Abstract
1. The specific activity of rat and pig liver nuclear-envelope nucleoside triphosphatase (EC 3.6.1.3) decreases when the system is depleted of RNA. The activity can be restored by adding high concentrations of yeast RNA to the assay medium. 2. Exogenous RNA also increases the activity of the enzyme in control envelopes (not RNA-depleted). The effect appears to be largely specific for poly(A) and poly(G); it is not stimulated by rRNA or tRNA preparations, ribonuclease-hydrolysed RNA, AMP, or double- or single-stranded DNA. 3. Inhibitors of the enzyme, in concentrations at which half-maximal inhibition of the enzyme is achieved, do not affect the percentage stimulation of the enzyme by yeast RNA. 4. The simulation is abolished by the inclusion of 150 mM-KCl or -NaCl in the assay medium, but not by increasing the assay pH to 8.5. 5. The results are discussed in the light of the possible role of the nucleoside triphosphatase in vivo in nucleo-cytoplasmic ribonucleoprotein translocation. 6. It is proposed that poly(G)-stimulated Mg2+-activated adenosine triphosphatase activity should be adopted as an enzymic marker for the nuclear envelope.
Full text
PDF

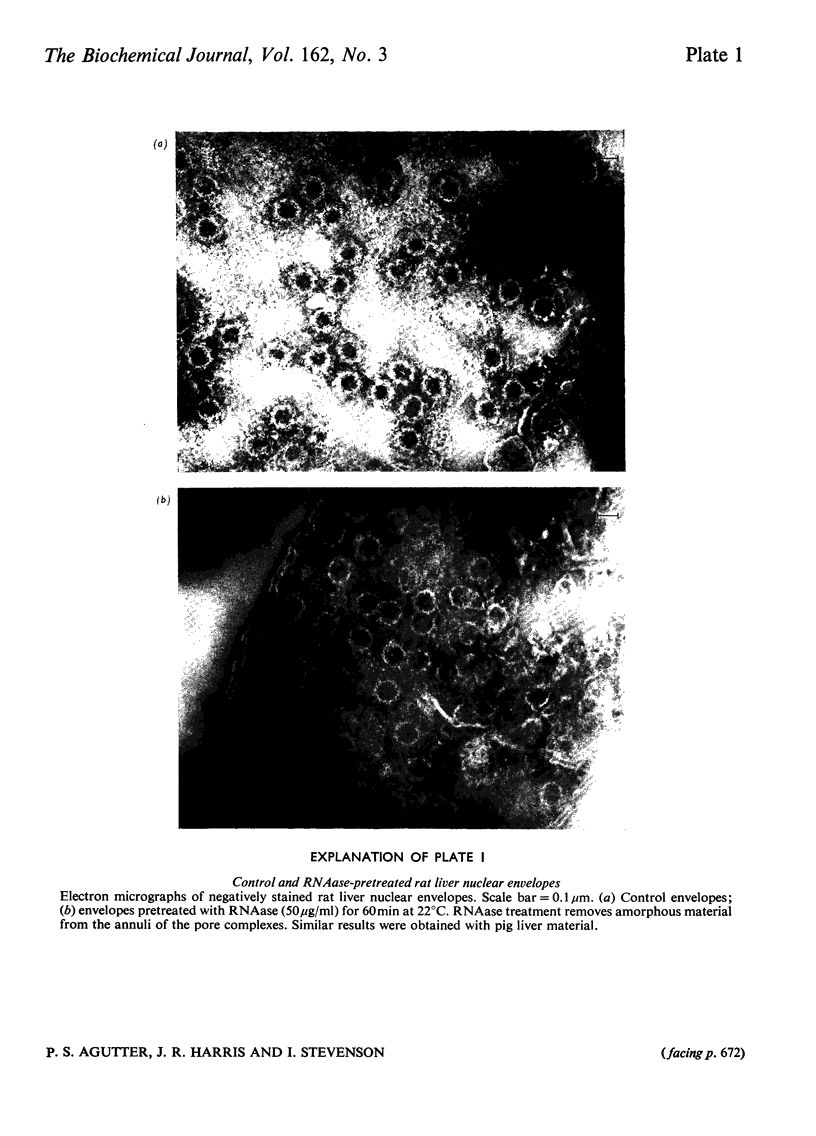

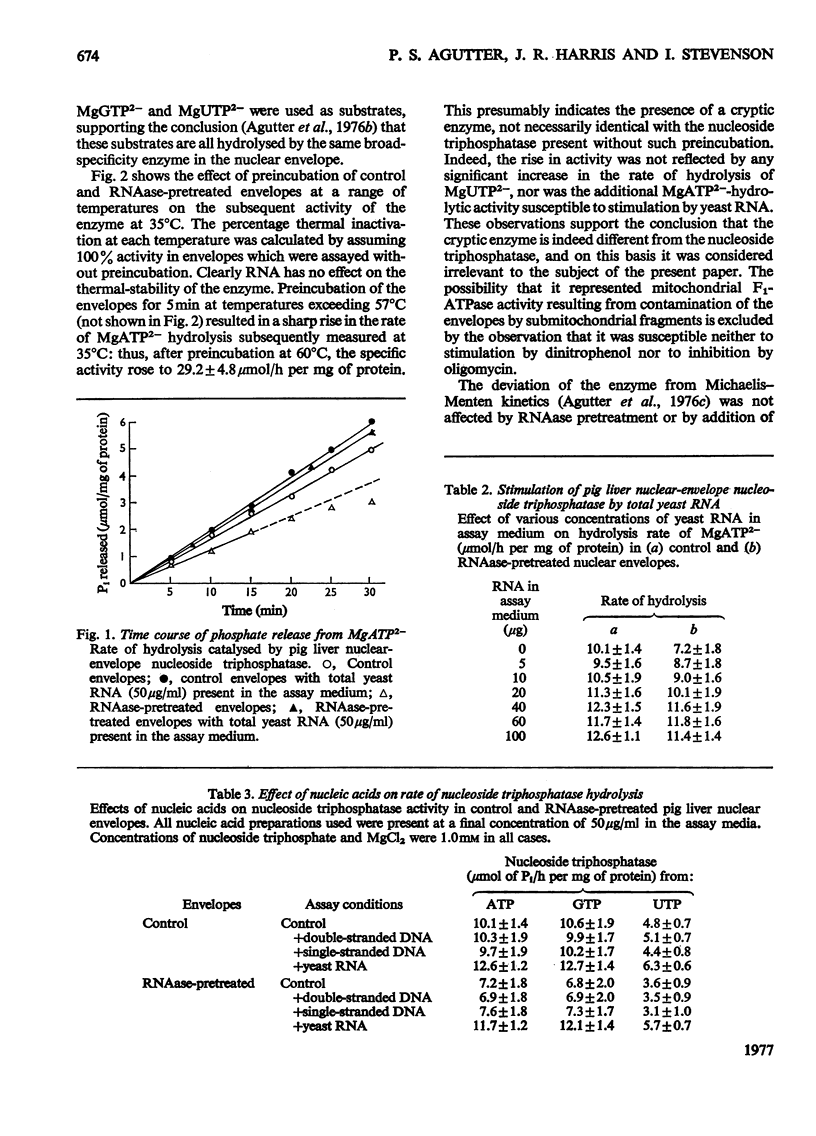
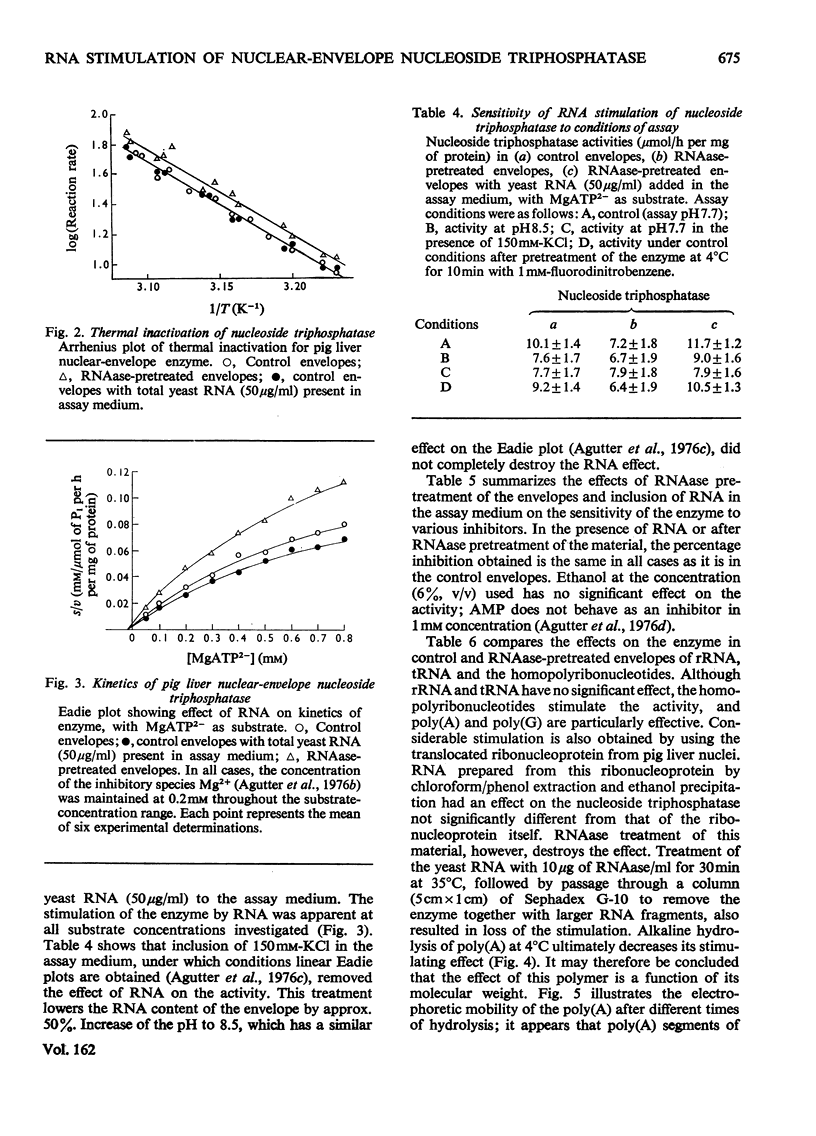



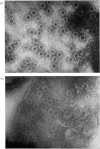
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agutter P. S., McArdle H. J., McCaldin B. Evidence for involvement of nuclear envelope nucleoside triphosphatase in nucleocytoplasmic translocation of ribonucleoprotein. Nature. 1976 Sep 9;263(5573):165–167. doi: 10.1038/263165a0. [DOI] [PubMed] [Google Scholar]
- Agutter P. S. The isolation of the envelopes of rat liver nuclei. Biochim Biophys Acta. 1972 Jan 17;255(1):397–401. doi: 10.1016/0005-2736(72)90039-9. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Funk L. K., Crane F. L. The isolation of nuclear membrane from a large-scale preparation of bovine liver nuclei. Biochim Biophys Acta. 1970 Jun 2;203(3):531–546. doi: 10.1016/0005-2736(70)90190-2. [DOI] [PubMed] [Google Scholar]
