Abstract
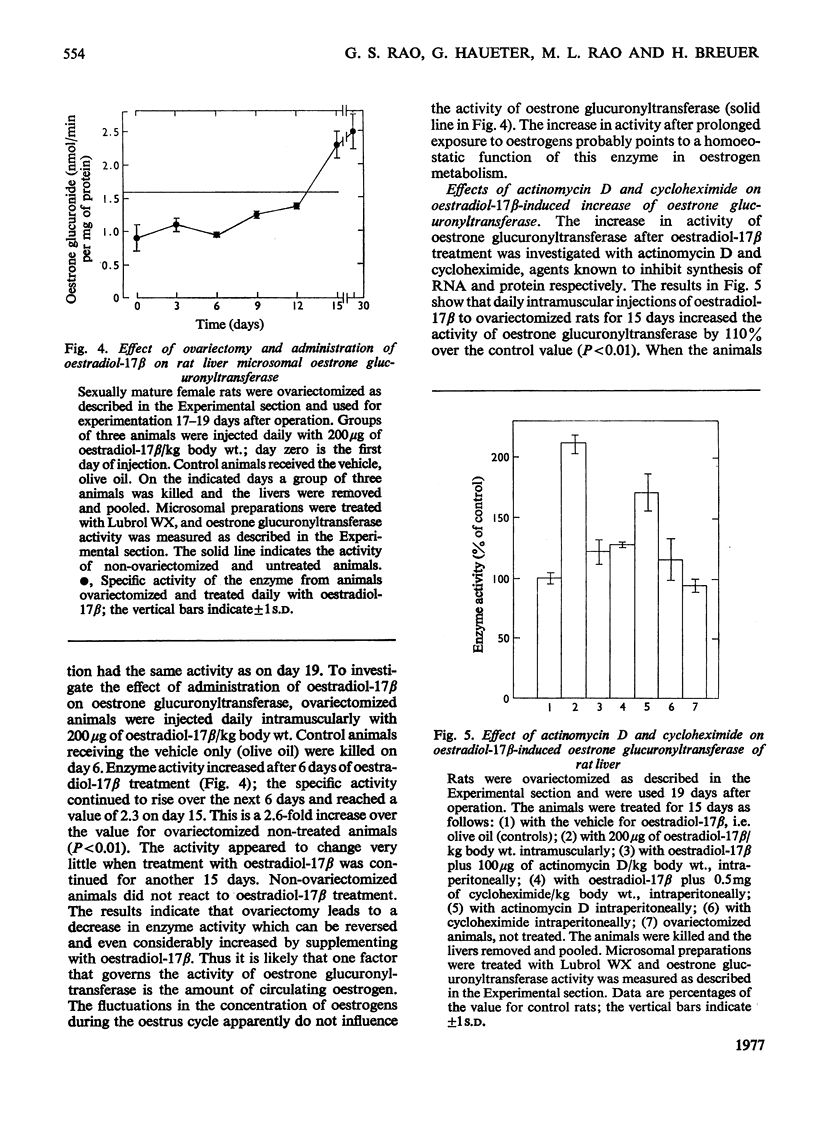
1. Microsomal preparations from rat liver, kidney and intestine were tested for UDP-glucuronyltransferase activity by using oestrone, oestradiol-17 beta, oestriol, testosterone, cortisol, cortisone, corticosterone, aldosterone, tetrahydrocortisol and tetrahydrocortisone as substrates. The microsomal preparation from the liver glucuronidated oestrone, oestradiol-17 beta and testosterone. 2. The specific activity of the enzyme was significantly higher in livers from female rats than in those from male rats. 3. Testosterone was actively glucuronidated by both sexes. Cortisol, cortisone, corticosterone, aldosterone, tetrahydrocortisol and tetrahydrocortisone were not glucuronidated by any of the three tissues. 4. The non-ionic detergent Lubrol WX activates liver microsomal UDP-glucuronyltransferase 2-3-fold with oestrone and testosterone as substrates. 5. Oestrone glucuronyltransferase was inhibited by oestradiol-17 beta, predominantly competitively and by testosterone non-competitively. Bilirubin was a non-competitive inhibitor of oestrone glucuronidation. p-Nitrophenol had no effect. 6. Oestrone glucuronyltransferase could not be stimulated by either acute or prolonged treatment of animals with phenobarbital, whereas a single dose of 3-methylcholanthrene led to a moderate stimulation. 7. Ovariectomy leads to a 56% decrease in oestrone glucuronyltransferase activity; administration of oestradiol-17 beta induces the enzyme to normal activity after 12 days, and after 15 days the activity is twice the control value. Actinomycin D and cycloheximide block the oestradiol-17 beta-induced increase in enzyme activity. 8. Castration has no effect on the activity of testosterone glucuronyltransferase, nor does administration of testosterone influence enzyme activity. The results provide strong evidence for the existence of multiple steroid glucuronyltransferases in the liver of the rat.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARIAS I. M., GARTNER L. M., SEIFTER S., FURMAN M. PROLONGED NEONATAL UNCONJUGATED HYPERBILIRUBINEMIA ASSOCIATED WITH BREAST FEEDING AND A STEROID, PREGNANE-3(ALPHA), 20(BETA)-DIOL, IN MATERNAL MILK THAT INHIBITS GLUCURONIDE FORMATION IN VITRO. J Clin Invest. 1964 Nov;43:2037–2047. doi: 10.1172/JCI105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K. W., Fröhling W., Remmer H., Rexer B. Effects of phenobarbital and 3-methylcholanthrene on substrate specificity of rat liver microsomal UDP-glucuronyltransferase. Biochim Biophys Acta. 1973 Nov 15;327(1):46–56. doi: 10.1016/0005-2744(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Dallner G., Siekevitz P., Palade G. E. Biogenesis of endoplasmic reticulum membranes. I. Structural and chemical differentiation in developing rat hepatocyte. J Cell Biol. 1966 Jul;30(1):73–96. doi: 10.1083/jcb.30.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. A., Taurog A. Enhanced biliary excretion of thyroxine glucuronide in rats pretreated with benzpyrene. Biochem Pharmacol. 1968 Jun;17(6):1049–1065. doi: 10.1016/0006-2952(68)90363-8. [DOI] [PubMed] [Google Scholar]
- Götze W., Grube E., Rao G. S., Rao M. L., Breuer H. Steroidglucuronyltransferasen. II. Solubilisierung, Anreicherung und kinetische Eigenschaften einer Ostradiol-17 -3-Glucuronyltransferase aus der Mikrosomen-Fraktion des Schweinedünndarmes. Hoppe Seylers Z Physiol Chem. 1971 Sep;352(9):1223–1230. [PubMed] [Google Scholar]
