Summary
Background
The association between congenital heart disease (CHD) and non-optimal temperatures has received limited investigation. We aimed to investigate the impact of extreme temperatures on CHD mortality.
Methods
We reviewed the National Mortality Surveillance System of China and retrieved death records attributable to CHD from 2013 to 2021. Temperature and air pollutants data were obtained from the ERA5-Land reanalysis dataset and the ChinaHighAirPollutants database. A two-stage case-crossover study design was implemented. Sensitivity and subgroup analyses were performed to test the robustness of findings and determine the vulnerable population.
Findings
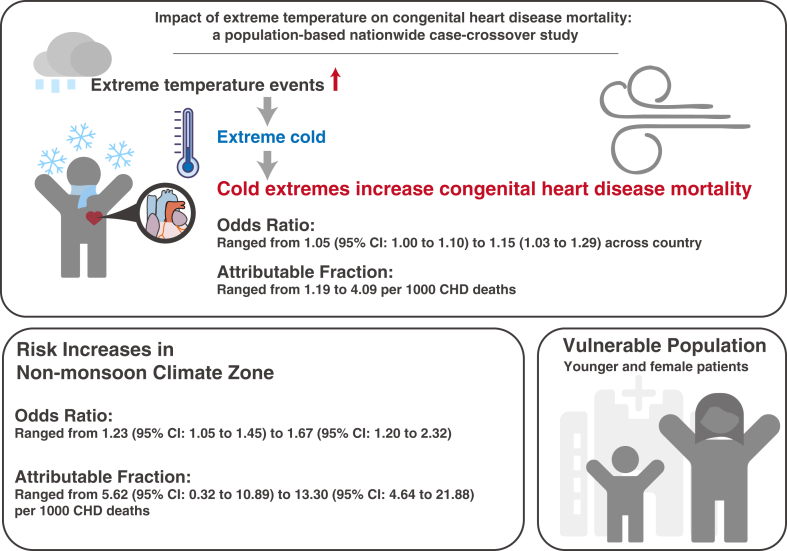
A total of 32,168 CHD deaths were included, showing a significant association between cold and CHD mortality, while there was little effect for heat. The odd ratio (OR) ranged from 1.05 (95% confidence interval: 1.00–1.10) to 1.15 (1.03–1.29) across country, with a more pronounced impact in non-monsoon regions up to 1.67 (1.20–2.32). Cold extremes accounted for an attributable fraction of 4.09 per 1000 CHD death nationwide and 13.30 per 1000 CHD deaths in non-monsoon regions. Sensitivity analyses utilizing apparent temperature and adjusting for air pollutants confirmed the robustness of the main findings. Female and pediatric CHD patients were identified as the vulnerable population to cold extremes.
Interpretation
For the first time, this nationwide study demonstrated the significant impact of cold extremes on CHD mortality, particularly in non-monsoon regions, and among female and pediatric subgroups. These findings may suggest that healthcare professionals advise CHD patients to avoid exposure to cold extremes, and provide insight into healthcare policy adjustment.
Funding
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS, 2023-I2M-C&T-B-059), the Capital Health Research and Development of Special Fund (2022-1-4032) and the National High Level Hospital Research Funding (2022-GSP-GG-19).
Keywords: Congenital heart disease, Extreme temperature, Mortality, Cold extreme
Research in context.
Evidence before this study
Extensive research has consistently shown a strong link between non-optimal temperatures and cardiovascular diseases, such as ischemic heart disease, stroke, heart failure, arrhythmia, and aortic disease, in terms of both morbidity and mortality. Congenital heart disease (CHD), a common cardiovascular condition in children, has been the focus of previous studies examining the impact of exposure to non-optimal temperatures during early pregnancy on the risk of CHD in offspring. However, limited research has been conducted on the association between CHD mortality and non-optimal temperatures.
Added value of this study
Utilizing data from China's National Mortality Surveillance System, we conducted a comprehensive two-stage, individual-level, nationwide case-crossover study to examine the relationship between ambient temperature and mortality due to CHD. Our findings revealed a significant association between CHD mortality and cold extremes rather than high temperatures, with the risk escalating in correlation with the severity and duration of cold spells.
Implications of all the available evidence
This study could offer valuable insights in the field of pediatric cardiology concerning the effects of environmental exposures, prompting healthcare professionals to advise patients with CHD to avoid exposure to extreme cold temperatures. Additional multi-national studies are necessary to validate the results of this research.
Introduction
Climate change is intricately linked to human health and can cause temperature extremes, an ongoing challenge that adversely undermines the well-being of individuals and increases the vulnerability of populations to life-threatening conditions.1 A multi-country study reported that 5,083,173 deaths per year from 2000 to 2019 were attributed to non-optimal temperatures, and 90.4% of these fatalities were linked to cold-related conditions.2 Due to anthropogenic influence, the frequency and magnitude of weather extremes tend to be continuously increased.3 Thus, the association between temperature extremes and human health is still the focus of research. Non-optimal temperature-related morbidity and mortality are multifactorial, which was speculated to be increased cardiac demand, dehydration, and pulmonary stress during heat exposure, as well as diminished cardiac output, hypotension and subsequent organ failure during cold exposure.2,4,5 In addition, the cardiovascular system is one of the most susceptible systems to non-optimal temperature, resulting in elevated risk and premature mortality.6
Congenital heart disease (CHD), one of the most prevalent cardiovascular diseases for children, exhibited an average increase of 14% in children and 70% in adults in the prevalence between 1985 and 2010.7,8 With the continuous advancement in CHD treatment, there will be more individuals living with CHD and engaging in social activity.9 Thus, there may be increasing possibility for patients with CHD to experience extreme temperature. Substantial research has reported the robust association between non-optimal temperatures and cardiovascular diseases, including ischemic heart disease, stroke, heart failure, arrhythmia, and aortic disease in the aspects of morbidity and mortality.10, 11, 12, 13 For CHD, previous studies mainly focused on the association between exposure to non-optimal temperatures during early pregnancy and the risk of CHD in offsprings.14, 15, 16, 17, 18, 19 However, the association between CHD mortality and non-optimal temperatures has received limited investigation. A recent American Heart Association Statement has pointed out that children are more vulnerable to environmental exposures due to the developmental plasticity,20 and CHD is the foremost congenital defect with the highest burden of mortality, accounting for over 50% of noncommunicable disease deaths under 20 years old in 2019.21 Taken together, exploring the association between CHD mortality and ambient temperature could be beneficial to understanding the contributing factors and further improve the lifespan of these populations.
