Abstract
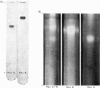
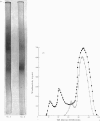
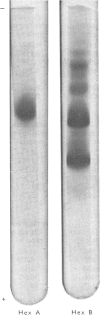
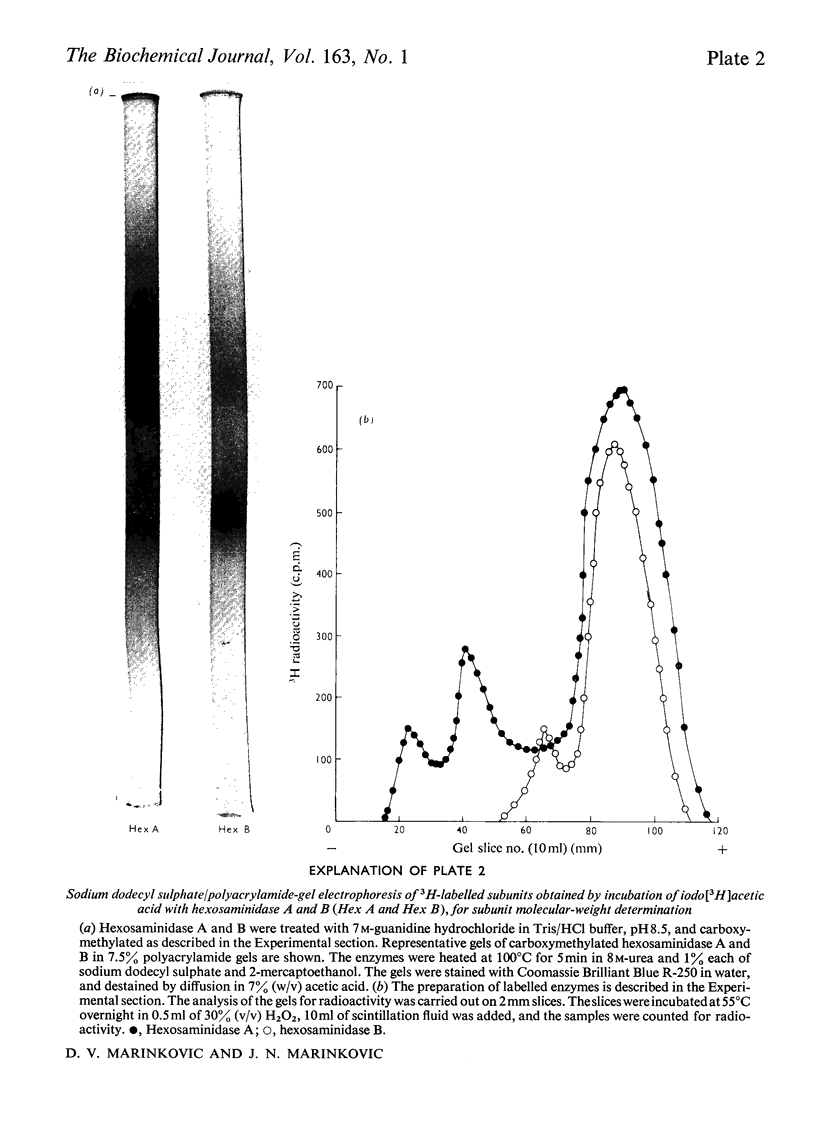
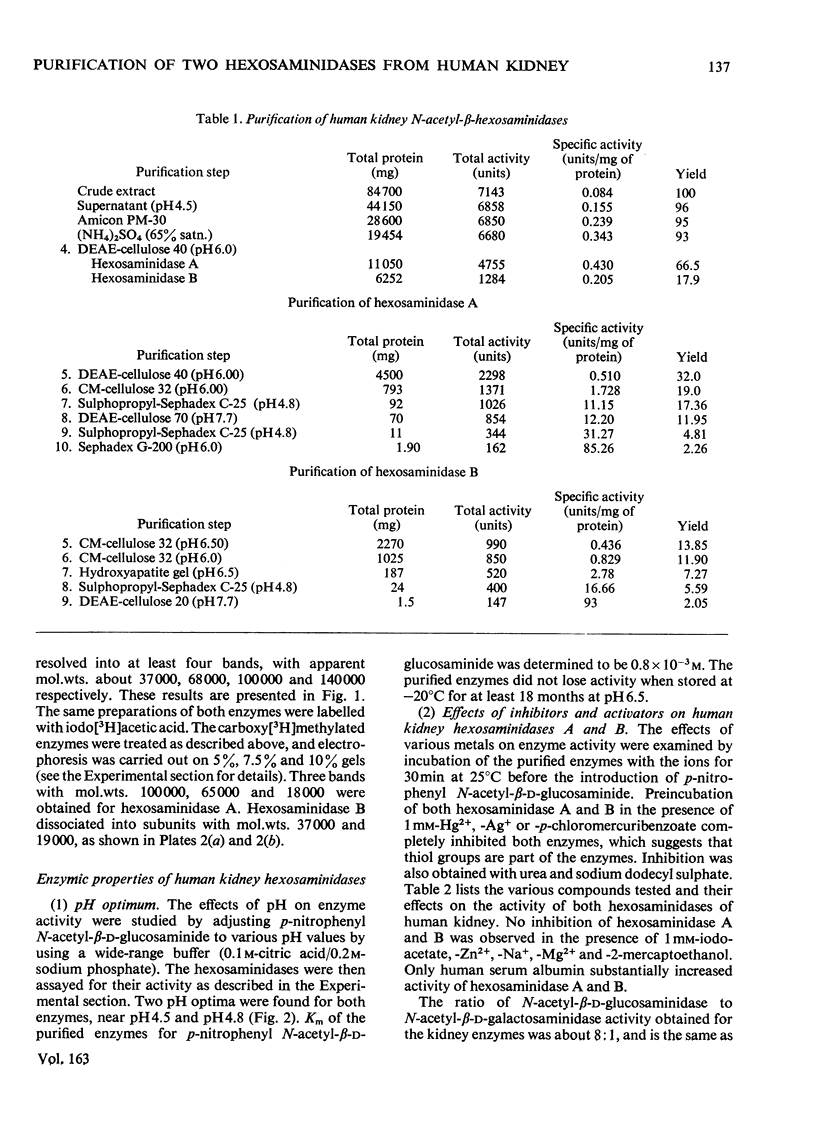
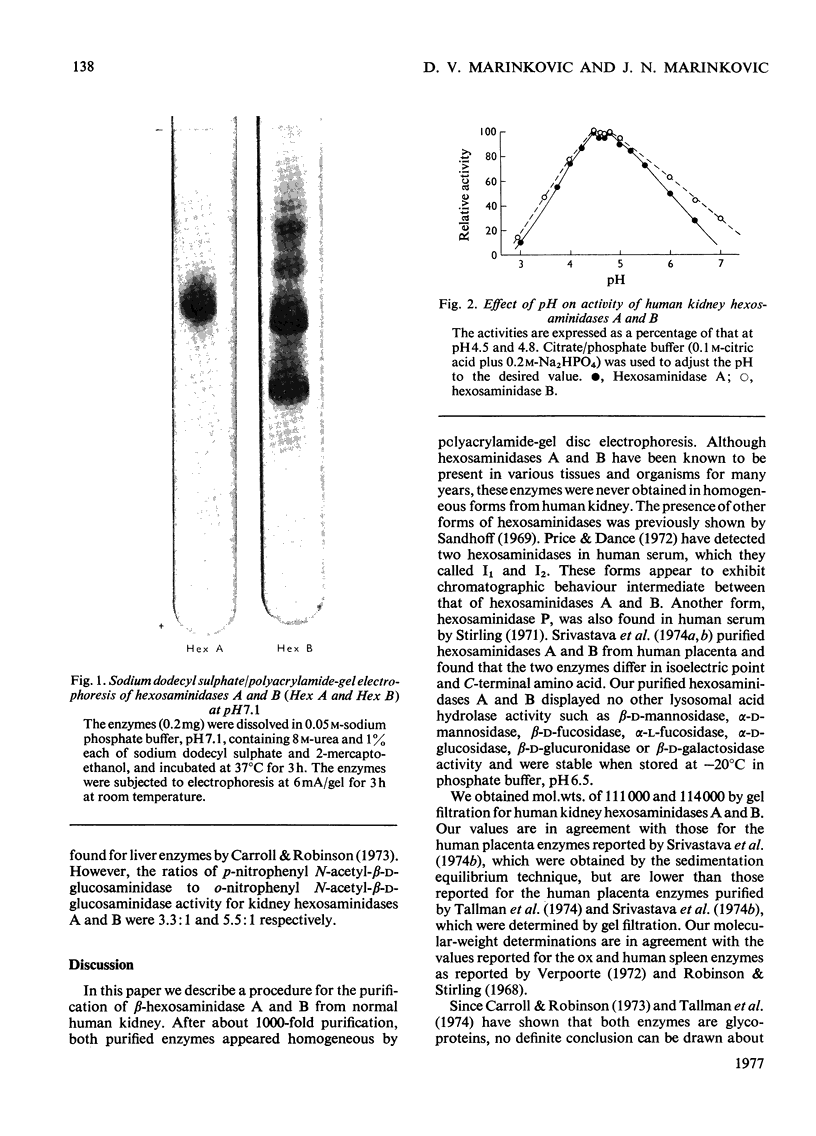
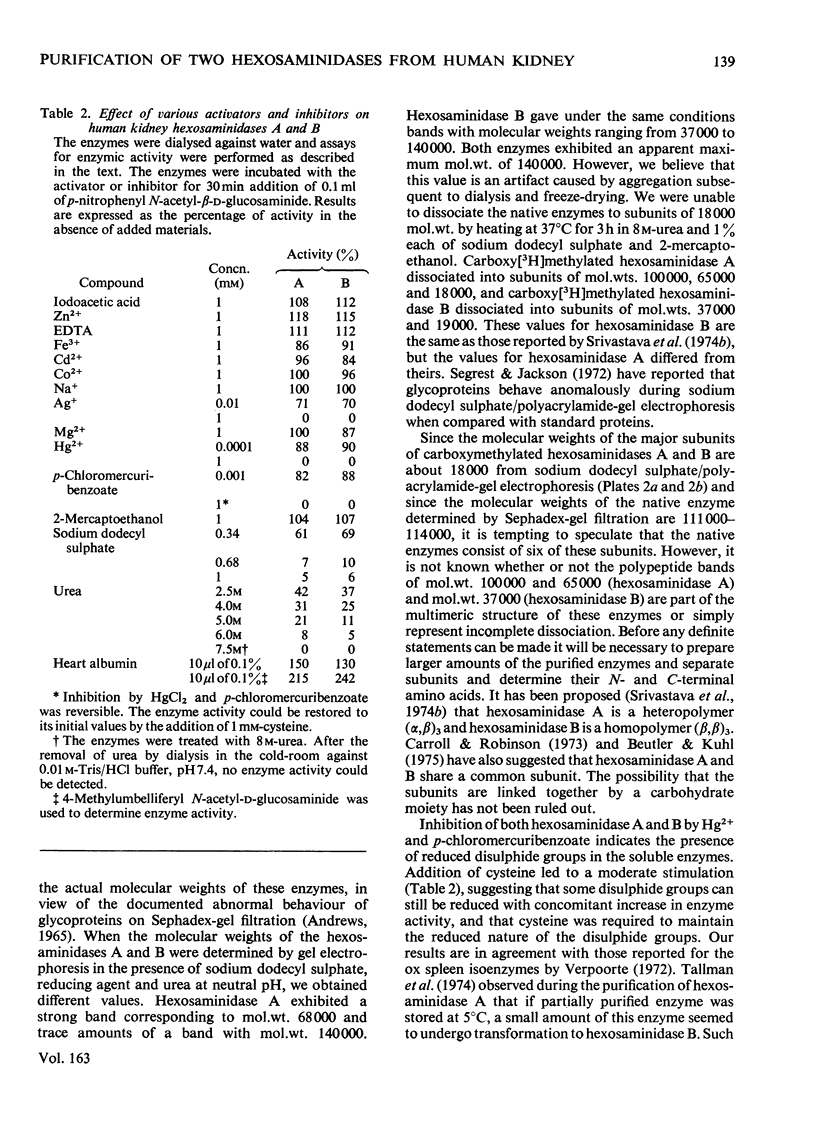
Hexosaminidase forms A and B were isolated from human kidney in a homogeneous state as demonstrated by electrophoretic and enzymic criteria. The enzymes were stable for at least 18 months when stored at -20 degrees C in 0.025 M-phosphate buffer, pH 6.5. The molecular weights of forms A and B were estimated by gel filtration to be 111 000 +/- 1500 and 114 000 +/- 1600 respectively. The molecular weights of hexosamidase A and B subunits were determined by using polyacrylamide-gel electrophoresis in the presence of sodium dodecyl sulphate. Hexosaminidase A dissociated into one subunit with mol.wt. 68 000. Hexosaminidase B dissociated into three subunits with mol. wts. 100 000, 68 000 and 37000 respectively, and one protein band of mol.wt. 140 000. After treatment of hexosaminidases A and B with iodoacetic acid, the molecular weights of the carboxymethylated polypeptide subunits were also estimated. Carboxymethylated hexosaminidase A dissociated into one major subunit of mol.wt. 18 000 and two other protein bands of mol.wts. 65 000 and 100 000. Carboxymethylated hexosaminidase B dissociated into one major subunit for mol.wt. 19 000 and an additional band of mol.wt. 37 000. The Km of the enzymes for the synthetic substrate p-nitrophenyl 2-acetamido-2-deoxy-beta-D-glucopyranoside was 0.8 mM. Both enzymes were inhibited or activated by various metal ions. Double pH optima for the enzymes were found at pH 4.5 and 4.8.
