Abstract
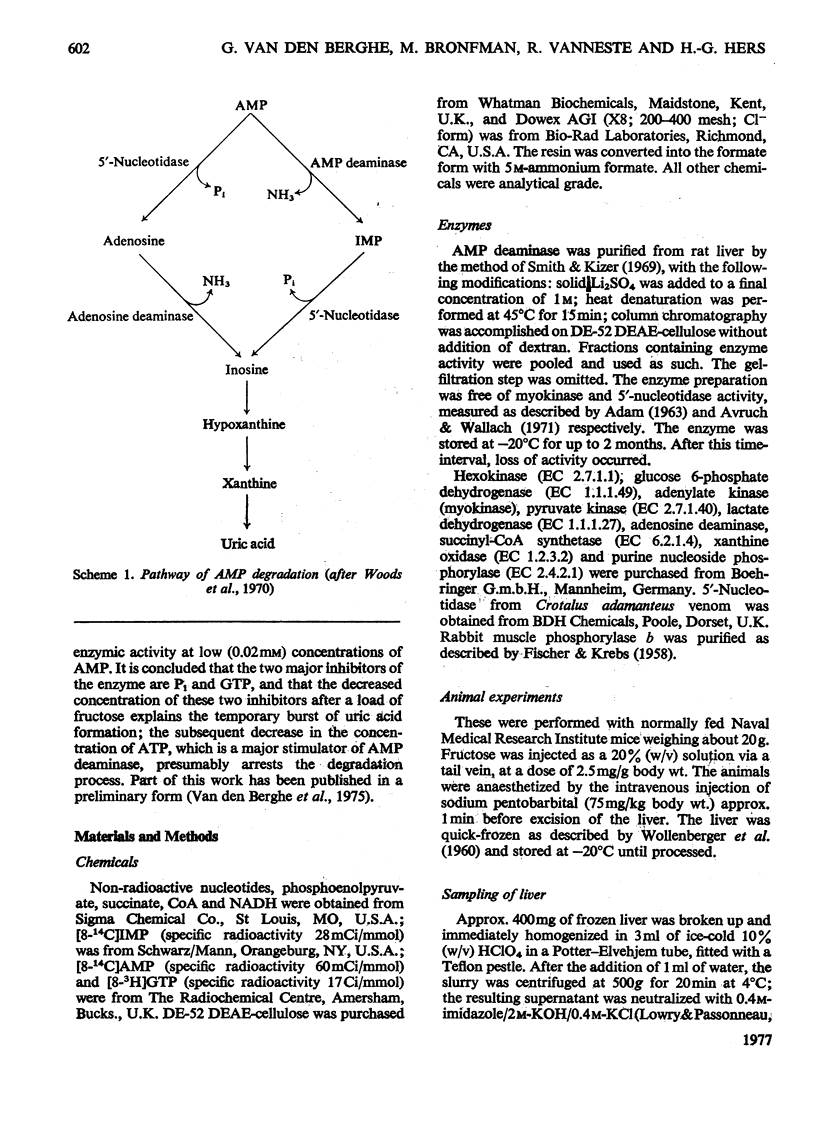
1. The hepatic concentration of several nucleotides and metabolites was measured during the first few minutes after an intravenous load of fructose to mice. The first changes, observed at 30s, were a decrease in the concentration of Pi and a simultaneous accumulation of fructose 1-phosphate. The decrease in the concentrations of ATP and GTP proceeded more slowly. An increase in the concentration of IMP was detected only after 1 min and could therefore not be considered to be the cause of the accumulation of fructose 1-phosphate. 2. To explain the temporary burst of adenine nucleotide breakdown that occurs after a load of fructose, the kinetics of AMP deaminase (EC 3.5.4.6) from rat liver were reinvestigated at physiological (0.2 mM) concentration of substrate. For this purpose, a new radiochemical-assay procedure was developed. At 0.2mM-AMP a low activity could be measured, which was more than 90% inhibited by 5mM-Pi. ATP (3MM) increased the enzyme activity over 200-fold. Pi alone did not influence the ATP-activated enzyme, but 0.5mM-GTP caused a 60% inhibition. The combined effect of both inhibitors at their physiological concentrations reached 95%. 3. It is proposed that the rapid degradation of adenine nucleotides that occurs after a load of fructose is caused by a decrease in the concentration of both inhibitors, Pi and GTP, soon counteracted by the decrease in the concentration of ATP. 4. Some of the kinetic parameters of liver AMP deaminase were computed in terms of the concerted transition theory of Monod, Wyman & Changeux (1965) (J. Mol. Biol. 12, 88-118).
