Abstract
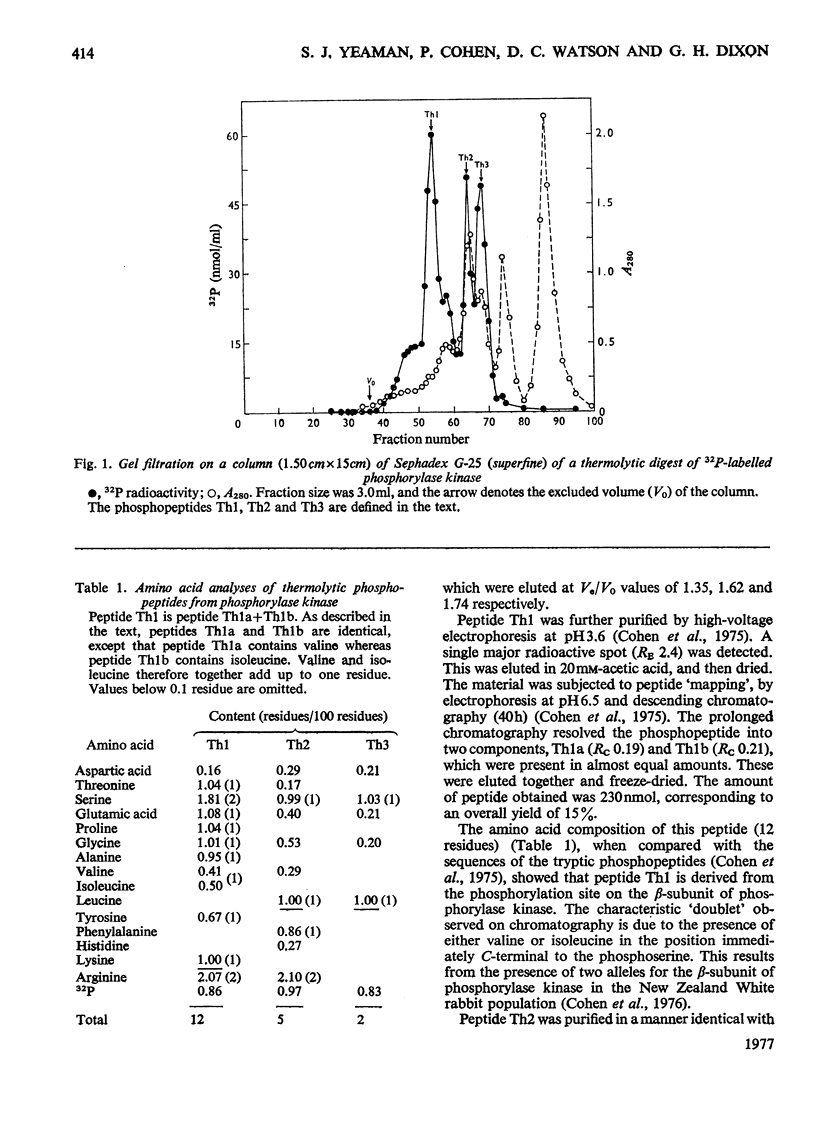
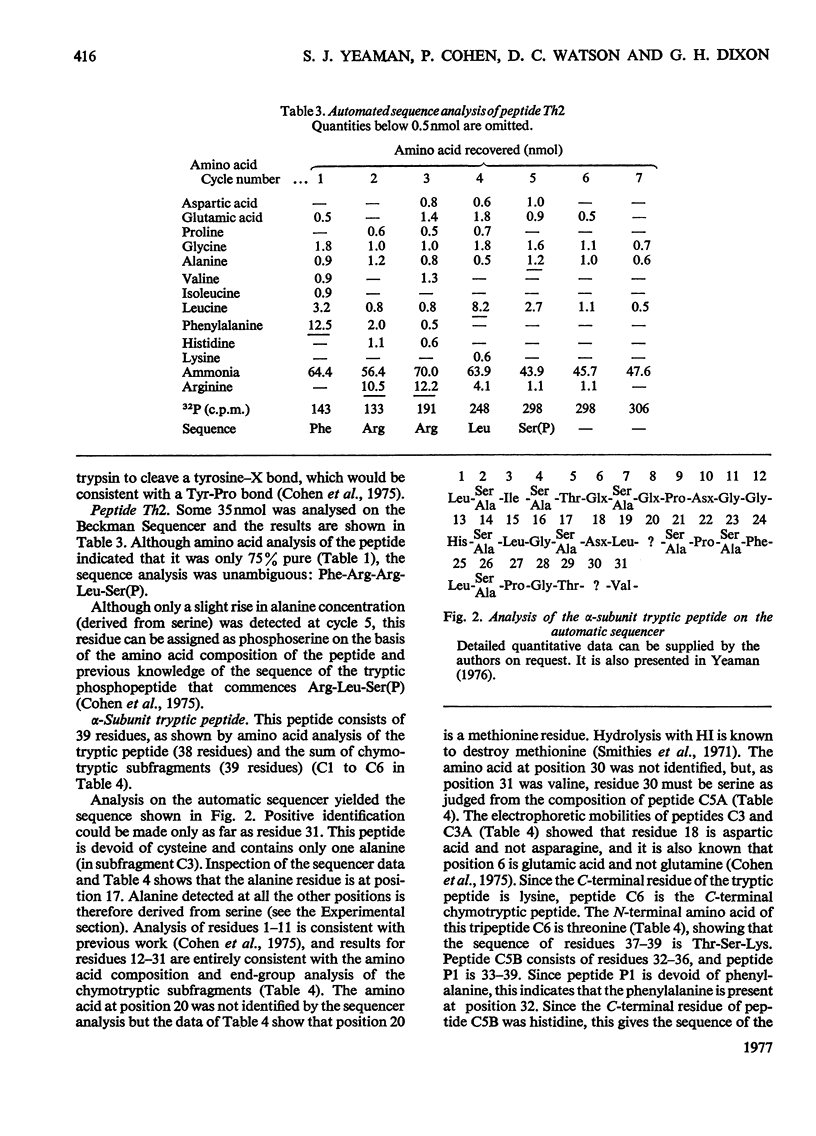
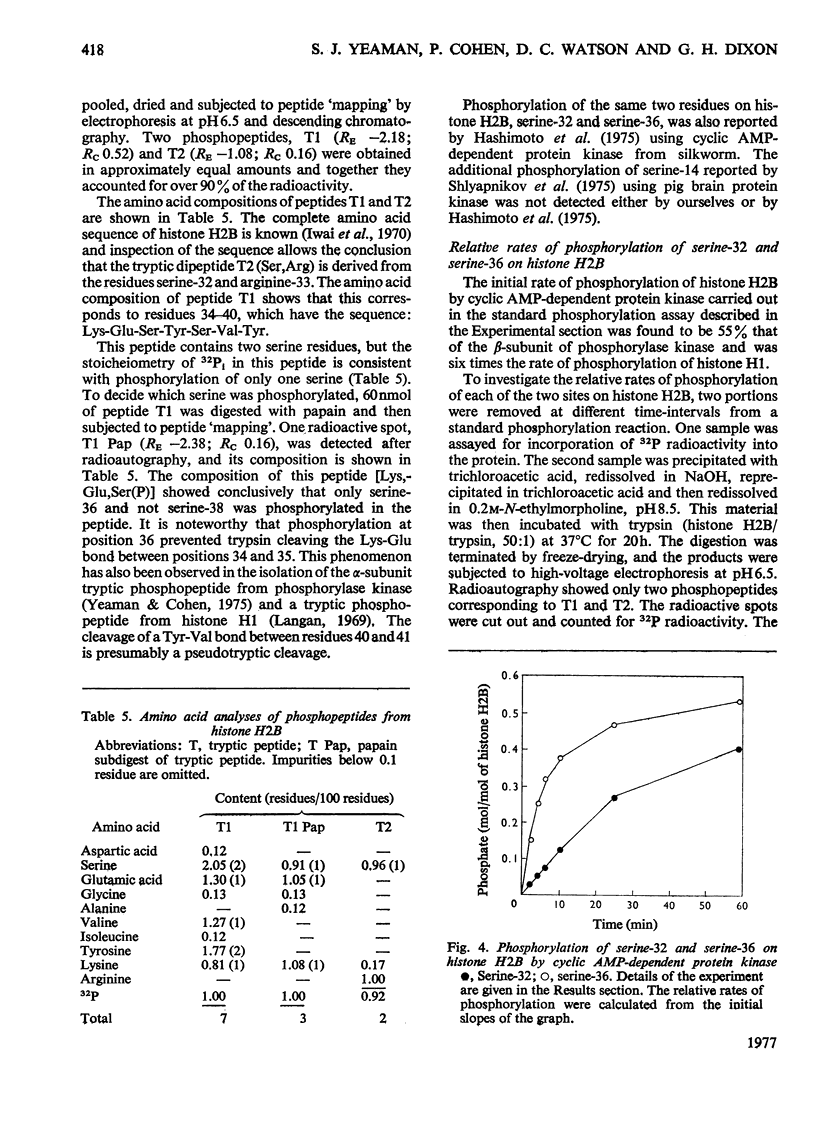
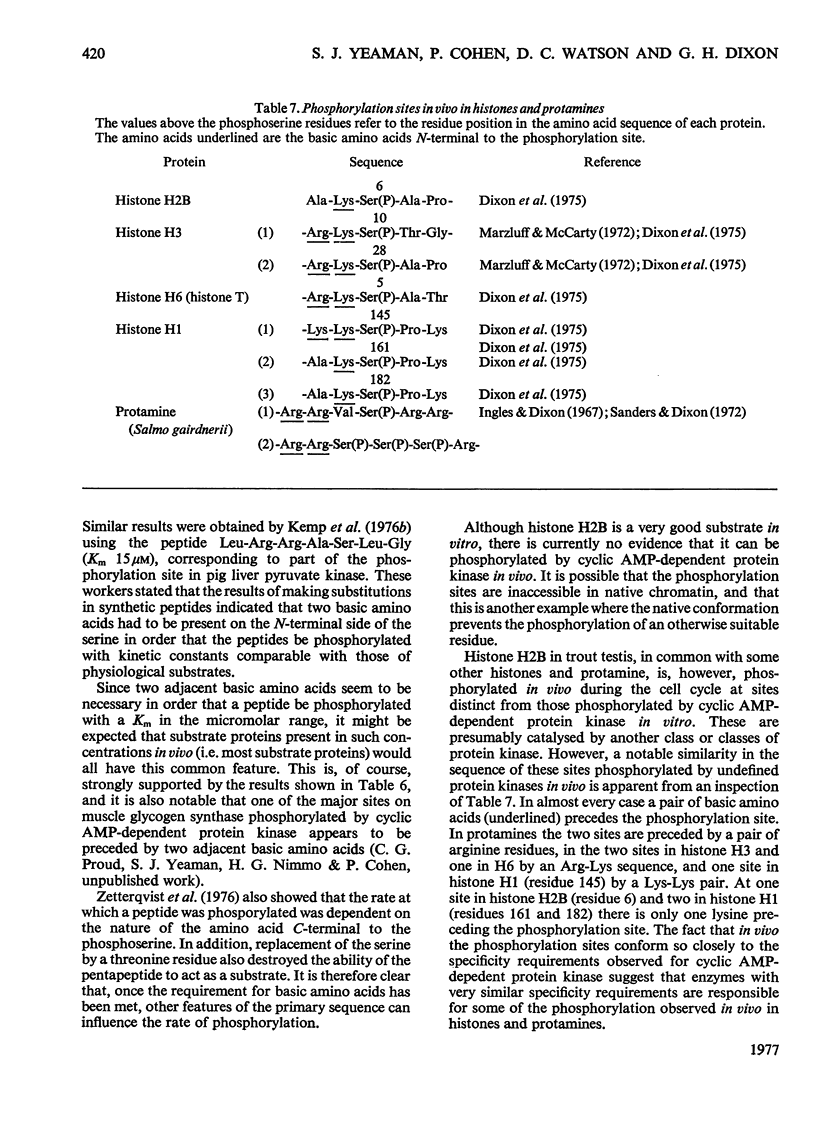
The known amino acid sequences at the two sites on phosphorylase kinase that are phosphorylated by cyclic AMP-dependent protein kinase were extended. The sequences of 42 amino acids around the phosphorylation site on the alpha-subunit and of 14 amino acids around the phosphorylation site on the beta-subunit were shown to be: alpha-subunit Phe-Arg-Arg-Leu-Ser(P)-Ile-Ser-Thr-Glu-Ser-Glx-Pro-Asx-Gly-Gly-His-Ser-Leu-Gly-Ala-Asp-Leu-Met-Ser-Pro-Ser-Phe-Leu-Ser-Pro-Gly-Thr-Ser-Val-Phe(Ser,Pro,Gly)His-Thr-Ser-Lys; beta-subunit, Ala-Arg-Thr-Lys-Arg-Ser-Gly-Ser(P)-VALIle-Tyr-Glu-Pro-Leu-Lys. The sites on histone H2B which are phosphorylated by cyclic AMP-dependent protein kinase in vitro were identified as serine-36 and serine-32. The amino acid sequence in this region is: Lys-Lys-Arg-Lys-Arg-Ser32(P)-Arg-Lys-Glu-Ser36(P)-Tyr-Ser-Val-Tyr-Val- [Iwai, K., Ishikawa, K. & Hayashi, H. (1970) Nature (London) 226, 1056-1058]. Serine-36 was phosphorylated at 50% of the rate at which the beta-subunit of phosphorylase kinase was phosphorylated, and it was phosphorylated 6-7-fold more rapidly than was serine-32. The amino acid sequences when compared with those at the phosphorylation sites