Based on the National Mortality Surveillance System of China, we performed a two-stage, individual-level, nationwide case-crossover study to systemically analyze the association between CHD mortality and ambient temperature. The modification effect of humidity and air pollutants on ambient temperature was considered in the model, and several subgroup analyses were conducted to explore the vulnerable subpopulations. In response to the American Heart Association Statement, this study may provide novel evidence-based insights into the life-long caring of CHD populations and health policy adjustment.
Methods
Data sources
The death records from the National Mortality Surveillance System of China, which was operated under the administration of the Chinese Center for Disease Control and Prevention,22,23 were reviewed for the period between January 1st, 2013 and December 31st, 2021. The underlying causes of deaths attributed to CHD were determined based solely on the primary diagnosis coded by the 10th revision of the International Classification of Diseases, including Q20 to Q28. The date of death, date of birth, gender, and geocoded residential address were also collected from the records.
The gridded ambient temperature data were obtained from the ERA5-Land hourly climate reanalysis dataset, which has a spatial resolution of 0.1° × 0.1°,24 and has been validated and widely adopted in previous global studies.25,26 Geocoded residential addresses of each identified CHD record from the National Mortality Surveillance System were converted into gridded form. Subsequently, we extracted hourly 2m dewpoint and 2m temperature data for each identified CHD death record from the nearest grid cell in the ERA5-Land dataset. The daily temperature was determined by calculating the average of the 24-h temperature. The corresponding relative humidity of each grid cell was also collected to calculate the apparent temperature as previously described.27
The gridded ambient air pollutants data were obtained from the ChinaHighAirPollutants (CHAP) database (available at https://weijing-rs.github.io/product.html), which was generated from the spatiotemporal artificial intelligence models combined with ground measurements, satellite remotes sensing products, and atmospheric reanalysis.28, 29, 30, 31 The corresponding daily PM2.5, O3, NO2, SO2, and CO data for each CHD death record at his or her geocoded residential address were extracted for downstream analysis. The spatial resolution was 1 × 1 km for PM2.5 and O3, and 10 × 10 km for NO2, SO2, and CO.
Human development index (HDI), a well-known index used to assess the well-being levels with a combination of health, education and standard of living, was obtained from the Subnational Human Development Database.32 The HDI ranges from 0 to 1, with a value approximate to 1 reflecting a higher level of human development. A HDI below 0.550 indicates low well-being level; between 0.550 and 0.699 is considered moderate well-being level; between 0.700 and 0.799 is classified as high well-being level; and a HDI equal to or greater than 0.800 is considered very high well-being level.33
Study design
We applied a two-stage, individual-level, time-stratified case-crossover design to investigate the association between CHD mortality and ambient temperature, which could better address the effect of long-term trend and seasonality of exposure and control for individual-level factors (such as age, sex, lifestyle, comorbidities and socioeconomic status).34 In the first stage, we obtained the province-specific temperature-mortality associations using a time-stratified case-crossover design. In this design, the day of death for each subject was designated as the case day; any other dates that fell within the same year, month, and weekday were designated as the matching control days. For example, if a patient passed away on Wednesday, June 10th, 2021, that date would be designated as the case day and the other Wednesdays in June would serve as the control days (June 3rd, 17th, 24th). Due to the vast territories of China, the optimal temperature for populations in different provinces may vary. Therefore, in the second stage, we obtained the overall estimate across China by using a meta-analytic model with random effect to pool provincial estimates.35 The informed consent was not applicable due to the nature of second-hand data, and this study was approved by the ethics committee of Fuwai hospital (No. 2024-2324).
Exposure assessment
The initial step was to standardize address-specific ambient temperatures by converting absolute values into percentiles. We subsequently derived the patient-specific location thresholds for cold extremes at the 5th, 7.5th, 10th, 12.5th percentiles, as well as heat extremes at the 87.5th, 90th, 92.5th and 95th percentiles of daily temperatures from 2013 to 2021. Three exposure patterns were measured: i) moving average effect: 2–5 days moving average of temperatures before CHD death was used to estimate the association36; ii) single lag effect: a range of lags from 0 to 21 days was used to estimate the temperature-mortality association37; iii) cumulative effect: cold or heat extremes last for 2–5 days (such as P5_5d, indicating temperature remained below the 5th percentile for 5 consecutive days, and P95_3d, indicating temperature remained above the 95th percentile for 3 consecutive days) were used to estimate the association.11
Statistical analysis
The association between CHD mortality and ambient temperature (as continuous variables) was visualized using a restricted cubic spline with 3 degrees of freedom. A crude conditional logistic regression model was used to examine the association between exposure to temperature extremes and CHD deaths, with odds ratio (OR) and its 95% confidence interval (CI) employed to quantify the associations. Each subject had one case day and three or four control days in the time stratum. The formula was as follows:
where indicates the ith observation of the hth stratum. indicates the constant term for the hth stratum.
