Abstract
Apomyoglobin was prepared by an extremely mild modification of the acid/butanone technique, and the kinetics of the recombination reaction between this preparation and alkaline haematin were studied. The recombination has been shown to be precisely second-order and mono-phasic. Rate constants obtained from the study are in good agreement with values obtained previously by an indirect technique not involving separation of haem and apoprotein.
Full text
PDF
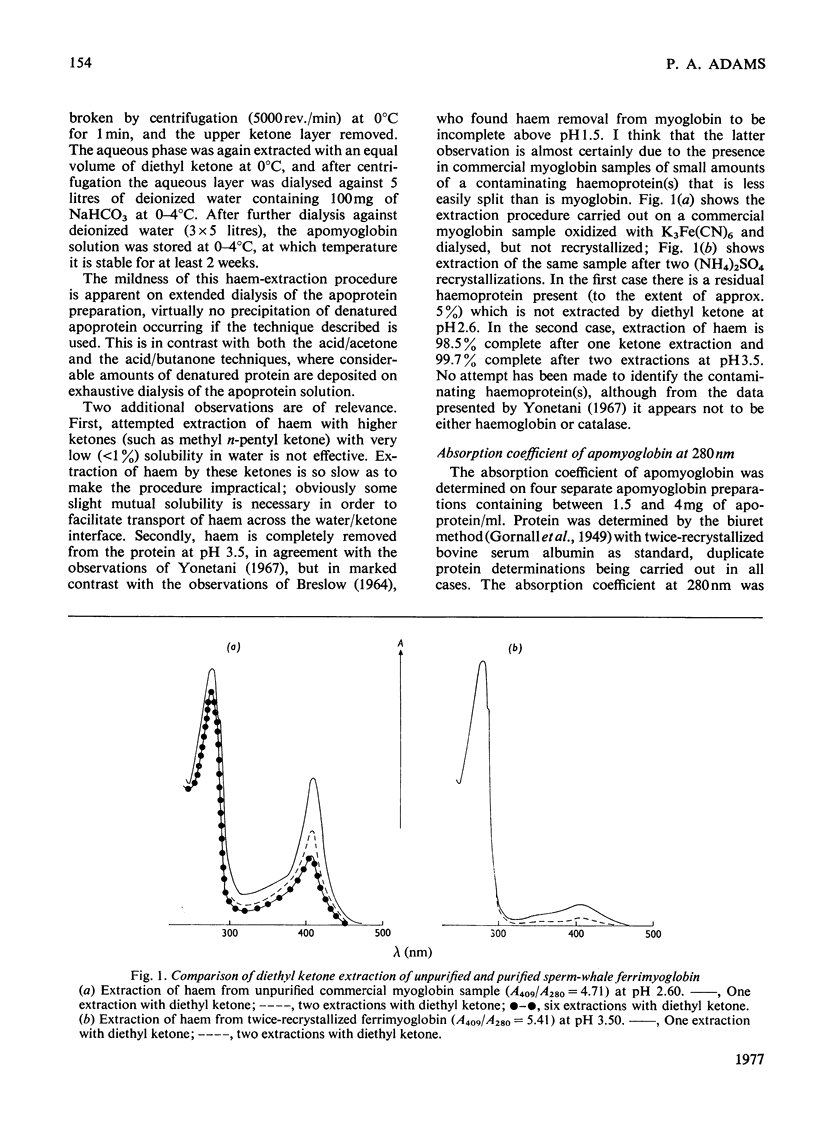
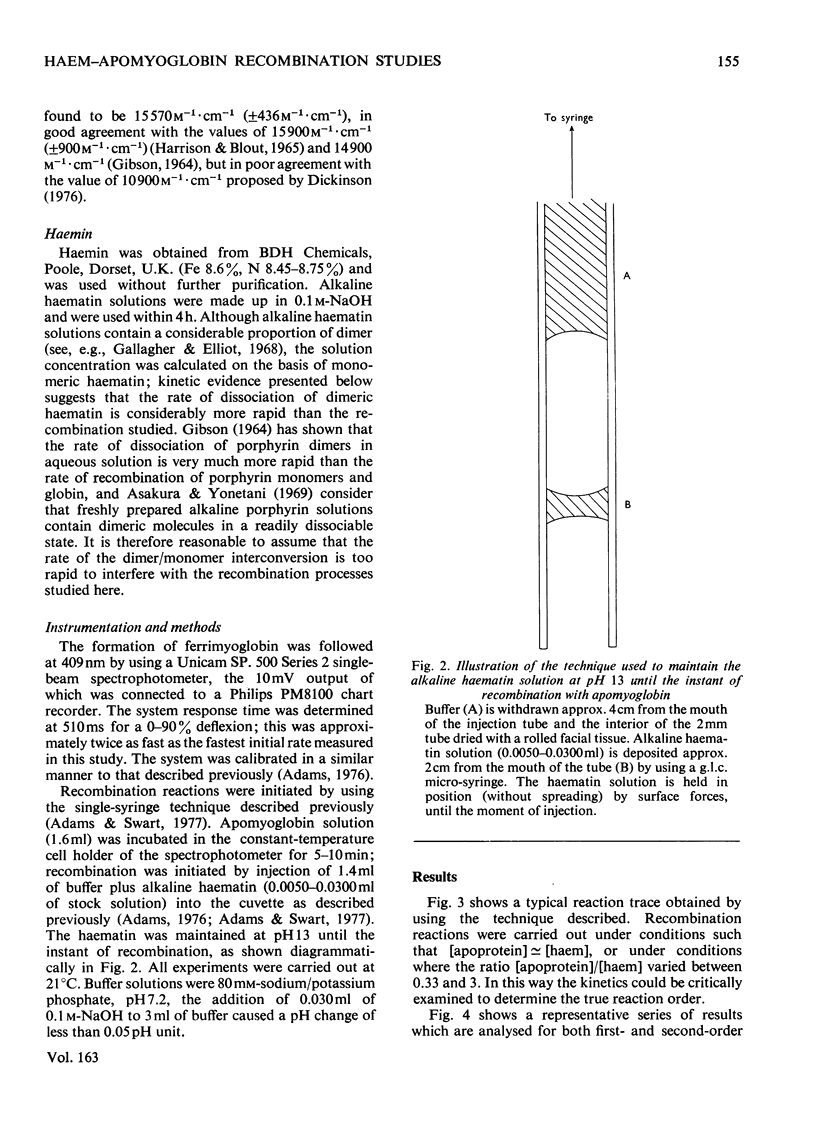
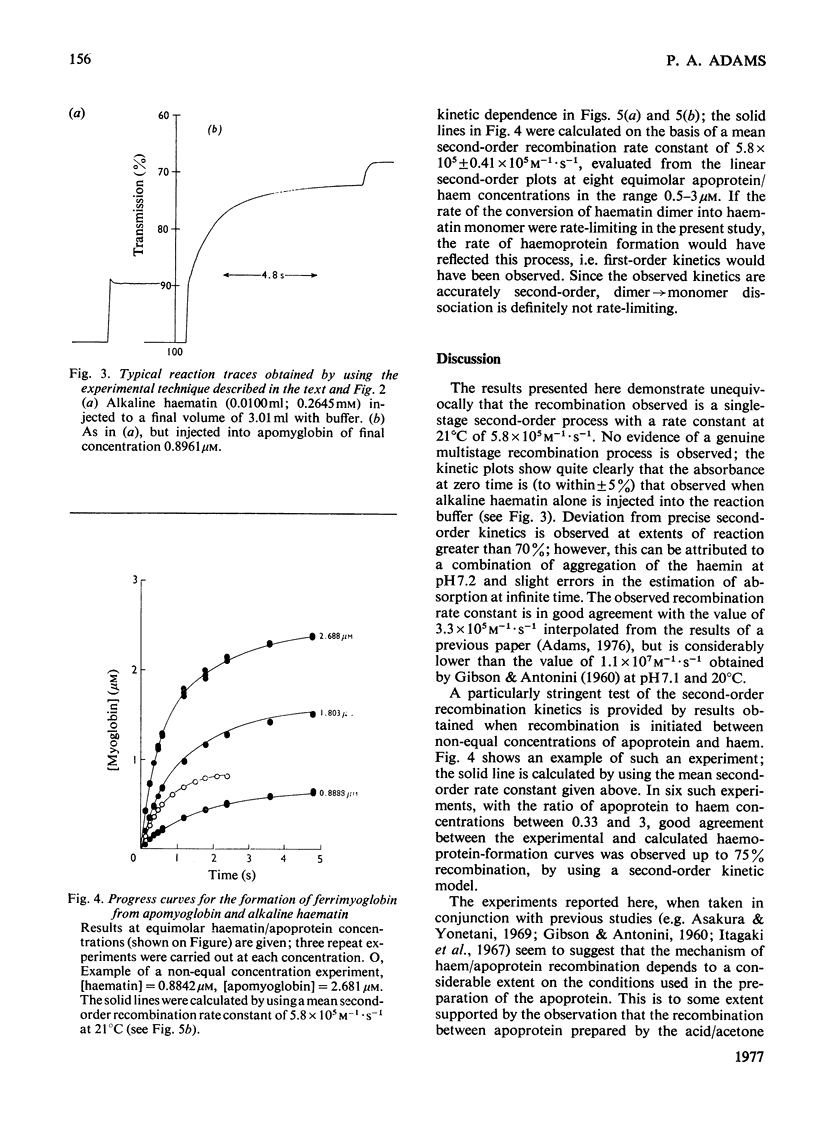
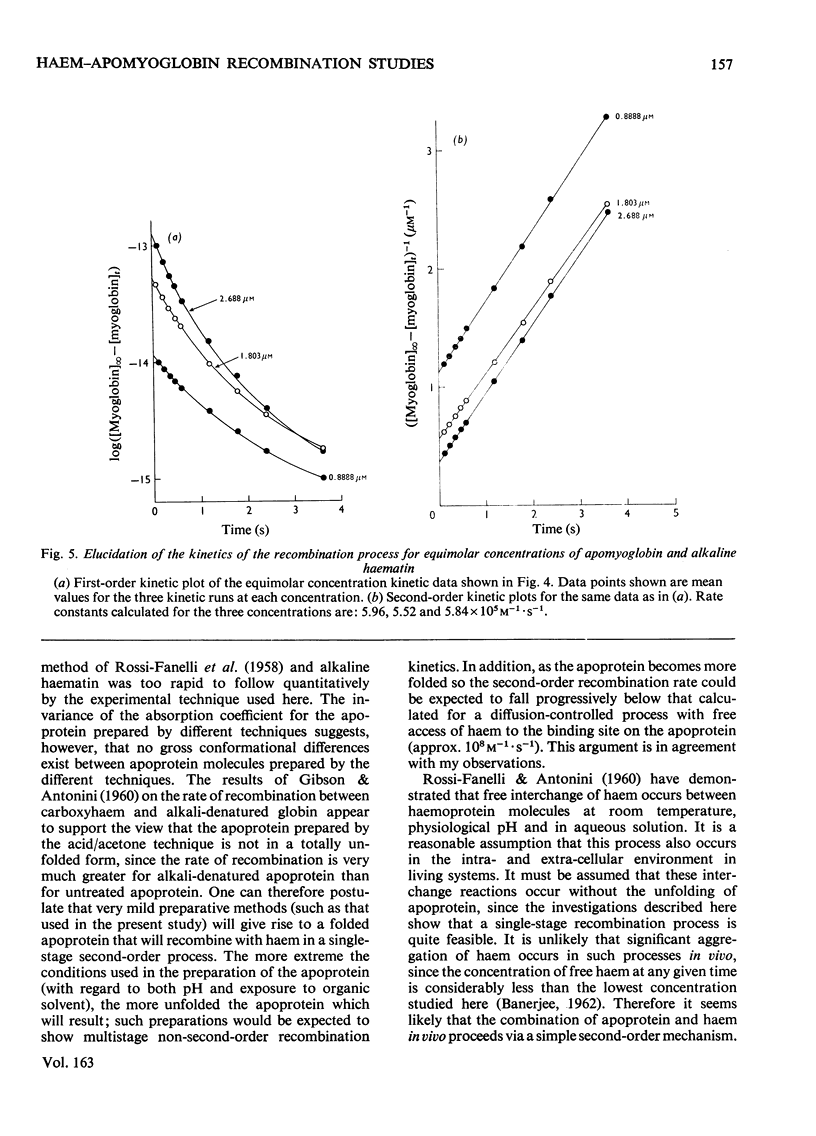

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. A., Swart E. R. The effect of temperature on the individual stages of the hydrolysis of non-specific-p-nitrophenol esters by