Full text
PDF



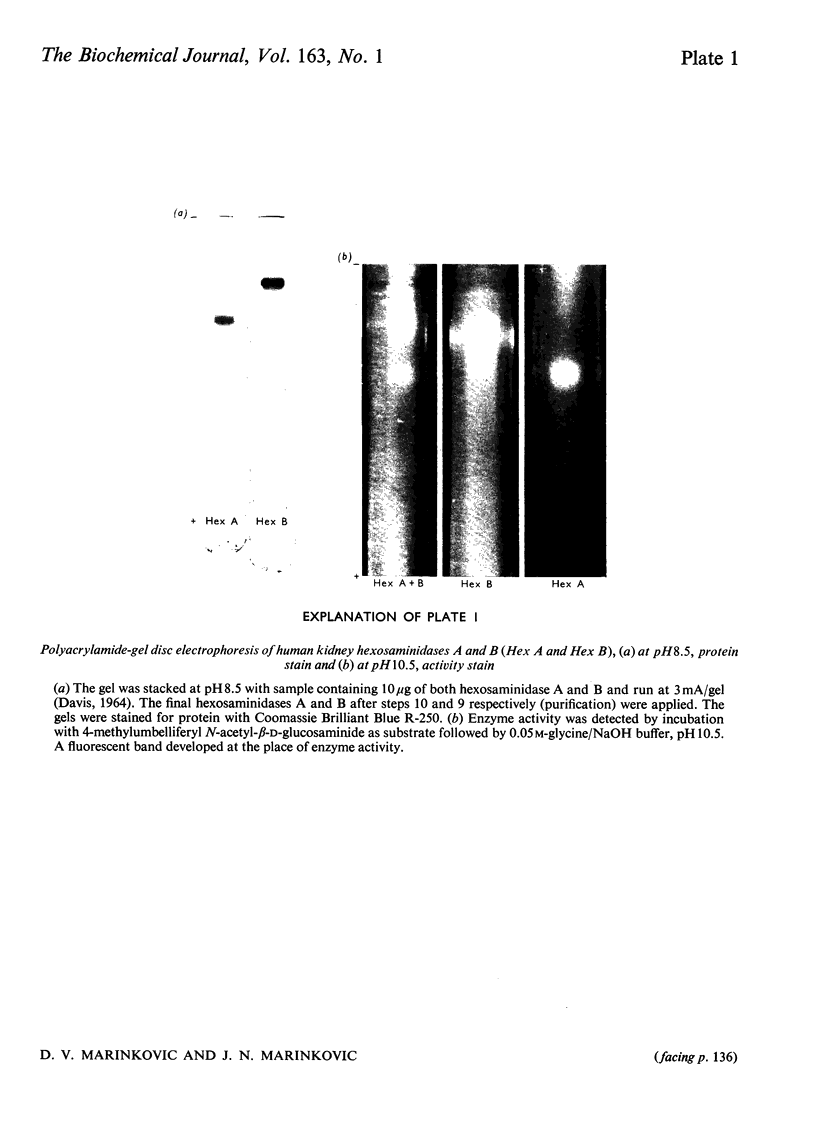

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- Beutler E., Kuhl W. Subunit structure of human hexosaminidase verified: interconvertibility of hexosaminidase isozymes. Nature. 1975 Nov 20;258(5532):262–264. doi: 10.1038/258262a0. [DOI] [PubMed] [Google Scholar]
- Braidman I., Carroll M., Dance N., Robinson D. Separation and properties of human brain hexosaminidase C. Biochem J. 1974 Nov;143(2):295–301. doi: 10.1042/bj1430295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Robinson D. A low-molecular-weight protein cross-reacting with human liver N-acetyl-beta-D-glucosaminidase. Biochem J. 1974 Feb;137(2):217–221. doi: 10.1042/bj1370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Robinson D. Immunological properties of N-acetyl-beta-D-glucosaminidase of normal human liver and of GM2-gangliosidosis liver. Biochem J. 1973 Jan;131(1):91–96. doi: 10.1042/bj1310091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R. L., Scroggs R. A., Whittington A. Purification and properties of beta-N-acetylglucosaminidase from bovine uterus. Biochim Biophys Acta. 1967 Sep 12;146(1):290–292. doi: 10.1016/0005-2744(67)90097-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dance N., Price R. G., Robinson D. Differential assay of human hexosaminidases A and B. Biochim Biophys Acta. 1970 Dec 29;222(3):662–664. doi: 10.1016/0304-4165(70)90193-5. [DOI] [PubMed] [Google Scholar]
- Dance N., Price R. G., Robinson D., Stirling J. L. Beta-galactosidase, beta-glucosidase and N-acetyl-beta-glucosaminidase in human kidney. Clin Chim Acta. 1969 May;24(2):189–197. doi: 10.1016/0009-8981(69)90311-8. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny E. H., Brady R. O., Volk B. W. Demonstration of an alteration of ganglioside metabolism in Tay-Sachs disease. Biochem Biophys Res Commun. 1969 Oct 22;37(3):526–531. doi: 10.1016/0006-291x(69)90947-4. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Mazzotta M. Y., Wan C. C., Orth R., Li S. C. Hydrolysis of Tay-Sachs ganglioside by beta-hexosaminidase A of human liver and urine. J Biol Chem. 1973 Nov 10;248(21):7512–7515. [PubMed] [Google Scholar]