To quantify the number of deaths attributable to temperature extremes, we separately estimated the attributable fraction (population) for different thresholds of cold extremes encompassing daily temperatures at the 5th, 7.5th, 10th, and 12.5th percentiles, as well as their cumulative effect. The formula was as follows:
where indicates the risk of CHD deaths in the whole population, indicates the risk of CHD deaths in the population that is not exposed to temperature extremes.
Sensitivity analyses were conducted to test the robustness of the main findings, including pooling the estimates at climate zones’ level in the second stage,38 analyzing the association of apparent temperatures with CHD mortality and adjusting air pollutants (PM2.5, O3, NO2, SO2, and CO) as the covariates in the conditional logistic regression model. To identify potentially vulnerable CHD populations to cold extremes, we conducted stratified analyses by climates (monsoon, non-monsoon), sex (male, female), age (<1 year, ≥1 year; <5 years, ≥5 years; <18 years, ≥18 years), and HDI (<0.7, ≥0.7). The stratification in climates was due to the climatic distribution and distinct characteristics between monsoon and non-monsoon climates39; age was mainly based on the clinical significance of CHD that 1-year is a critical turning point of CHD treatment,40 5-year is an essential milestone to assess the health of pediatrics within a country,41,42 and 18-year is another significant period to separate the CHD management between pediatric and adult population43; HDI was categorized as low or high well-being level with the cutting point of 0.7.33 We also divided the study period by 2020 to investigate whether the COVID-19 pandemic had an impact on the relationship between extreme temperatures and CHD mortality.44 P for interaction was also tested by adding the interaction term in the model. The data collection process was conducted using Python (v.3.9) and all the analyses were performed utilizing R (v.4.0.3) and SAS (v 9.4).
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation, writing of the report.
Results
Descriptive results
From 2013 to 2021, a total of 32,168 case days of CHD deaths were identified through the National Mortality Surveillance System of China, with no missing data and corresponding 109,201 control days matched utilizing the case-crossover design (Table 1). Among all deaths attributed to CHD, neonates and adults emerged as the predominant age groups, with most cases being male patients (55.2%). The case of CHD deaths remained relatively stable throughout the four seasons, with a slightly higher occurrence observed in spring and winter. The majority of case days occurred in regions with monsoon climates, and consistent patterns were observed across genders, age groups and seasons. The summarized results were shown in Central illustration.
Table 1.
Characteristics of the study subjects in China, from 2013 to 2021.
| Nationwide | Non-monsoon climate | Monsoon climate | |
|---|---|---|---|
| All CHD deaths (case days) | 32,168 | 2739 | 29,429 |
| Control days | 109,201 | 9315 | 99,886 |
| Age | |||
| <28 days | 10,776 (33.5%) | 944 (34.5%) | 9832 (33.4%) |
| 28 days to 1-year | 1396 (4.3%) | 106 (3.9%) | 1290 (4.4%) |
| 1 to 5-year | 6382 (19.8%) | 655 (23.9%) | 5727 (19.5%) |
| 5 to 18-year | 3914 (12.2%) | 327 (11.9%) | 3587 (12.2%) |
| ≧18-year | 9700 (30.2%) | 707 (25.8%) | 8993 (30.6%) |
| Sex | |||
| Male | 17,743 (55.2%) | 1457 (53.2%) | 16,286 (55.3%) |
| Female | 14,425 (44.8%) | 1282 (46.8%) | 13,143 (44.7%) |
| Season at death | |||
| Spring (March to May) | 8508 (26.4%) | 715 (26.1%) | 7793 (26.5%) |
| Summer (June to August) | 7274 (22.6%) | 583 (21.3%) | 6691 (22.7%) |
| Autumn (September to November) | 7283 (22.6%) | 609 (22.2%) | 6674 (22.7%) |
| Winter (December to February) | 9103 (28.3%) | 832 (30.4%) | 8271 (28.1%) |
Abbreviation: CHD, congenital heart disease.
Values are presented with number (percentage).
Central illustration.
Cold extremes increase the risk of mortality due to congenital heart disease, with higher risk among younger individuals, females, and in non-monsoon regions.
Association between ambient temperature and CHD mortality
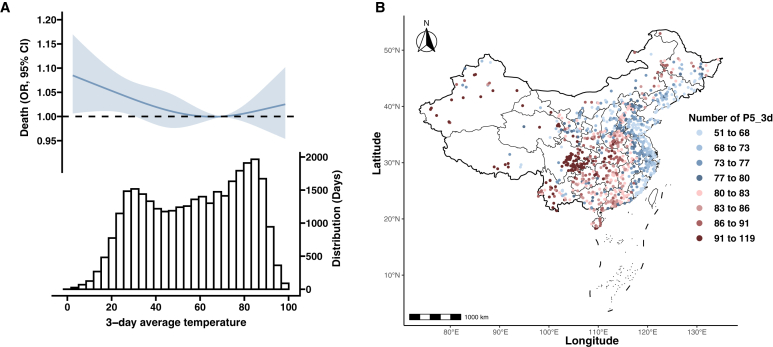
The relationship between 2-day to 5-day average temperature and OR of CHD mortality exhibited a common U-shape pattern (Fig. 1A and Supplementary Figure S1). The risk of CHD mortality was significantly associated with cold temperatures, while no significant association was observed with heat temperatures despite the presence of notable point estimates. Negative results were also observed in the cumulative effects of heat extremes on CHD mortality (Supplementary Tables S1 and S2). Thus, we focused on exploring the association between cold temperatures and CHD mortality. The number of cold extremes exhibited a gradual increase as the elevated percentiles of thresholds (P5_3d to P12.5_3d), primarily concentrated in the western and central regions of the country (Fig. 1B and Supplementary Figure S2).
Fig. 1.
