Abstract
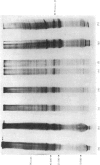
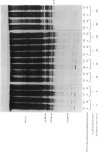
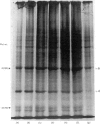
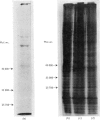
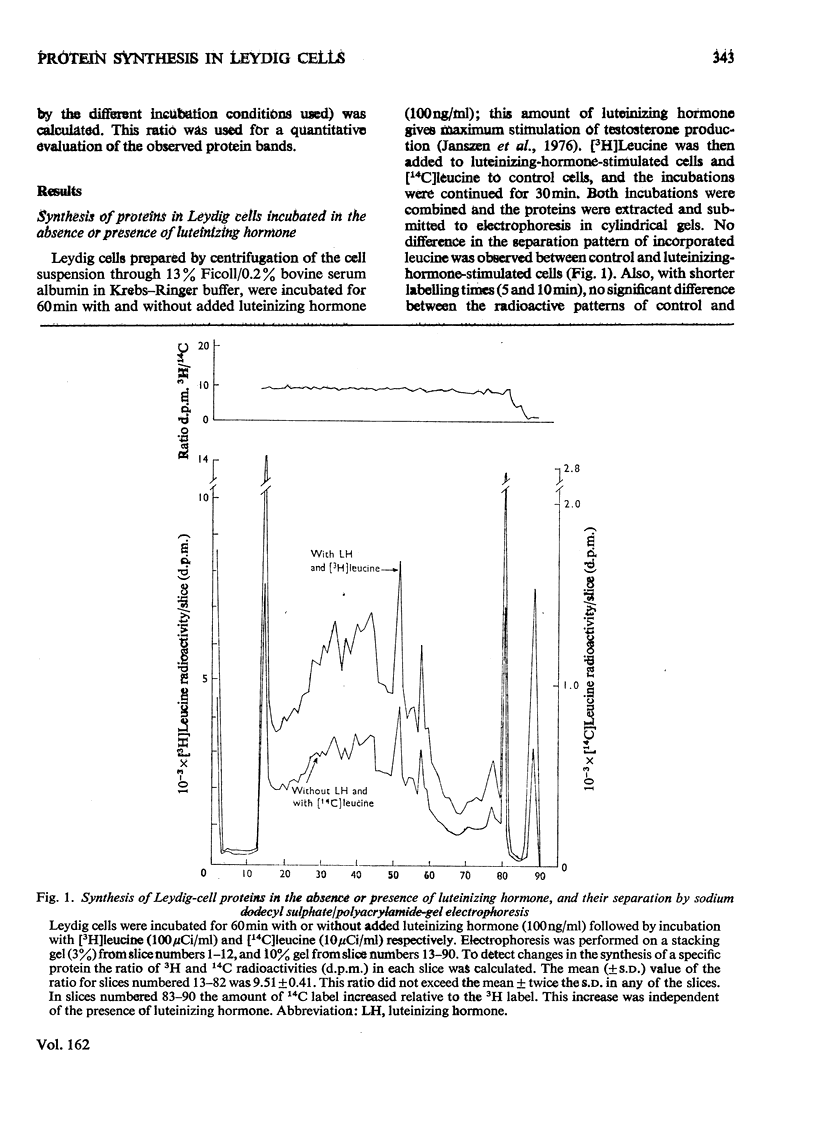
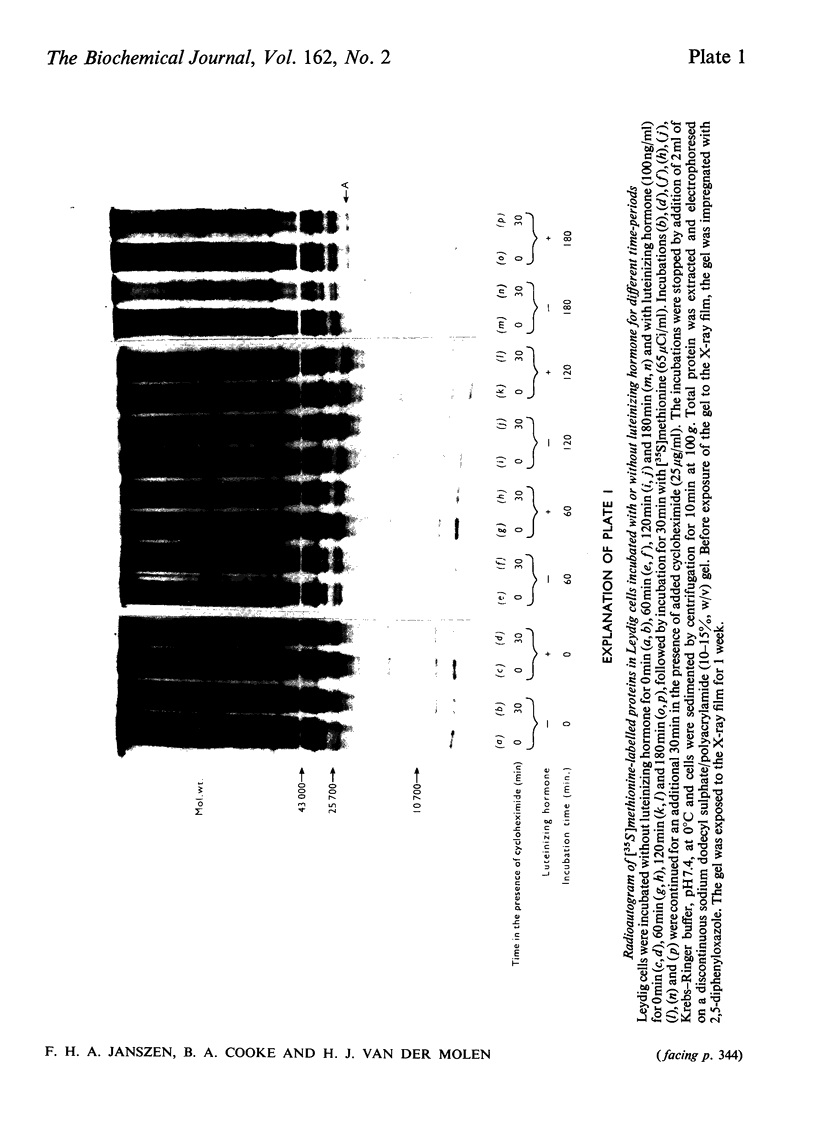
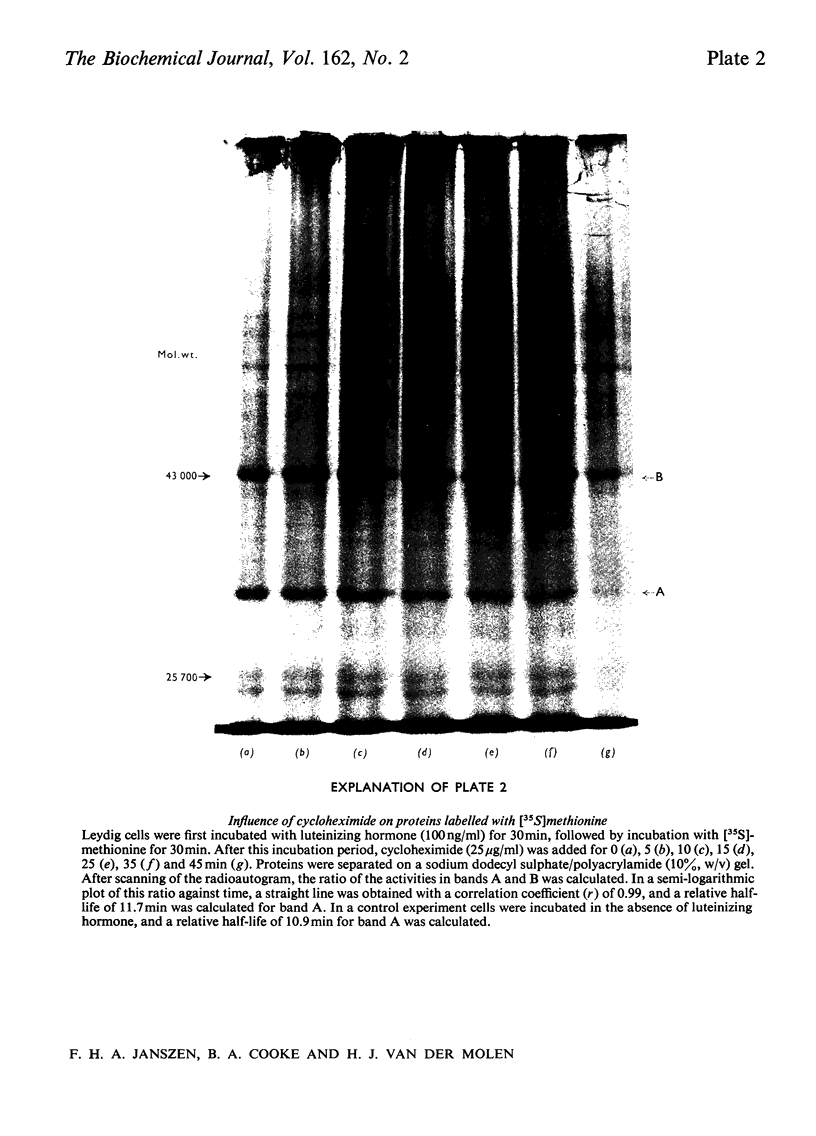
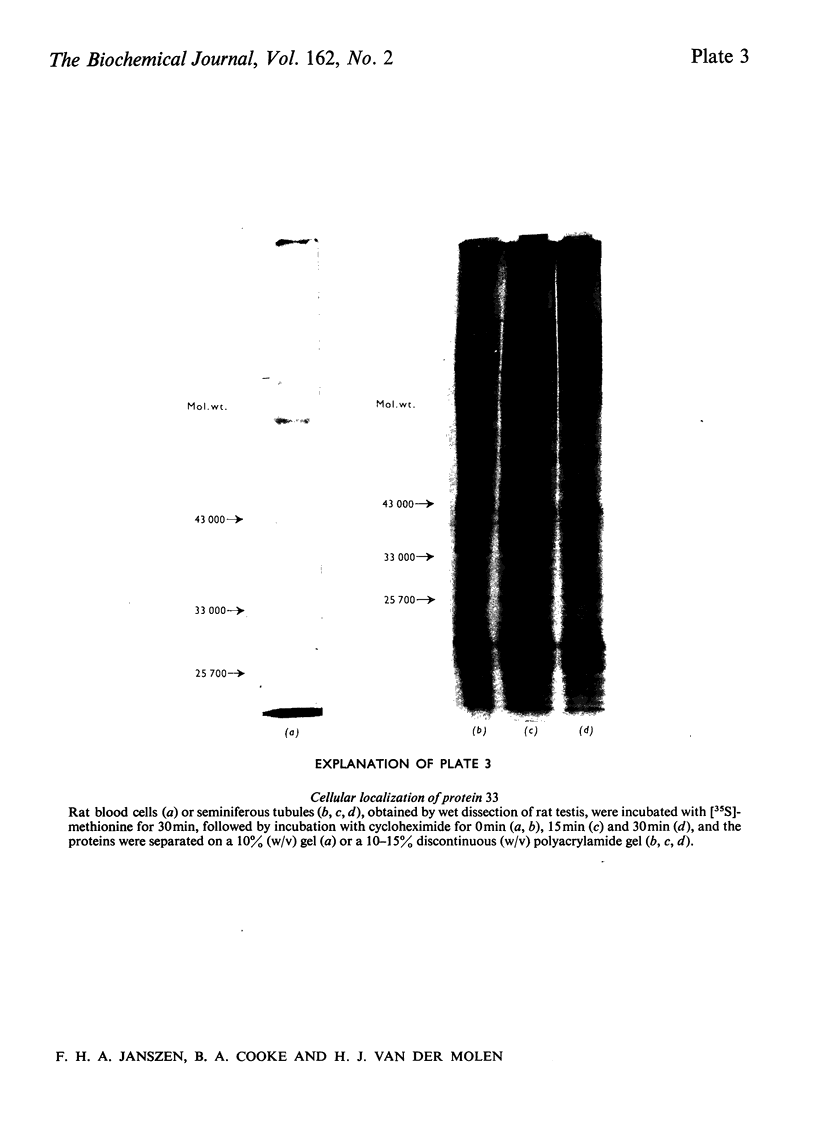
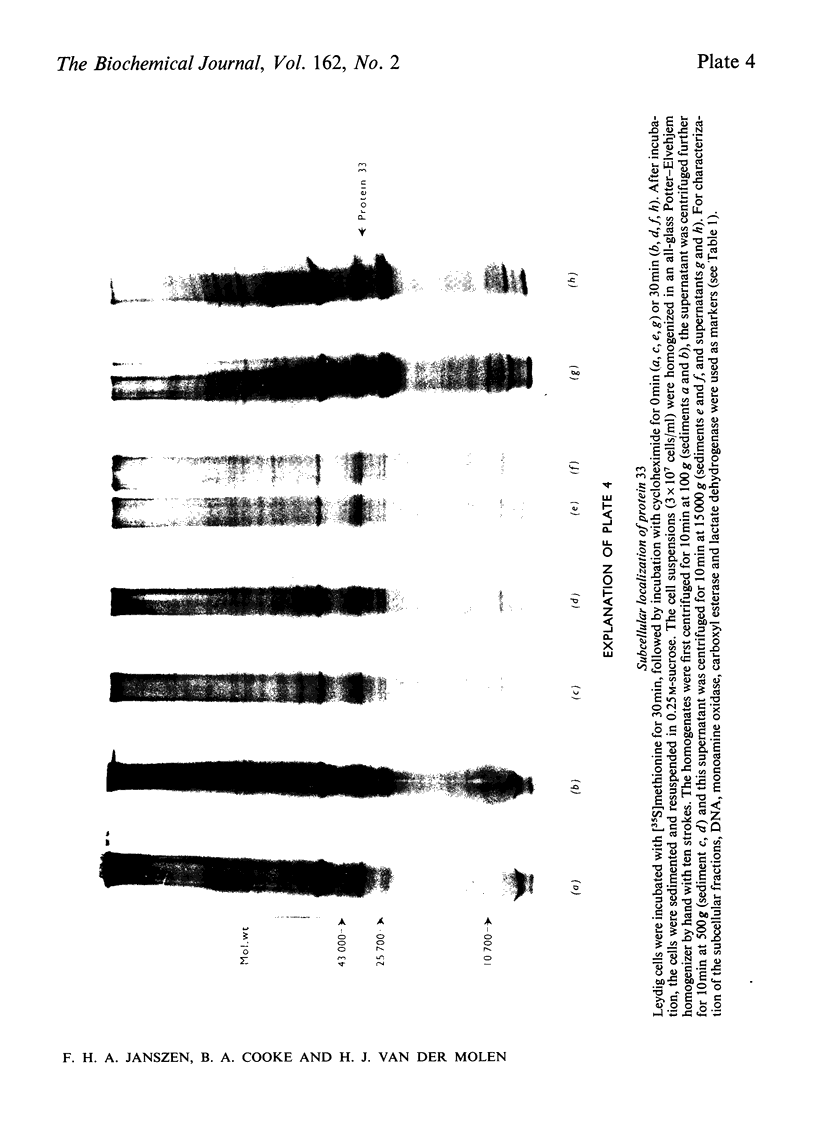
The effect of luteinizing hormone (luteotropin) and cycloheximide on specific protein synthesis in rat testis Leydig cells has been investigated. Proteins were labelled with either I114C]leucine, [3H]leucine or [35S]methionine during incubation with Leydig-cell suspensions in vitro. Total protein was extracted from the cells and separated by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. No detectable increase in the synthesis of specific proteins could be observed after incubation of Leydig cells with luteinizing hormone for up to 1 h. However, after a 2h incubation period, an increase in [35S]methionine incorporation was observed in a protein with an apparent mol.wt. of 21000 (referred to as 'protein 21"). When, after labelling of this protein with [35S]-methionine, Leydig cells were incubated for another 30min with cycloheximide, no decrease in radioactivity of this