- Bul'diaeva T. V., Kuz'mina S. N., Zbarskii I. B. Okislitel'noe fosforilirovanie v izolirovannykh iadernykh obolochkakh i iadrakh kletok pecheni krysy. Dokl Akad Nauk SSSR. 1972 Jan 11;202(2):467–470. [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Delektorskaia L. N., Perevoshchikov K. A. Adenozintrifosfataznaia aktivnost' iadernykh obolochek i iader kletok pechenii krys, gepatomy Zazhdelia krys i astsitnogo raka Erlikha myshei. Biokhimiia. 1969 Jan-Feb;34(1):199–203. [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Franke W. W., Deumling B., Baerbelermen, Jarasch E. D., Kleinig H. Nuclear membranes from mammalian liver. I. Isolation procedure and general characterization. J Cell Biol. 1970 Aug;46(2):379–395. doi: 10.1083/jcb.46.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Scheer U. The ultrastructure of the nuclear envelope of amphibian oocytes: a reinvestigation. II. The immature oocyte and dynamic aspects. J Ultrastruct Res. 1970 Feb;30(3):317–327. doi: 10.1016/s0022-5320(70)80065-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W. Structure, biochemistry, and functions of the nuclear envelope. Int Rev Cytol. 1974;Suppl 4:71–236. [PubMed] [Google Scholar]
- Ishikawa K., Kuroda C., Ogata K. Release of ribonucleoprotein particles containing rapidly labeled ribonucleic acid from rat liver nuclei. Effect of adenosine 5'-triphosphate and some properties of the particles. Biochim Biophys Acta. 1969 Apr 22;179(2):316–331. doi: 10.1016/0005-2787(69)90040-9. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Kuroda C., Ueki M., Ogata K. Messenger ribonucleoprotein complexes released from rat liver nuclei by ATP. I. Characterization of the RNA moiety of messenger ribonucleoprotein complexes. Biochim Biophys Acta. 1970 Aug 8;213(2):495–504. doi: 10.1016/0005-2787(70)90056-0. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Franke W. W. Is cytochrome oxidase a constituent of nuclear membranes? J Biol Chem. 1974 Nov 25;249(22):7245–7254. [PubMed] [Google Scholar]