alpha-chymotrypsin. Biochem J. 1977 Jan 1;161(1):83–92. doi: 10.1042/bj1610083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. A. The kinetics and mechanism of the recombination reaction between apomyoglobin and haemin. Biochem J. 1976 Nov;159(2):371–376. doi: 10.1042/bj1590371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T., Yonetani T. Studies on cytochrome c peroxidase. 13. Crystalline complexes of apoenzyme with porphyrins. J Biol Chem. 1969 Feb 25;244(4):537–544. [PubMed] [Google Scholar]
- BANERJEE R. [Thermodynamic study of the heme-globin association. I. Dissociation equilibrium of metmyoglobin: thermodynamic data]. Biochim Biophys Acta. 1962 Oct 22;64:368–384. doi: 10.1016/0006-3002(62)90746-1. [DOI] [PubMed] [Google Scholar]
- BRESLOW E. CHANGES IN SIDE CHAIN REACTIVITY ACCOMPANYING THE BINDING OF HEME TO SPERM WHALE APOMYOGLOBIN. J Biol Chem. 1964 Feb;239:486–496. [PubMed] [Google Scholar]
- Dickinson L. C. Metal replaced hemoproteins. A review with introductory laboratory preparation of cobaltmyoglobin. J Chem Educ. 1976 Jun;53(6):381–385. doi: 10.1021/ed053p381. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., ANTONINI E. Kinetic studies on the reaction between native globin and haem derivatives. Biochem J. 1960 Nov;77:328–341. doi: 10.1042/bj0770328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H. THE COMBINATION OF PORPHYRINS WITH NATIVE HUMAN GLOBIN. J Biol Chem. 1964 Oct;239:3282–3287. [PubMed] [Google Scholar]
- Gallagher W. A., Elliott W. B. Alkaline haematin and nitrogenous ligands. Biochem J. 1968 Jun;108(1):131–136. doi: 10.1042/bj1080131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON S. C., BLOUT E. R. REVERSIBLE CONFORMATIONAL CHANGES OF MYOGLOBIN AND APOMYOGLOBIN. J Biol Chem. 1965 Jan;240:299–303. [PubMed] [Google Scholar]
- Itagaki E., Palmer G., Hager L. P. Studies on cytochrome b562 of Escherichia coli. II. Reconstitution of cytochrome b562 from apoprotein and hemin. J Biol Chem. 1967 May 10;242(9):2272–2277. [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Studies on cytochrome c peroxidase. IX. The reaction of ferrimyoglobin with hydroperoxides and a comparison of peroxide-induced compounds of ferrimyoglobin and cytochrome c peroxidase. J Biol Chem. 1967 Apr 25;242(8):1974–1979. [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. X. Crystalline apo-and reconstituted holoenzymes. J Biol Chem. 1967 Nov 10;242(21):5008–5013. [PubMed] [Google Scholar]