protein band was observed, indicating that it does not have a short half-life. However, another protein band was detected, which after incubation with cycloheximide disappeared rapidly, the reaction following first-order kinetics, with a half-life of about 11 min. This protein, with an apparent mol.wt. of 33000 (referred to as "protein 33"), was found to be located in the particulate fraction of the Leydig cell, and could not be demonstrated in other rat testis-cell types or blood cells. No effect of luteinizing hormone on molecular weight, subcellular localization or half-life of protein 33 was observed. A possible role for protein 33 and protein 21 in the mechanism of action of luteinizing hormone on testosterone production of Leydig cells is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Janszen F. H., Clotscher W. F., van der Molen H. J. Effect of protein-synthesis inhibitors on testosterone production in rat testis interstitial tissue and Leydig-cell preparations. Biochem J. 1975 Sep;150(3):413–418. doi: 10.1042/bj1500413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B. A., Lindh M. L., Janszen F. H. Correlation of protein kinase activation and testosterone production after stimulation of Leydig cells with luteinizing hormone. Biochem J. 1976 Dec 15;160(3):439–446. doi: 10.1042/bj1600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke B. A., van der Kemp A. J. Protein kinase activity in rat testis interstitial tissue. Effect of luteinizing hormone and other factors. Biochem J. 1976 Feb 15;154(2):371–378. doi: 10.1042/bj1540371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Catt K. J. Gonadotropic stimulation of interstitial cell functions of the rat testis in vitro. Methods Enzymol. 1975;39:252–271. doi: 10.1016/s0076-6879(75)39024-1. [DOI] [PubMed] [Google Scholar]
- FERGUSON J. J., Jr PROTEIN SYNTHESIS AND ADRENOCORTICOTROPIN RESPONSIVENESS. J Biol Chem. 1963 Aug;238:2754–2759. [PubMed] [Google Scholar]
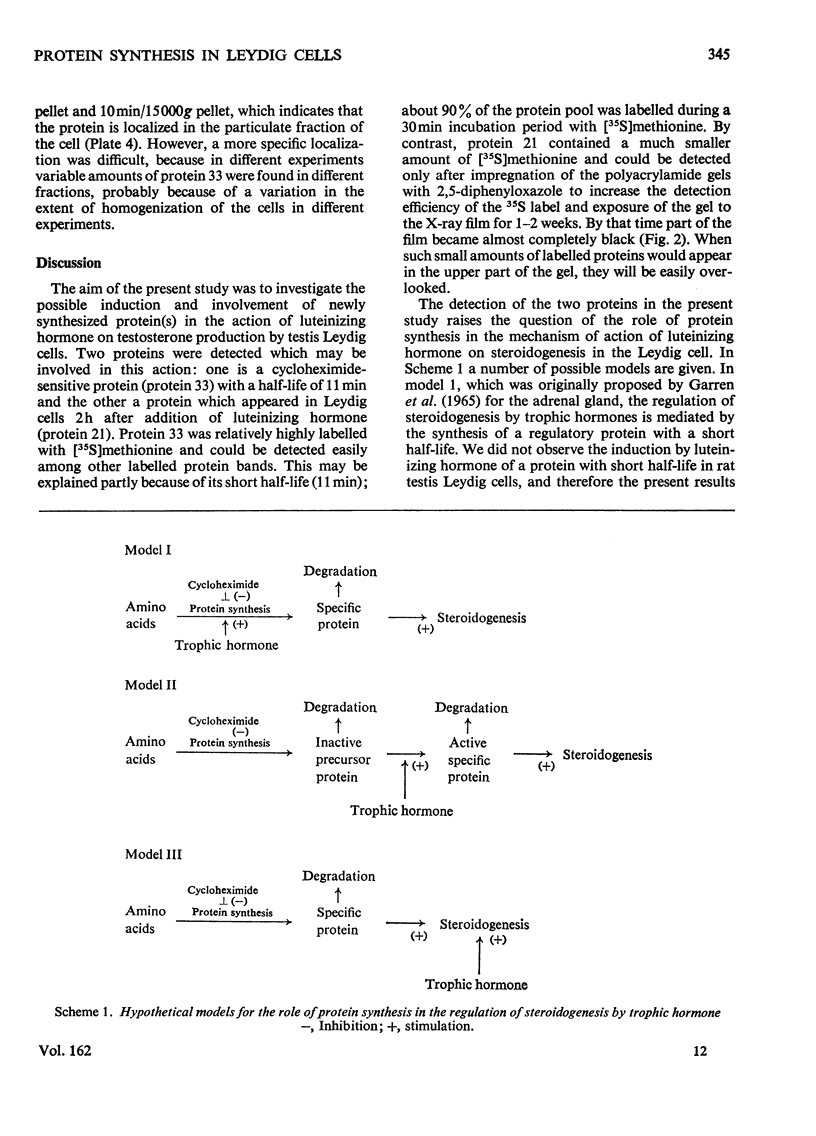
- Garren L. D., Ney R. L., Davis W. W. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL P. F., EIK-NES K. B. The action of gonadotropic hormones upon rabbit testis in vitro. Biochim Biophys Acta. 1962 Oct 8;63:411–422. doi: 10.1016/0006-3002(62)90105-1. [DOI] [PubMed] [Google Scholar]
- Hermier C., Combarnous Y., Jutisz M. Role of a regulating protein and molecular oxygen in the mechanism of action of luteinizing hormone. Biochim Biophys Acta. 1971 Sep 21;244(3):625–633. doi: 10.1016/0304-4165(71)90080-8. [DOI] [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., van Driel M. J., van der Molen H. J. Purification and characterization of Leydig cells from rat testes. J Endocrinol. 