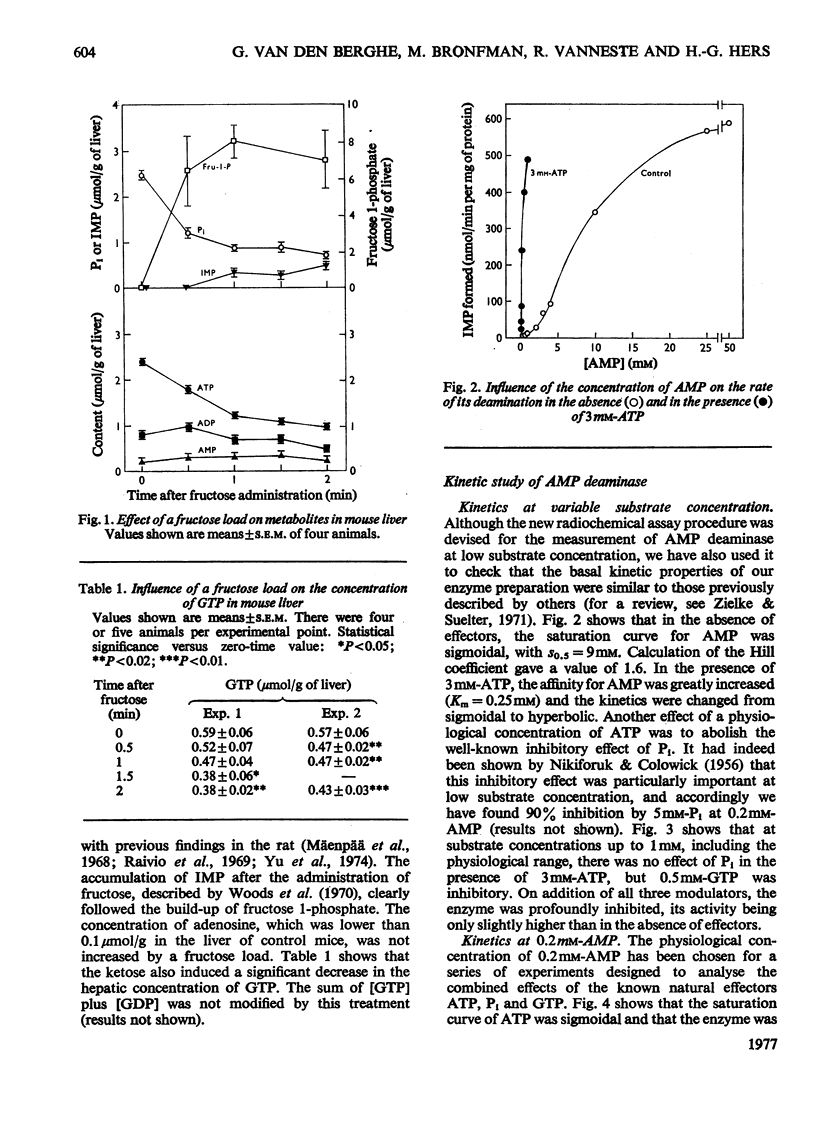
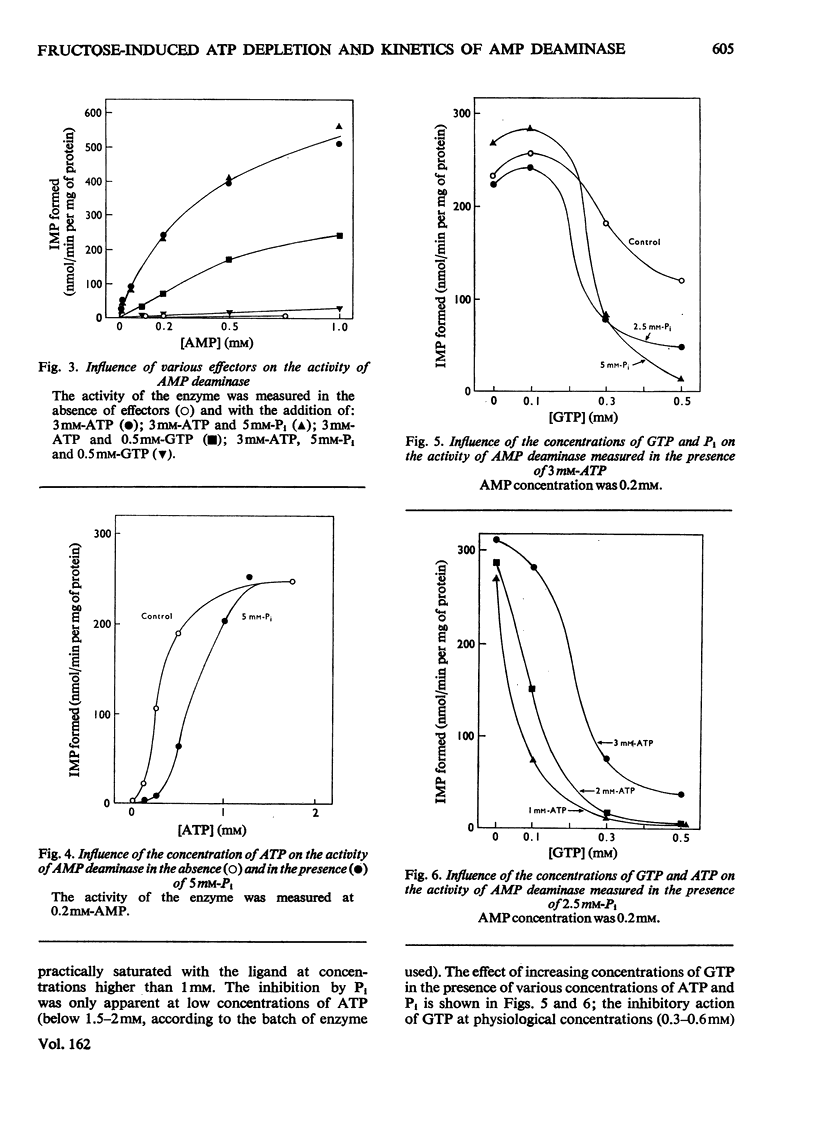
Full text
PDF

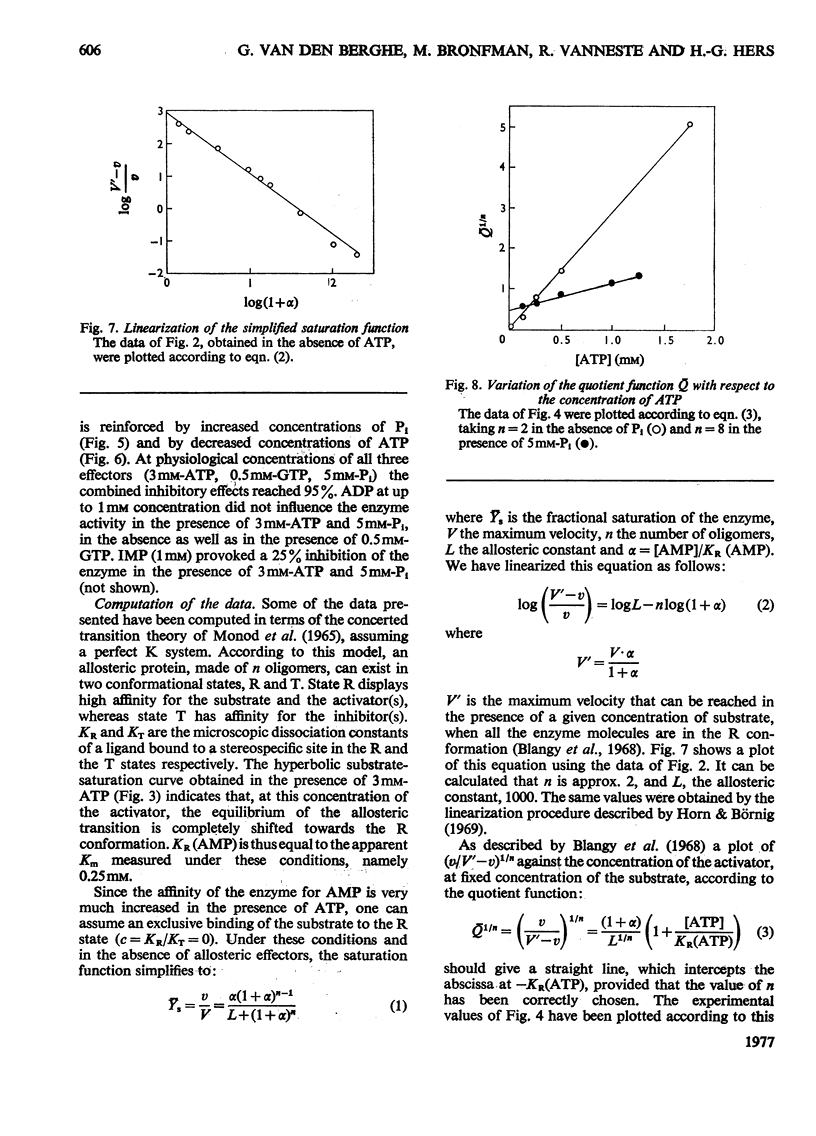



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R. C., Ballard F. J., Weinhouse S. Purification and properties of rat liver fructokinase. J Biol Chem. 1967 Jul 25;242(14):3360–3365. [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Blangy D., Buc H., Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):13–35. doi: 10.1016/0022-2836(68)90051-x. [DOI] [PubMed] [Google Scholar]
- Boosman A., Sammons D., Chilson O. An investigation of the subunit structure and AMP-deaminases from rabbit and chicken muscle. Biochem Biophys Res Commun. 1971 Nov;45(4):1025–1032. doi: 10.1016/0006-291x(71)90440-2. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM B., LOWENSTEIN J. M. REGULATION OF ADENYLATE DEAMINASE BY ADENOSINE TRIPHOSPHATE. Biochim Biophys Acta. 1965 Mar 22;96:535–537. doi: 10.1016/0005-2787(65)90575-7. [DOI] [PubMed] [Google Scholar]
- Cha S., Cha C. J. Microdetermination of guanine ribonucleotides by an enzymic amplification technique. Anal Biochem. 1970 Jan;33(1):174–192. doi: 10.1016/0003-2697(70)90451-3. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Atkinson D. E. Stabilization of adenylate energy charge by the adenylate deaminase reaction. J Biol Chem. 1973 Dec 10;248(23):8309–8312. [PubMed] [Google Scholar]