- Jarasch E. D., Reilly C. E., Comes P., Kartenbeck J., Franke W. W. Isolation and characterization of nuclear membranes from calf and rat thymus. Hoppe Seylers Z Physiol Chem. 1973 Aug;354(8):974–986. doi: 10.1515/bchm2.1973.354.2.974. [DOI] [PubMed] [Google Scholar]
- Kashnig D. M., Kasper C. B. Isolation, morphology, and composition of the nuclear membrane from rat liver. J Biol Chem. 1969 Jul 25;244(14):3786–3792. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F., Gesteland R. F. The origin and destiny of adenovirus proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):581–590. doi: 10.1101/sqb.1974.039.01.072. [DOI] [PubMed] [Google Scholar]
- Livne A., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. V. Interaction of coupling factor 1 from chloroplasts with ribonucleic acid and lipids. J Biol Chem. 1969 Mar 10;244(5):1332–1338. [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- MIYAZAWA Y., THOMAS C. A., Jr NUCLEOTIDE COMPOSITION OF SHORT SEGMENTS OF DNA MOLECULES. J Mol Biol. 1965 Feb;11:223–237. doi: 10.1016/s0022-2836(65)80053-5. [DOI] [PubMed] [Google Scholar]
- Maale G., Stein G., Mans R. Effects of cordycepin and cordycepintriphosphate on polyadenylic and ribonucleic acid-synthesising enzymes from eukaryotes. Nature. 1975 May 1;255(5503):80–82. doi: 10.1038/255080a0. [DOI] [PubMed] [Google Scholar]
- Maddy A. H., Spooner R. L. Ox erythrocyte agglutinability. 1. Variation in the membrane protein. Vox Sang. 1970 Jan;18(1):34–41. doi: 10.1111/j.1423-0410.1970.tb01427.x. [DOI] [PubMed] [Google Scholar]