Association of CHD mortality with ambient temperatures. A) Restricted cubic spline for the association between 3-day average temperature and risk of CHD mortality; B) The number of P5_3d presenting at the site of each CHD death. P5_3d indicated temperature less than the 5th percentile for 3 consecutive days. OR, odds ratio; CI, confidence interval.
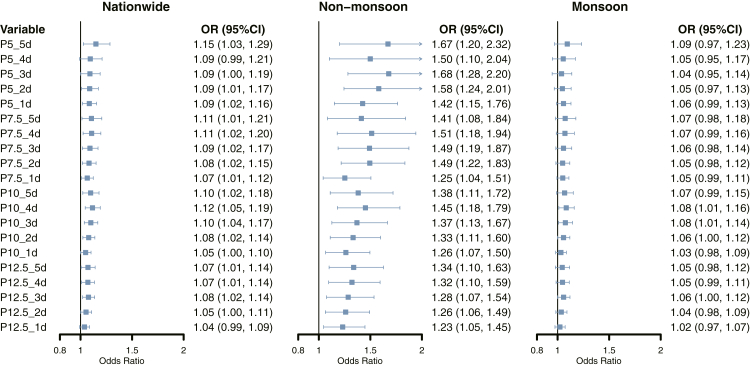
The lag responses of each threshold for cold extremes were examined up to 21 days, revealing that the significant association between cold extremes and CHD mortality occurred within a lag period of 5 days (Supplementary Figure S3). Therefore, we further explored the cumulative effects of cold extremes on CHD mortality. Approximately 90% of CHD deaths occurred in regions with monsoon climates at various thresholds of cold extremes (Table 2). A significant association between cold extremes and CHD mortality was observed across the country, with the highest risk in P5_5d (OR: 1.15, 95% CI: 1.03–1.29) (Fig. 2). The positive contribution was found to be more pronounced in the non-monsoon zone and exhibited an increase with lower temperature thresholds compared with the monsoon zone, with the OR ranging from 1.23 (95% CI: 1.05–1.45) for P12.5_1d to 1.67 (95% CI: 1.20–2.32) for P5_5d. The sensitivity analysis pooling estimates at climate zones’ level (Supplementary Table S3) and utilizing apparent temperature yielded consistent results with the main findings (Supplementary Table S4). In addition, sensitivity analysis adjusted by air pollutants exhibited a slightly more positive contribution of cold extremes on CHD mortality (Supplementary Table S5). Stratified analysis did not find interaction between year and the exposure (2013–2019 vs. 2020–2021), albeit to the larger point estimates in the 2020–2021 group (Supplementary Table S6).
Table 2.
Number of CHD deaths during cold extremes in China, from 2013 to 2021.
| Exposurea | No. of CHD deaths (%) |
||
|---|---|---|---|
| Nationwide | Non-monsoon climate | Monsoon climate | |
| P5_5d | 448 | 59 (13.2) | 389 (86.8) |
| P5_4d | 567 | 66 (11.6) | 501 (88.4) |
| P5_3d | 771 | 94 (12.2) | 677 (87.8) |
| P5_2d | 1095 | 121 (11.1) | 974 (88.9) |
| P5_1d | 1580 | 153 (9.7) | 1427 (90.3) |
| P7.5_5d | 778 | 93 (12.0) | 685 (88.0) |
| P7.5_4d | 990 | 112 (11.3) | 878 (88.7) |
| P7.5_3d | 1285 | 140 (10.9) | 1145 (89.1) |
| P7.5_2d | 1736 | 177 (10.2) | 1559 (89.8) |
| P7.5_1d | 2504 | 225 (9.0) | 2279 (91.0) |
| P10_5d | 1207 | 141 (11.7) | 1066 (88.3) |
| P10_4d | 1502 | 168 (11.2) | 1334 (88.8) |
| P10_3d | 1878 | 194 (10.3) | 1684 (89.7) |
| P10_2d | 2420 | 234 (9.7) | 2186 (90.3) |
| P10_1d | 3314 | 300 (9.1) | 3014 (90.9) |
| P12.5_5d | 1696 | 197 (11.6) | 1499 (88.4) |
| P12.5_4d | 2055 | 223 (10.9) | 1832 (89.1) |
| P12.5_3d | 2521 | 253 (10.0) | 2268 (90.0) |
| P12.5_2d | 3142 | 301 (9.6) | 2841 (90.4) |
| P12.5_1d | 4005 | 362 (9.0) | 3643 (91.0) |
Abbreviation: CHD, congenital heart disease.
Indicates different cold extremes. For example, P5_5d indicates temperatures less than the 5th percentile of the patient-specific location last for 5 days.
Fig. 2.
Risk of CHD mortality and different thresholds of cold extremes across country, regions with monsoon and non-monsoon climates. P value for interaction as follows: 0.015 for P5_5d, 0.028 for P5_4d, 0.001 for P5_3d, 0.001 for P5_2d, 0.008 for P5_1d. 0.027 for P7.5_5d, 0.005 for P7.5_4d, 0.003 for P7.5_3d, 0.001 for P7.5_2d, 0.076 for P7.5_1d, 0.025 for P10_5d, 0.008 for P10_4d, 0.017 for P10_3d, 0.014 for P10_2d, 0.025 for P10_1d, 0.014 for P12.5_5d, 0.016 for P12.5_4d, 0.035 for P12.5_3d, 0.025 for P12.5_2d, 0.028 for P12.5_1d. OR, odds ratio; CI, confidence interval.