- Cheng Y. C., Robison B., Parks R. E., Jr Demonstration of the heterogeneity of nucleoside diphosphokinase in rat tissues. Biochemistry. 1973 Jan 2;12(1):5–10. doi: 10.1021/bi00725a002. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Frandsen E. K., Grunnet N. Kinetic properties of triokinase from rat liver. Eur J Biochem. 1971 Dec 10;23(3):588–592. doi: 10.1111/j.1432-1033.1971.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Grunst J., Dietze G., Wicklmayr M., Hoppe F., Mehnert H. Einfluss parenteraler Fruktoxe- bzw. Glukosezufuhr auf die Harnsäurebildung und Phosphataufnahme der menschlichen Leber. Z Ernahrungswiss. 1975 Dec;14(4):259–267. doi: 10.1007/BF02025863. [DOI] [PubMed] [Google Scholar]
- Heinz F., Junghänel J. Metabolitmuster in Rattenleber nach Fructoseapplikation. Hoppe Seylers Z Physiol Chem. 1969 Jul;350(7):859–866. [PubMed] [Google Scholar]
- Horn A., Börnig H. Analysis of kinetic data of allosteric enzymes by a linear plot. FEBS Lett. 1969 Jun;3(5):325–329. doi: 10.1016/0014-5793(69)80169-9. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- LEHNINGER A. L., SICE J., JENSEN E. V. Effect of substrate structure on the aldolase equilibrium. Biochim Biophys Acta. 1955 Jun;17(2):285–287. doi: 10.1016/0006-3002(55)90368-1. [DOI] [PubMed] [Google Scholar]
- Lee Y. P., Wang M. H. Studies of the nature of the inhibitory action of inorganic phosphate, fluoride, and detergents on 5'-adenylic acid deaminase activity and on the activation by adenosine triphosphate. J Biol Chem. 1968 May 10;243(9):2260–2265. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- NIKIFORUK G., COLOWICK S. P. The purification and properties of 5-adenylic acid deaminase from muscle. J Biol Chem. 1956 Mar;219(1):119–129. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Becker 7. A., Meyer L. J., Greene M. L., Nuki G., Seegmiller J. E. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975 Jul;24(7):861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Kekomäki M. P., Mäenpä P. H. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem Pharmacol. 1969 Oct;18(10):2615–2624. doi: 10.1016/0006-2952(69)90192-0. [DOI] [PubMed] [Google Scholar]
- Ronca-Testoni S., Raggi A., Ronca G. Muscle AMP aminohydrolase. 3. A comparative study on the regulatory properties of skeletal muscle enzyme from various species. Biochim Biophys Acta. 1970 Jan 14;198(1):101–112. doi: 10.1016/0005-2744(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Scopes R. K., Penny I. F. Subunit sizes of muscle proteins, as determined by sodium dodecyl sulphate gel electrophoresis. Biochim Biophys Acta. 1971 May 25;236(2):409–415. doi: 10.1016/0005-2795(71)90221-2. [DOI] [PubMed] [Google Scholar]
- Setlow B., Burger R., Lowenstein J. M. Adenylate deaminase. I. The effects of adenosine and guanosine triphosphates on activity and the organ distribution of the regulated enzyme. J Biol Chem. 1966 Mar 10;241(5):1244–1245. [PubMed] [Google Scholar]
- Setlow B., Lowenstein J. M. Adenylate deaminase. II. Purification and some regulatory properties of the enzyme from calf brain. J Biol Chem. 1967 Feb 25;242(4):607–615. [PubMed] [Google Scholar]
- Setlow B., Lowenstein J. M. Adenylate deaminase. V. Effect of alkali metal and magnesium ions on activity. J Biol Chem. 1968 Dec 10;243(23):6216–6221. [PubMed] [Google Scholar]
- Smiley K. L., Suelter C. H. Univalent cations as allosteric activators of muscle adenosine 5'-phosphate deaminase. J Biol Chem. 1967 Apr 25;242(8):1980–1981. [PubMed] [Google Scholar]