- Hill A. V. A new mathematical treatment of changes of ionic concentration in muscle and nerve under the action of electric currents, with a theory as to their mode of excitation. J Physiol. 1910 May 11;40(3):190–224. doi: 10.1113/jphysiol.1910.sp001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. M., Levin W., Conney A. H. Studies on bilirubin and steroid glucuronidation by rat liver microsomes. Biochem Pharmacol. 1975 Mar 15;24(6):655–662. doi: 10.1016/0006-2952(75)90240-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lucier G. W., McDaniel O. S., Hook G. E. Nature of the enhancement of hepatic uridine diphosphate glucuronyltransferase activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Biochem Pharmacol. 1975 Feb 1;24(3):325–334. doi: 10.1016/0006-2952(75)90213-0. [DOI] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Spontaneous and detergent activation of a glucuronyltransferase in vitro. Arch Biochem Biophys. 1967 Apr;120(1):198–203. doi: 10.1016/0003-9861(67)90614-5. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Heath E. C., Easton K. H., Dingell J. V. Effect of triton X-100 on the conjugation of tetrahydrocortisone, in vitro. Biochem Pharmacol. 1973 Sep 15;22(18):2319–2327. doi: 10.1016/0006-2952(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Mulder G. J. Bilirubin and heterogeneity of microsomal uridine diphosphate glucuronyltransferase from rat liver. Biochim Biophys Acta. 1972 Dec 7;289(2):284–292. doi: 10.1016/0005-2744(72)90079-4. [DOI] [PubMed] [Google Scholar]
- Potrepka R. F., Spratt J. L. Bilirubin glucuronidation by hepatic microsomal subfractions and the effect of 3-methylcholanthrene. Biochem Pharmacol. 1971 Sep;20(9):2247–2252. doi: 10.1016/0006-2952(71)90224-3. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Breuer H. Partial purification and kinetic properties of a soluble estrogen glucuronyltransferase from pig intestine. J Biol Chem. 1969 Oct 25;244(20):5521–5527. [PubMed] [Google Scholar]
- Rao G. S., Haueter G., Rao M. L., Breuer H. An improved assay for steroid glucuronyltransferase in rat liver microsomes. Anal Biochem. 1976 Jul;74(1):35–40. doi: 10.1016/0003-2697(76)90307-9. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Breuer H. Partial purification and kinetics of oestriol 16 alpha-glucuronyltransferase from the cytosol fraction of human liver. Biochem J. 1970 Jul;118(4):625–634. doi: 10.1042/bj1180625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Breuer H. Studies on a solubilized oestriol 16 -glucuronyltransferase from human liver microsomes. J Steroid Biochem. 1972 Jan;3(1):1–10. doi: 10.1016/0022-4731(72)90005-2. [DOI] [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Breuer H. Studies on a testosterone glucuronyltransferase from the cytosol fraction of human liver. Biochem J. 1970 Oct;119(4):635–642. doi: 10.1042/bj1190635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. S., Rao M. L., Haueter G., Breuer H. Steroidglucuronyltransferases, V. Formation and hydrolysis of oestrogen glucuronides by the liver, kidney and intestine of the pig. Hoppe Seylers Z Physiol Chem. 1974 Jul;355(7):881–890. doi: 10.1515/bchm2.1974.355.2.881. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsnes A. Studies on the activation in vitro of glucuronyltransferase. Biochim Biophys Acta. 1969 Nov 4;191(2):279–291. doi: 10.1016/0005-2744(69)90247-2. [DOI] [PubMed] [Google Scholar]
- Zhivkov V. Measurement of uridine diphosphate glucuronic acid concentrations and synthesis in animal tissues. Biochem J. 1970 Dec;120(3):505–508. doi: 10.1042/bj1200505. [DOI] [PMC free article] [PubMed] [Google Scholar]