Vulnerable CHD population to cold extremes
Subgroup analyses were conducted to investigate the vulnerable CHD population to cold extremes. Compared to male patients, female patients appeared to exhibit a higher susceptibility to cold extremes, with the OR ranging from 1.08 (95% CI: 1.01–1.16) for P10_1d to 1.20 (95% CI: 1.01–1.43) for P5_5d (Supplementary Table S7). The vulnerability to cold extremes in CHD patients with different age was revealed to be discrepant that younger patients exhibiting a relatively higher risk when compared with others (Supplementary Tables S8–S10). The point estimates in lower HDI regions exhibited a greater susceptibility to cold extremes in comparison to those with higher HDI, and prolonged duration of cold extremes might exacerbate the impacts on mortality (Supplementary Table S11).
Attribution fraction of CHD deaths due to cold extremes
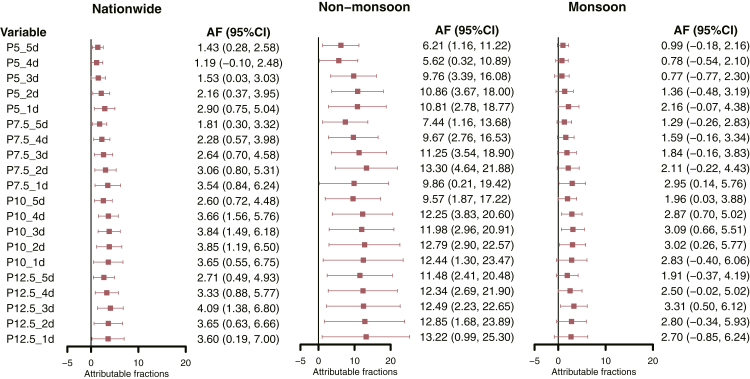
The attribution fraction of CHD deaths to cold extremes was explored and ranged from 1.19 to 4.09 per 1000 CHD deaths (Fig. 3). The attribution fraction exhibited an upward trend as the thresholds increased and the duration of cold extremes decreased. Similarly, the vulnerability of CHD patients to cold extremes was found to be higher in the non-monsoon regions compared to monsoon regions, ranging from 5.62 per 1000 CHD deaths for P5_4d to 13.30 per 1000 CHD deaths for P7.5_2d.
Fig. 3.
Attributable fractions of CHD deaths due to cold extremes across country, regions with monsoon and non-monsoon climates. AF, attributable fraction; CI, confidence interval.
Discussion
In this case-crossover study based on the National Mortality Surveillance System of China, we systematically investigated the association between ambient temperatures and CHD mortality. We found that the CHD mortality was significantly associated with cold extremes rather than ambient heat, and the risk increased with the intensity and duration of cold extremes. The sensitivity analyses, which substituted the ambient temperatures with the apparent temperatures or incorporated air pollutants as adjustment, demonstrated consistent results with the major findings. In addition, CHD patients who were female, younger, and lived in regions with low HDI regions were more vulnerable to cold extremes.
Enormous research has confirmed the significant association between ambient temperature and death due to different cardiovascular diseases.10, 11, 12, 13 However, little is known about the relationship between ambient temperature and CHD mortality, albeit several studies exploring the morbidity of CHD to ambient temperature.14, 15, 16, 17, 18 The decreasing birth rate of neonates with CHD, combined with improvements in treatment outcomes, has resulted in a substantial decrease in number of CHD-related deaths,9,21 leading to an inadequate sample size for a thorough analysis. To our knowledge, this is the first study designed to illustrate the impact of ambient temperature on CHD mortality. Our study reviewed the National Mortality Surveillance System of China and included 32,168 CHD death records from 2013 to 2021 spanning almost a decade from a nation comprising nearly one-fifth of the world's population. Due to the availability of personal death records, we were able to assess the association at the individual level while controlling for time-varying personal confounding factors and seasonality. In addition, people living in different climate zones may have different tolerances to extreme temperatures.37,45 Thus, we utilized location-specific standardized temperatures represented as percentiles as the exposure and implemented a two-stage statistical approach to accommodate local variations by pooling estimates across multiple cities for more accurate and reliable results. Furthermore, the vast territories of China with various climates may enhance the generalization of our study findings.
Our results revealed that only cold extremes had a significant impact on CHD mortality, which was partially inconsistent with previous studies reporting that both cold and heat extremes contributed to extra cardiovascular mortality.10,13 These might be explained in several aspects: i) Cold extremes may have more severe impacts on CHD mortality as a series of multi-country studies suggested that cold extremes may cause extra mortality than heat events.2,37,45 In addition, pediatrics is the major population in patients with CHD, and there are distinct physical variances between the children and adult populations. A recent review has indicated that most studies have not found a correlation between heat and cardiovascular diseases in pediatrics46; ii) For CHD with left-to-right shunt, increased pulmonary blood flow may render individuals more susceptible to cold environment, increasing the risk of respiratory tract infection47; iii) For CHD with right-to-left shunt, increased metabolism and oxygen consumption in cold environment may exacerbate the hypoxia in multi-organs.48 Collectively, CHD patients should be alerted to the potential extra risk of premature death caused by cold extremes.
Compared to men, women generally demonstrate reduced sweating, lower mean skin temperature and may feel more discomfort in cold temperatures.49,50 Xiong et al. found that women might be more sensitive in thermal sensation, and feeling discomfort in cold temperatures might cause cytokines level fluctuation.49 In addition, women might have reduced ability to generate heat in cold environments,51 and experience greater heat loss due to increased evaporative efficiency of sweating resulting from a higher ratio of body surface to body mass,50 leading to lower skin temperature and vicious cycle. Moreover, reproductive hormones have significant effects on thermoregulation, as estrogen typically promotes vasodilation, heat dissipation, and lower body temperature, while progestins generally exhibit opposing effects.52 Thus, the fluctuations in reproductive hormones due to menstrual cycle or the use of oral contraceptives may lead to differences in thermoregulation between women and men. Collectively, the aforementioned processes may pose additional challenges for female CHD patients when encountering cold extremes, and future study to verify this theory is warrant.