1976 Sep;70(3):345–359. doi: 10.1677/joe.0.0700345. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowry P. J., McMartin C. Measurement of the dynamics of stimulation and inhibition of steroidogenesis in isolated rat adrenal cells by using column perfusion. Biochem J. 1974 Aug;142(2):287–294. doi: 10.1042/bj1420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Dependence of gonadotropin-induced steroidogenesis upon RNA and protein synthesis in the interstitial cells of the rat testis. Biochim Biophys Acta. 1975 Dec 5;411(2):222–230. doi: 10.1016/0304-4165(75)90302-5. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Moudgal N. R., Greep R. O. Cessation of steroidogenesis in Leydig cell tumors after removal of luteinizing hormone and adenosine cyclic 3',5'-monophosphate. J Biol Chem. 1971 Aug 25;246(16):4978–4982. [PubMed] [Google Scholar]
- Moyle W. R., Ramachandran J. Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology. 1973 Jul;93(1):127–134. doi: 10.1210/endo-93-1-127. [DOI] [PubMed] [Google Scholar]
- Purvis J. L., Canick J. A., Rosenbaum J. H., Hologgitas J., Latif S. A. Control of cytochrome P-450 in rat testis mitochondria by human chorionic gonadotrophin. Arch Biochem Biophys. 1973 Nov;159(1):31–38. [PubMed] [Google Scholar]
- Rommerts F. F., Cooke B. A., van der Molen H. J. The role of cyclic AMP in the regulation of steroid biosynthesis in testis tissue. J Steroid Biochem. 1974 May;5(3):279–285. doi: 10.1016/0022-4731(74)90143-5. [DOI] [PubMed] [Google Scholar]
- Rommerts F. F., van Doorn L. G., Galjaard H., Cooke B. A., van der Molen H. J. Dissection of wet tissue and of freeze-dried sections in the investigation of seminiferous tubules and interstitial tissue from rat testis. J Histochem Cytochem. 1973 Jun;21(6):572–579. doi: 10.1177/21.6.572. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Jaanus S. D., Carchman R. A., Puig M. Reversible inhibition of ACTH-induced corticosteroid release by cycloheximide: evidence for an unidentified cellular messenger. Endocrinology. 1973 Sep;93(3):575–580. doi: 10.1210/endo-93-3-575. [DOI] [PubMed] [Google Scholar]
- Schulster D., Richardson M. C., Palfreyman J. W. The role of protein synthesis in adrenocorticotrophin action: effects of cycloheximide and puromycin on the steroidogenic response of isolated adrenocortical cells. Mol Cell Endocrinol. 