- Smith L. D., Kizer D. E. Purification and properties of rat liver AMP deaminase. Biochim Biophys Acta. 1969 Nov 4;191(2):415–424. doi: 10.1016/0005-2744(69)90260-5. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y., Wolfenden R. Binding of guanosine triphosphate and adenosine triphosphate by rabbit muscle adenosine monophosphate deaminase. Biochemistry. 1970 Aug 18;9(17):3400–3404. doi: 10.1021/bi00819a017. [DOI] [PubMed] [Google Scholar]
- Van Den Berghe G., Hue L., Hers H. G. Effect of administration of the fructose on the glycogenolytic action of glucagon. An investigation of the pathogeny of hereditary fructose intolerance. Biochem J. 1973 Jun;134(2):637–645. doi: 10.1042/bj1340637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Berghe G., Vanneste R., Hers H. G. A new radioactive assay for purified AMP-aminohydrolase. Its application to the physiological regulation of the liver enzyme. Arch Int Physiol Biochim. 1975 Dec;83(5):1010–1011. [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wolfenden R., Tomozawa Y., Bamman B. Takadiastase adenosine deaminase, calf duodenal adenosine deaminase, and rabbit muscle adenosine monophosphate deaminase. A comparison of physical properties and amino acid composition. Biochemistry. 1968 Nov;7(11):3964–3970. doi: 10.1021/bi00851a025. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. T., Burch H. B., Phillips M. J. Pathogenesis of fructose hepatotoxicity. Lab Invest. 1974 Jan;30(1):85–92. [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