Most CHD patients require surgical repair in their early life, placing pediatric patients at risk of fatal postoperative complications. The overwhelming major adverse cardiovascular events occurred within the first year of life in CHD patients,53 meaning that patients in this postoperative period may be more potentially vulnerable to environmental exposure. In addition, children are more susceptible to cold extremes and less capable of regulating body temperature.54 A national survey in China reported that decreasing temperatures were associated with fluctuation in blood pressure among pediatric patients.55 Collectively, these factors may render pediatric CHD patients at higher risk when experiencing cold extremes.
In subgroup analysis of HDI, the insufficient sample size in low-HDI regions may lead to the non-significant results, despite exhibiting higher point estimates compared to high-HDI regions. According to the China Statistical Yearbook from the National Bureau of Statistics,56 the resident population in low-HDI regions accounted for approximately 10% of the country's total population over the past decade. In addition, more patients prefer to seek care in high-HDI regions due to the regionalization of CHD-related medical resources.9 Thus, the interpretation of results stratified by HDI should be cautious, and further studies investigating this aspect are warranted.
A multi-country study investigating the association between cardiovascular mortality and extreme cold temperatures below the 2.5th percentile reported an excess of 9.09 deaths per 1000 all-cause cardiovascular deaths, ranging from 6.87 for arrhythmia to 12.80 for heart failure.10 However, CHD deaths were not included in its scope. The attributable fractions of CHD deaths due to cold extremes ranged from 1.43 per 1000 CHD deaths for P5_5d to 4.09 per 1000 CHD deaths for P12.5_3d in our studies and increased up to 13.30 per 1000 CHD deaths when focusing on non-monsoon climate zones. This study represents the first quantification of attributable fractions of CHD deaths due to cold extremes, filling a gap in understanding the burden of CHD mortality associated with ambient temperature and providing significant implications for public health. With the advancement of treatment, the life expectancy of CHD population has significantly increased, allowing more patients surviving until adulthood.21 As a result, the focus may transition from enhancing treatment to fulfilling lifelong care and improving the quality of life.57 Based on our findings, in addition to regular medical follow-up, it is crucial to be vigilant towards fluctuations in weather conditions, particularly cold extremes. Our study may serve as important evidence for healthcare professionals to advise CHD patients to avoid exposure to cold extremes or consider using artificial warm-keeping measures, such as air conditioner, and the results could potentially inform health policy adjustment.
Limitation
Several limitations should be addressed in this study. First, the limited sample size of CHD deaths may hinder the obtainment of statistically significant results in various analyses, indicating the necessity of multi-country study to verify the results and improve the generalizability to other populations. Second, similar to the majority of studies, ambient temperatures and air pollutants were derived from grid datasets reporting ambient values rather than individual measurement, potentially leading to exposure misclassifications. For instance, there was a lack of individual adaptive behaviors, such as uncertainty in the use of air conditioner or movement trajectory.58 Due to variations in the diagnosis and treatment capabilities of CHD, seeking care from primary home cities to regions with superior medical capacities is common. However, the case-crossover design may reduce the effect of inter-individual discrepancy. Third, CHD encompasses a broad spectrum of diseases characterized by distinct intracardiac defects, and we could not obtain the specific types of defects because of the limited medical capacity of several medical institutions and the culture of reluctance to autopsy. Thus, subgroup analyses to determine the specific CHD with the highest vulnerability were unavailable. Fourth, surgical repair may directly cause mortality, but we could not identify the details about the surgery in database. Fifth, the impact of Covid-19 pandemic on the results was not identified, while a recent study pointed out that temperature-related mortality exceeded Covid-19 mortality and the pandemic might amplify the effect of temperature on mortality.44
Conclusion
In this nationwide, two-stage, individual-level case-crossover study, we found that cold extremes were significantly associated with CHD mortality. The risks of CHD mortality due to cold extremes increased with the intensity and duration of extreme cold events. Female and pediatric CHD patients were identified as the vulnerable subgroups to cold extremes. This study may provide significant data in pediatric cardiology regarding the impact of environmental exposures that healthcare professionals should suggest CHD patients refrain from exposure to cold extremes. Further multi-country studies are required to substantiate the findings of this research.
Contributors
QH: conceptualization, data curation, data analysis, manuscript drafting, manuscript revision and data verification; XL: data curation, data analysis, manuscript drafting, manuscript revision; HS: conceptualization, manuscript drafting, manuscript revision; JL: data curation and access to the raw data; JW: data curation, manuscript revision; XL: manuscript revision; KM, ZD, YL: investigation; ZZ, MZ, SL: conceptualization, supervision, manuscript revision and responsible for the decision to submit for publication.
Data sharing statement
Data from the National Mortality Surveillance System are publicly unavailable, and other data used in this study could be obtained via reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
All author declared no conflict of interest.
Acknowledgements
We thank all participants contributing to the databases used in this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101244.
Contributor Information
Zhou Zhou, Email: zhouzhou@fuwaihospital.org.
Maigeng Zhou, Email: zhoumaigeng@ncncd.chinacdc.cn.
Shoujun Li, Email: drlishoujunfw@163.com.
