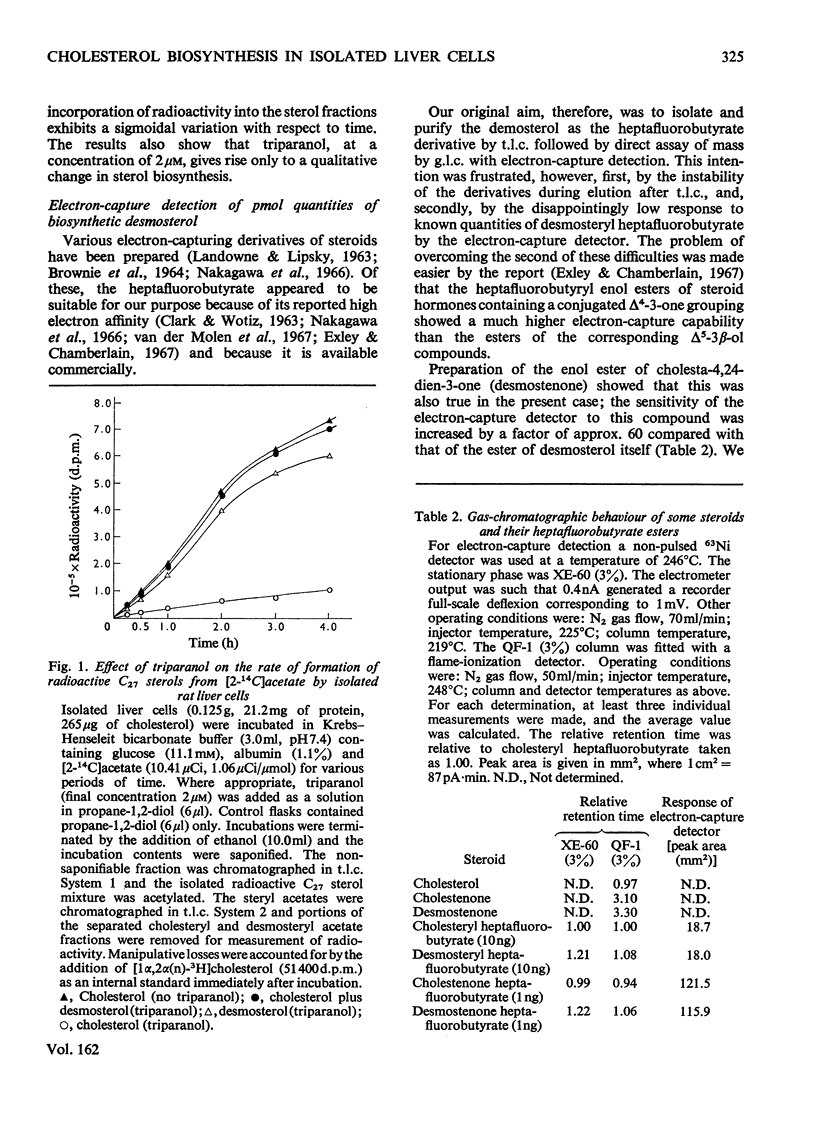
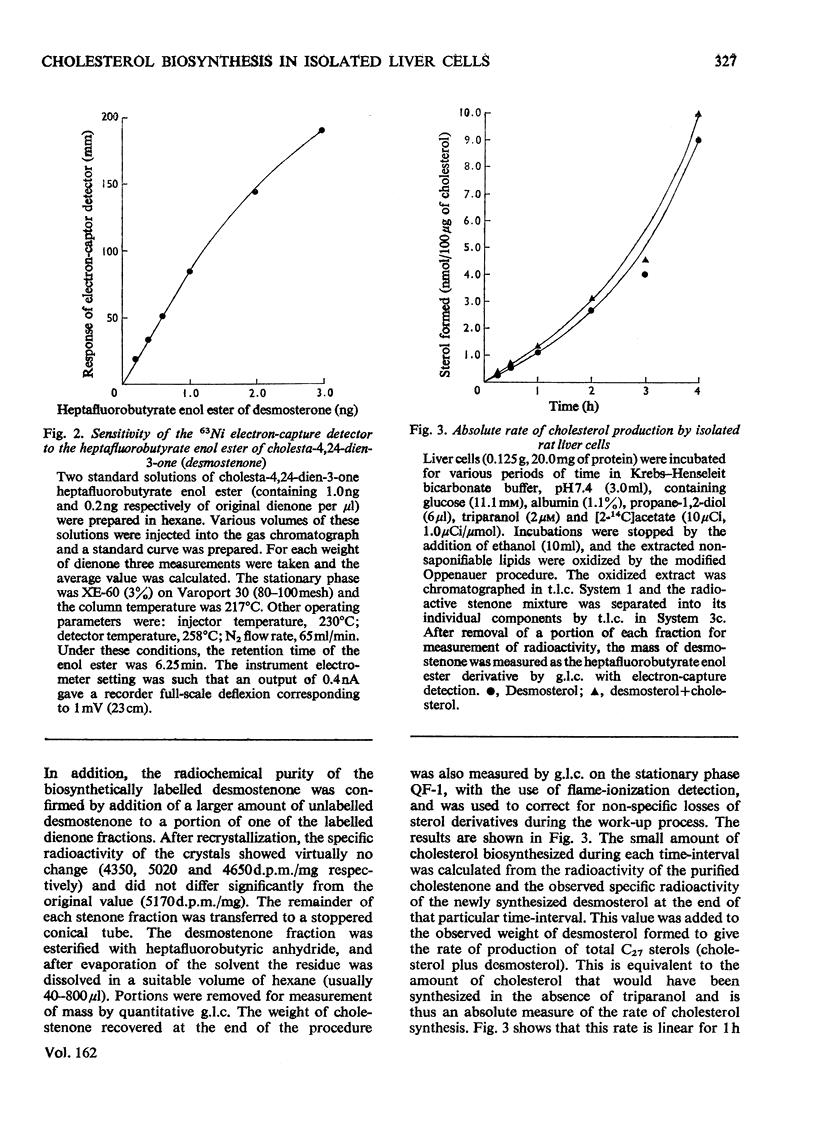
Triparanol [2-(4-chlorophenyl)-1-(4-diethylaminoethoxyphenyl)-1-p-tolylethanol] at a concentration of 2 micronm has no effect on the overall conversion of [2=14C]acetate into C27 sterols by isolated liver cells. In the presence of triparanol, however, the formation of radioactive cholesterol is inhibited by 85-90% and the balance of radioactivity appears in the C27 sterol desmosterol (cholesta-5,24-dien-3beta-ol). The very small weights of desmosterol which accumulate under these conditions were, as a routine, quantitatively converted into the heptafluorobutyrate 3-enol ester of cholesta-4,24-dien-3-one. This derivative has a high electron-capturing capability, a property that enables extremely small quantities (less than 0.25pmol) of the material to be accurately measured by gas chromatography with electron-capture detection. Measurements of the mass and specific radioactivity of the newly biosynthesized desmosterol formed in the presence of triparanol provides an accurate assessment of the amount of cholesterol that would be synthesized by the liver cells in the absence of the drug.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J., GOODMAN D. S., STEINBERG D. Studies of cholesterol biosynthesis. IV. Reduction of lanosterol to 24,25-dihydrolanosterol by rat liver homogenates. J Biol Chem. 1963 Apr;238:1283–1286. [PubMed] [Google Scholar]
- AVIGAN J., STEINBERG D., VROMAN H. E., THOMPSON M. J., MOSETTIG E. Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. J Biol Chem. 1960 Nov;235:3123–3126. [PubMed] [Google Scholar]
- BLOHM T. R., MACKENZIE R. D. Specific inhibition of cholesterol biosynthesis by a synthetic compound (MER-29). Arch Biochem Biophys. 1959 Nov;85:245–249. doi: 10.1016/0003-9861(59)90467-9. [DOI] [PubMed] [Google Scholar]
- BROWNIE A. C., VAN DER MOLEN H. J., NISHIZAWA E. E., EIK-NES K. B. DETERMINATION OF TESTOSTERONE IN HUMAN PERIPHERAL BLOOD USING GAS-LIQUID CHROMATOGRAPHY WITH ELECTRON CAPTURE DETECTION. J Clin Endocrinol Metab. 1964 Nov;24:1091–1102. doi: 10.1210/jcem-24-11-1091. [DOI] [PubMed] [Google Scholar]