of other physiological substrates suggest that the presence of two adjacent basic amino acids on the N-terminal side of the susceptible serine residue may be critical for specific substrate recognition in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bylund D. B., Krebs E. G. Effect of denaturation on the susceptibility of proteins to enzymic phosphorylation. J Biol Chem. 1975 Aug 25;250(16):6355–6361. [PubMed] [Google Scholar]
- Cohen P. T., Burchell A., Cohen P. The molecular basis of skeletal muscle phosphorylase kinase deficiency. Eur J Biochem. 1976 Jul 1;66(2):347–356. doi: 10.1111/j.1432-1033.1976.tb10524.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Watson D. C., Dixon G. H. The hormonal control of activity of skeletal muscle phosphorylase kinase. Amino-acid sequences at the two sites of action of adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1975 Feb 3;51(1):79–92. doi: 10.1111/j.1432-1033.1975.tb03909.x. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R. Peptides from myelin basic protein as substrates for adenosine 3', 5'-cyclic monophosphate-dependent protein kinases. Biochem Biophys Res Commun. 1974 Dec 11;61(3):852–858. doi: 10.1016/0006-291x(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Daile P., Carnegie P. R., Young J. D. Synthetic substrate for cyclic AMP-dependent protein kinase. Nature. 1975 Oct 2;257(5525):416–418. doi: 10.1038/257416a0. [DOI] [PubMed] [Google Scholar]