Appendix A. Supplementary data
References
- 1.Romanello M., Napoli C.D., Green C., et al. The 2023 report of the Lancet Countdown on health and climate change: the imperative for a health-centred response in a world facing irreversible harms. Lancet. 2023;402(10419):2346–2394. doi: 10.1016/S0140-6736(23)01859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Q., Guo Y., Ye T., et al. Global, regional, and national burden of mortality associated with non-optimal ambient temperatures from 2000 to 2019: a three-stage modelling study. Lancet Planet Health. 2021;5(7):e415–e425. doi: 10.1016/S2542-5196(21)00081-4. [DOI] [PubMed] [Google Scholar]
- 3.IPCC Climate change 2021: the physical science basis. 2021. https://www.ipcc.ch/report/ar6/wg1/downloads/report/IPCC_AR6_WGI_TS.pdf
- 4.Ebi K.L., Capon A., Berry P., et al. Hot weather and heat extremes: health risks. Lancet. 2021;398(10301):698–708. doi: 10.1016/S0140-6736(21)01208-3. [DOI] [PubMed] [Google Scholar]
- 5.Rowell L.B. Cardiovascular aspects of human thermoregulation. Circ Res. 1983;52(4):367–379. doi: 10.1161/01.res.52.4.367. [DOI] [PubMed] [Google Scholar]
- 6.Burkart K.G., Brauer M., Aravkin A.Y., et al. Estimating the cause-specific relative risks of non-optimal temperature on daily mortality: a two-part modelling approach applied to the Global Burden of Disease Study. Lancet. 2021;398(10301):685–697. doi: 10.1016/S0140-6736(21)01700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marelli A.J., Mackie A.S., Ionescu-Ittu R., Rahme E., Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163–172. doi: 10.1161/CIRCULATIONAHA.106.627224. [DOI] [PubMed] [Google Scholar]
- 8.Marelli A.J., Ionescu-Ittu R., Mackie A.S., Guo L., Dendukuri N., Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130(9):749–756. doi: 10.1161/CIRCULATIONAHA.113.008396. [DOI] [PubMed] [Google Scholar]
- 9.Ma K., He Q., Dou Z., et al. Current treatment outcomes of congenital heart disease and future perspectives. Lancet Child Adolesc Health. 2023;7(7):490–501. doi: 10.1016/S2352-4642(23)00076-7. [DOI] [PubMed] [Google Scholar]
- 10.Alahmad B., Khraishah H., Roy√© D., et al. Associations between extreme temperatures and cardiovascular cause-specific mortality: results from 27 countries. Circulation. 2023;147(1):35–46. doi: 10.1161/CIRCULATIONAHA.122.061832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu R., Huang S., Shi C., et al. Extreme temperature events, fine particulate matter, and myocardial infarction mortality. Circulation. 2023;148(4):312–323. doi: 10.1161/CIRCULATIONAHA.122.063504. [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Gao Y., Jiang Y., et al. Low ambient temperature and temperature drop between neighbouring days and acute aortic dissection: a case-crossover study. Eur Heart J. 2022;43(3):228–235. doi: 10.1093/eurheartj/ehab803. [DOI] [PubMed] [Google Scholar]
- 13.Saucy A., Ragettli M.S., Vienneau D., et al. The role of extreme temperature in cause-specific acute cardiovascular mortality in Switzerland: a case-crossover study. Sci Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.147958. [DOI] [PubMed] [Google Scholar]
- 14.Agay-Shay K., Friger M., Linn S., Peled A., Amitai Y., Peretz C. Ambient temperature and congenital heart defects. Hum Reprod. 2013;28(8):2289–2297. doi: 10.1093/humrep/det244. [DOI] [PubMed] [Google Scholar]
- 15.Van Zutphen A.R., Hsu W.H., Lin S. Extreme winter temperature and birth defects: a population-based case-control study. Environ Res. 2014;128:1–8. doi: 10.1016/j.envres.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Auger N., Fraser W.D., Sauve R., Bilodeau-Bertrand M., Kosatsky T. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ Health Perspect. 2017;125(1):8–14. doi: 10.1289/EHP171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S., Lin Z., Ou Y., et al. Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects - a large US population-based, case-control study. Environ Int. 2018;118:211–221. doi: 10.1016/j.envint.2018.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Spero T.L., Nolte C.G., et al. Projected changes in maternal heat exposure during early pregnancy and the associated congenital heart defect burden in the United States. J Am Heart Assoc. 2019;8(3) doi: 10.1161/JAHA.118.010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd R., McMullen H., Beqaj H., Kalfa D. Environmental exposures and congenital heart disease. Pediatrics. 2022;149(1) doi: 10.1542/peds.2021-052151. [DOI] [PubMed] [Google Scholar]
- 20.Zachariah J.P., Jone P.N., Agbaje A.O., et al. Environmental exposures and pediatric cardiology: a scientific statement from the American heart association. Circulation. 2024;149(20):e1165–e1175. doi: 10.1161/CIR.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 21.Su Z., Zou Z., Hay S.I., et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990-2019: an age-period-cohort analysis for the Global Burden of Disease 2019 study. eClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu S., Wu X., Lopez A.D., et al. An integrated national mortality surveillance system for death registration and mortality surveillance, China. Bull World Health Organ. 2016;94(1):46–57. doi: 10.2471/BLT.15.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng X., Adair T., Wang L., et al. Measuring the completeness of death registration in 2844 Chinese counties in 2018. BMC Med. 2020;18(1):176. doi: 10.1186/s12916-020-01632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz Sabater J. ERA5-Land hourly data from 1950 to present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS) 2019. https://doi.org/10.24381/cds.e2161bac (Accessed on 18-11-2023) [Google Scholar]