- Barth C., Hackenschmidt J., Ullmann H., Decker K. Inhibition of cholesterol synthesis by (-)-hydroxycitrate in perfused rat liver. Evidence for an extramitochondrial mevalonate synthesis from acetyl coenzyme A. FEBS Lett. 1972 May 15;22(3):343–346. doi: 10.1016/0014-5793(72)80266-7. [DOI] [PubMed] [Google Scholar]
- Brunengraber H., Sabine J. R., Boutry M., Lowenstein J. M. 3- -Hydroxysterol synthesis by the liver. Arch Biochem Biophys. 1972 Jun;150(2):392–396. doi: 10.1016/0003-9861(72)90054-9. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. B., NELSON A. N., FRANTZ I. D., Jr THE SKIN STEROLS OF NORMAL AND TRIPARANOL-TREATED RATS. J Lipid Res. 1963 Apr;4:166–176. [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Dempsey M. E. Pathways of enzymic synthesis and conversion to cholesterol of delta-5,7,24-cholestatrien-3 beta-ol and other naturally occurring sterols. J Biol Chem. 1965 Nov;240(11):4176–4188. [PubMed] [Google Scholar]
- Dietschy J. M., Brown M. S. Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res. 1974 Sep;15(5):508–516. [PubMed] [Google Scholar]
- Dietschy J. M., McGarry J. D. Limitations of acetate as a substrate for measuring cholesterol synthesis in liver. J Biol Chem. 1974 Jan 10;249(1):52–58. [PubMed] [Google Scholar]
- Exley D., Chamberlain J. Properties of steroidal 3 enol heptafluorobutyrates. Steroids. 1967 Nov;10(5):509–526. doi: 10.1016/0039-128x(67)90126-2. [DOI] [PubMed] [Google Scholar]
- FRANTZ I. D., Jr, SANGHVI A. T., CLAYTON R. B. Detection of a sterol with the probable structure delta 5, 7, 24-cholestatrien-3 beta-ol in the intestinal wall of guinea pigs treated with triparanol. J Biol Chem. 1962 Nov;237:3381–3383. [PubMed] [Google Scholar]
- GOODMAN D. S., AVIGAN J., STEINBERG D. Studies of cholesterol biosynthesis. V. The time course and pathway of the later stages of cholesterol biosynthesis in the livers of intact rats. J Biol Chem. 1963 Apr;238:1287–1293. [PubMed] [Google Scholar]