1974 Dec;2(1):17–29. doi: 10.1016/0303-7207(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Schulster D., Tait S. A., Tait J. F., Mrotek J. Production of steroids by in vitro superfusion of endocrine tissue. 3. Corticosterone output from rat adrenals stimulated by adrenocorticotropin or cyclic 3',5'-adenosine monophosphate and the inhibitory effect of cycloheximide. Endocrinology. 1970 Mar;86(3):487–502. doi: 10.1210/endo-86-3-487. [DOI] [PubMed] [Google Scholar]
- Shin S. I. Studies on interstitial cells in tissue culture: steroid biosynthesis in monolayers of mouse testicular interstitial cells. Endocrinology. 1967 Sep;81(3):440–448. doi: 10.1210/endo-81-3-440. [DOI] [PubMed] [Google Scholar]
- Tsafriri A., Lieberman M. E., Barnea A., Bauminger S., Lindner H. R. Induction by luteinizing hormone of ovum maturation and of steroidogenesis in isolated Graafian follicles of the rat: role of RNA and protein synthesis. Endocrinology. 1973 Dec;93(6):1378–1386. doi: 10.1210/endo-93-6-1378. [DOI] [PubMed] [Google Scholar]
- Younglai E. V. Steroid production by the isolated rabbit ovarian follicle. III. Actinomycin D-insensitive stimulation of steroidogenesis by LH. Endocrinology. 1975 Feb;96(2):468–474. doi: 10.1210/endo-96-2-468. [DOI] [PubMed] [Google Scholar]