- Edlund B., Andersson J., Titanji V., Dahlqvist U., Ekman P., Zetterqvist O., Engstoöm L. Amino acid sequence at the phosphorylated site of rat liver pyruvate kinase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1516–1521. doi: 10.1016/0006-291x(75)90198-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Takeda M., Nishizuka Y. Phosphorylated sites of calf thymus histone H2B by adenosine 3',5'-monophosphate-dependent protein kinase from silkworm. Biochem Biophys Res Commun. 1975 Sep 16;66(2):547–555. doi: 10.1016/0006-291x(75)90545-8. [DOI] [PubMed] [Google Scholar]
- Hjelmquist G., Andersson J., Edlund B., Engstroöm L. Amino acid sequence of a (32P) phosphopeptide from pig liver pyruvate kinase phosphorylated by cyclic 3',5'-AMP-stimulated protein kinase and gamma-(32P)ATP. Biochem Biophys Res Commun. 1974 Nov 27;61(2):559–563. doi: 10.1016/0006-291x(74)90993-0. [DOI] [PubMed] [Google Scholar]
- Humble E., Berglund L., Titanji V., Ljungström O., Edlund B., Zetterqvist O., Engström L. Non-dependence on native structure of pig liver pyruvate kinase when used as a substrate for cyclic 3',5'-AMP-stimulated protein kinase. Biochem Biophys Res Commun. 1975 Sep 16;66(2):614–621. doi: 10.1016/0006-291x(75)90554-9. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Dixon G. H. Phosphorylation of protamine during spermatogenesis in trout testis. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1011–1018. doi: 10.1073/pnas.58.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai K., Ishikawa K., Hayashi H. Amino-acid sequence of slightly lysine-rich histone. Nature. 1970 Jun 13;226(5250):1056–1058. doi: 10.1038/2261056b0. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Bylund D. B., Huang T. S., Krebs E. G. Substrate specificity of the cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3448–3452. doi: 10.1073/pnas.72.9.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan T. A. Cyclic AMP and histone phosphorylation. Ann N Y Acad Sci. 1971 Dec 30;185:166–180. doi: 10.1111/j.1749-6632.1971.tb45246.x. [DOI] [PubMed] [Google Scholar]
- Langan T. A. Phosphorylation of liver histone following the administration of glucagon and insulin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1276–1283. doi: 10.1073/pnas.64.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Marzluff W. F., Jr, McCarty K. S. Structural studies of calf thymus F3 histone. II. Occurrence of phosphoserine and -N-acetyllysine in thermolysin peptides. Biochemistry. 1972 Jul 4;11(14):2677–2681. doi: 10.1021/bi00764a020. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The phosphorylation of rabbit skeletal muscle glycogen synthase by glycogen synthase kinase-2 and adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1976 Sep;68(1):31–44. doi: 10.1111/j.1432-1033.1976.tb10762.x. [DOI] [PubMed] [Google Scholar]
- Sanders M. M., Dixon G. H. The biosynthesis of protamine in trout testis. IV. Sites of phosphorylation. J Biol Chem. 1972 Feb 10;247(3):851–855. [PubMed] [Google Scholar]
- Shlyapnikov S. V., Arutyunyan A. A., Kurochkin S. N., Memelova L. V., Nesterova M. V., Sashchenko L. P., Severin E. S. Investigation of the sites phosphorylated in lysine-rich histones by protein kinase from pig brain. FEBS Lett. 1975 May 15;53(3):316–319. doi: 10.1016/0014-5793(75)80045-7. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P. The hormonal control of activity of skeletal muscle phosphorylase kinase. Phosphorylation of the enzyme at two sites in vivo in response to adrenalin. Eur J Biochem. 1975 Feb 3;51(1):93–104. doi: 10.1111/j.1432-1033.1975.tb03910.x. [DOI] [PubMed] [Google Scholar]
- Zetterqvist O., Ragnarsson U., Humble E., Berglund L., Engström L. The minimum substrate of cyclic AMP-stimulated protein kinase, as studied by synthetic peptides representing the phosphorylatable site of pyruvate kinase (type L) of rat liver. Biochem Biophys Res Commun. 1976 Jun 7;70(3):696–703. doi: 10.1016/0006-291x(76)90648-3. [DOI] [PubMed] [Google Scholar]