- Galli G., Maroni S. Mass spectrometric investigations of some unsaturated sterols biosynthetically related to cholesterol. Steroids. 1967 Sep;10(3):189–197. doi: 10.1016/0039-128x(67)90046-3. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Mitropoulos K. A. Inhibition of cholesterol biosynthesis by carbon monoxide: accumulation of lanosterol and 24,25-dihydrolanosterol. Biochem J. 1972 Mar;127(1):315–317. doi: 10.1042/bj1270315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons G. F., Mitropoulos K. A., Ramananda K. A method for the rapid qualitative and quantitative analysis of 4,4-dimethyl sterols. J Lipid Res. 1973 Sep;14(5):589–591. [PubMed] [Google Scholar]
- Gibbons G. F., Mitropoulos K. A. The effect of carbon monoxide on the nature of the accumulated 4,4-dimethyl sterol precursors of cholesterol during its biosynthesis from (2-14C)mevalonic acid in vitro. Biochem J. 1973 Mar;132(3):439–448. doi: 10.1042/bj1320439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Biochem Soc Symp. 1968;27:61–86. [PubMed] [Google Scholar]
- Nakagawa K., McNiven N. L., Forchielli E., Vermeulen A., Dorfman R. I. Determination of testosterone by gas-liquid chromatography using an electron capture detector. I. Responses of halo-alkyl derivatives. Steroids. 1966 Apr;7(4):329–340. doi: 10.1016/0039-128x(66)90104-8. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Increased cholesterol-ester formation during forced cholesterol synthesis in rat hepatocytes. Eur J Biochem. 1975 Feb 21;51(2):337–342. doi: 10.1111/j.1432-1033.1975.tb03933.x. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Sundler R., Akesson B. Biosynthesis of fatty acids and cholesterol in isolated rat-liver parenchymal cells. Effect of albumin-bound fatty acids. Eur J Biochem. 1973 Nov 15;39(2):613–620. doi: 10.1111/j.1432-1033.1973.tb03160.x. [DOI] [PubMed] [Google Scholar]
- SCHROEPFER G. J., Jr Conversion of zymosterol-C-14 and zymostenol-H3 to cholesterol by rat liver homogenates and intact rats. J Biol Chem. 1961 Jun;236:1668–1673. [PubMed] [Google Scholar]
- Scallen T. J. Chemical synthesis of cholesta-5,7,24-trien-3-beta-ol and demonstration of its conversion to cholesterol in the rat. Biochem Biophys Res Commun. 1965 Oct 26;21(2):149–155. doi: 10.1016/0006-291x(65)90101-4. [DOI] [PubMed] [Google Scholar]
- Svoboda J. A., Thompson M. J. Separation of 24- and 25-dehydrocholesterols; and the impure state of commercial desmosterol preparations. J Lipid Res. 1967 Mar;8(2):152–154. [PubMed] [Google Scholar]