- Marinkovic D. V., Marinkovic J. N. Isolation and properties of alpha-D-mannosidase from human kidney. Biochem J. 1976 May 1;155(2):217–223. doi: 10.1042/bj1550217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. S., Okada S., Ho M. W., Fillerup D. L., Veath M. L., Adams K. Ganglioside storage diseases. Fed Proc. 1971 May-Jun;30(3):956–969. [PubMed] [Google Scholar]
- O'Donnell I. J., Frangione B., Porter R. R. The disulphide bonds of the heavy chain of rabbit immunoglobulin G. Biochem J. 1970 Jan;116(2):261–268. doi: 10.1042/bj1160261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The demonstration of multiple heat stable forms of N-acetyl- -glucosaminidase in normal human serum. Biochim Biophys Acta. 1972 Jun 22;271(1):145–153. doi: 10.1016/0005-2795(72)90142-0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Antibody against purified human hexosaminidase B cross-reacting with human hexosaminidase A. Biochem Biophys Res Commun. 1972 May 26;47(4):753–759. doi: 10.1016/0006-291x(72)90556-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Verpoorte J. A. Purification of two -N-acetyl-D-glucosaminidases from beef spleen. J Biol Chem. 1972 Aug 10;247(15):4787–4793. [PubMed] [Google Scholar]
- Wetmore S. J., Verpoorte J. A. The partial purification of two -N-acetyl-D-hexosaminidases from porcine kidney. Can J Biochem. 1972 May;50(5):563–573. doi: 10.1139/o72-078. [DOI] [PubMed] [Google Scholar]
- Young E. P., Ellis R. B., Lake B. D., Patrick A. D. Tay-sachs disease and related disorders: Fractionation of brain N-acetyl-beta-hexosaminidase on DEAE-cellulose. FEBS Lett. 1970 Jul 15;9(1):1–4. doi: 10.1016/0014-5793(70)80295-2. [DOI] [PubMed] [Google Scholar]