- McNamara D. J., Racevskis J., Schumm D. E., Webb T. E. Ribonucleic acid synthesis in isolated rat liver nuclei under conditions of ribonucleic acid processing and transport. Biochem J. 1975 May;147(2):193–197. doi: 10.1042/bj1470193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Nemer M., Dubroff L. M., Graham M. Properties of sea urchin embryo messenger RNA containing and lacking poly(A). Cell. 1975 Oct;6(2):171–178. doi: 10.1016/0092-8674(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Paine P. L. Nucleocytoplasmic movement of fluorescent tracers microinjected into living salivary gland cells. J Cell Biol. 1975 Sep;66(3):652–657. doi: 10.1083/jcb.66.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskas H. J. Release of adenovirus messenger RNA from isolated nuclei. Nat New Biol. 1971 Sep 29;233(39):134–136. doi: 10.1038/newbio233134a0. [DOI] [PubMed] [Google Scholar]
- Schumm D. E., McNamara D. J., Webb T. E. Cytoplasmic proteins regulating messenger RNA release from nuclei. Nat New Biol. 1973 Oct 17;245(146):201–203. doi: 10.1038/newbio245201a0. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- WAHLER B. E., WOLLENBERGER A. Zur Bestimmung des Orthophosphats neben säure-molybdat-labilen Phosphorsäureverbindungen. Biochem Z. 1958;329(6):508–520. [PubMed] [Google Scholar]
- Wiegers U., Hilz H. Rapid isolation of undegraded polysomal RNA without phenol. FEBS Lett. 1972 Jun 1;23(1):77–82. doi: 10.1016/0014-5793(72)80289-8. [DOI] [PubMed] [Google Scholar]
- Yasuzumi G., Tsubo I. The fine structure of nuclei as revealed by electron microscopy. 3. Adenosine triphosphatase activity in the pores of nuclear envelope of mouse choroid plexus epithelial cells. Exp Cell Res. 1966 Sep;43(2):281–292. doi: 10.1016/0014-4827(66)90055-3. [DOI] [PubMed] [Google Scholar]
- Zbarsky I. B., Perevoshchikova K. A., Delektorskaya L. N., Delektorsky V. V. Isolation and biochemical characteristics of the nuclear envelope. Nature. 1969 Jan 18;221(5177):257–259. doi: 10.1038/221257a0. [DOI] [PubMed] [Google Scholar]