- 25.Yilmaz M. Accuracy assessment of temperature trends from ERA5 and ERA5-Land. Sci Total Environ. 2023;856(Pt 2) doi: 10.1016/j.scitotenv.2022.159182. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher I.K., Grillet M.E., Moreno J.E., et al. Synergies between environmental degradation and climate variation on malaria re-emergence in southern Venezuela: a spatiotemporal modelling study. Lancet Planet Health. 2022;6(9):e739–e748. doi: 10.1016/S2542-5196(22)00192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halonen J.I., Zanobetti A., Sparrow D., Vokonas P.S., Schwartz J. Outdoor temperature is associated with serum HDL and LDL. Environ Res. 2011;111(2):281–287. doi: 10.1016/j.envres.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei J., Li Z., Lyapustin A., et al. Reconstructing 1-km-resolution high-quality PM2. 5 data records from 2000 to 2018 in China: spatiotemporal variations and policy implications. Rem Sens Environ. 2021;252 [Google Scholar]
- 29.Wei J., Li Z., Li K., et al. Full-coverage mapping and spatiotemporal variations of ground-level ozone (O3) pollution from 2013 to 2020 across China. Rem Sens Environ. 2022;270 [Google Scholar]
- 30.Wei J., Li Z., Wang J., Li C., Gupta P., Cribb M. Ground-level gaseous pollutants (NO2, SO2, and CO) in China: daily seamless mapping and spatiotemporal variations. Atmos Chem Phys. 2023;23(2):1511–1532. [Google Scholar]
- 31.Wei J., Liu S., Li Z., et al. Ground-level NO(2) surveillance from space across China for high resolution using interpretable spatiotemporally weighted artificial intelligence. Environ Sci Technol. 2022;56(14):9988–9998. doi: 10.1021/acs.est.2c03834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smits J., Permanyer I. The subnational human development database. Sci Data. 2019;6 doi: 10.1038/sdata.2019.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Bureau of Statistics of China How to determine human development index. 2023. https://www.stats.gov.cn/zs/tjws/tjjc/202301/t20230101_1903379.html
- 34.Carracedo-Martínez E., Taracido M., Tobias A., Saez M., Figueiras A. Case-crossover analysis of air pollution health effects: a systematic review of methodology and application. Environ Health Perspect. 2010;118(8):1173–1182. doi: 10.1289/ehp.0901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sera F., Gasparrini A. Extended two-stage designs for environmental research. Environ Health. 2022;21(1):41. doi: 10.1186/s12940-022-00853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilker E.H., Yeh G., Wellenius G.A., Davis R.B., Phillips R.S., Mittleman M.A. Ambient temperature and biomarkers of heart failure: a repeated measures analysis. Environ Health Perspect. 2012;120(8):1083–1087. doi: 10.1289/ehp.1104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gasparrini A., Guo Y., Hashizume M., et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet. 2015;386(9991):369–375. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H., Liu G., Han C., Yang Y., Chen R. Quantifying the trends and variations in the frost-free period and the number of frost days across China under climate change using ERA5-land reanalysis dataset. Rem Sens. 2022;14(10):2400. [Google Scholar]
- 39.Huang C., You S., Liu A., Li P., Zhang J., Deng J. High-resolution national-scale mapping of paddy rice based on sentinel-1/2 data. Rem Sens. 2023;15:4055. [Google Scholar]
- 40.Lopez K.N., Morris S.A., Sexson Tejtel S.K., Espaillat A., Salemi J.L. US mortality attributable to congenital heart disease across the lifespan from 1999 through 2017 exposes persistent racial/ethnic disparities. Circulation. 2020;142(12):1132–1147. doi: 10.1161/CIRCULATIONAHA.120.046822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Black R.E., Walker N., Laxminarayan R., Temmerman M. In: 3rd ed. Black R.E., Laxminarayan R., Temmerman M., Walker N., editors. Vol. 2. The International Bank for Reconstruction and Development/The World Bank © 2016 International Bank for Reconstruction and Development/The World Bank; Washington (DC): 2016. Reproductive, maternal, newborn, and child health: key messages of this volume. (Reproductive, Maternal, Newborn, And Child Health: Disease Control Priorities). [Google Scholar]
- 42.Perin J., Mulick A., Yeung D., et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6(2):106–115. doi: 10.1016/S2352-4642(21)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heery E., Sheehan A.M., While A.E., Coyne I. Experiences and outcomes of transition from pediatric to adult health care services for young people with congenital heart disease: a systematic review. Congenit Heart Dis. 2015;10(5):413–427. doi: 10.1111/chd.12251. [DOI] [PubMed] [Google Scholar]
- 44.Lo Y.T.E., Mitchell D.M., Gasparrini A. Compound mortality impacts from extreme temperatures and the COVID-19 pandemic. Nat Commun. 2024;15(1):4289. doi: 10.1038/s41467-024-48207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kephart J.L., Sánchez B.N., Moore J., et al. City-level impact of extreme temperatures and mortality in Latin America. Nat Med. 2022;28(8):1700–1705. doi: 10.1038/s41591-022-01872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uibel D., Sharma R., Piontkowski D., Sheffield P.E., Clougherty J.E. Association of ambient extreme heat with pediatric morbidity: a scoping review. Int J Biometeorol. 2022;66(8):1683–1698. doi: 10.1007/s00484-022-02310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Granbom E., Fernlund E., Sunnegårdh J., Lundell B., Naumburg E. Respiratory tract infection and risk of hospitalization in children with congenital heart defects during season and off-season: a Swedish national study. Pediatr Cardiol. 2016;37(6):1098–1105. doi: 10.1007/s00246-016-1397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hinde K., Lloyd R., Low C., Cooke C. The effect of temperature, gradient, and load carriage on oxygen consumption, posture, and gait characteristics. Eur J Appl Physiol. 2017;117(3):417–430. doi: 10.1007/s00421-016-3531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong J., Lian Z., Zhou X., You J., Lin Y. Investigation of gender difference in human response to temperature step changes. Physiol Behav. 2015;151:426–440. doi: 10.1016/j.physbeh.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 50.Kaciuba-Uscilko H., Grucza R. Gender differences in thermoregulation. Curr Opin Clin Nutr Metab Care. 2001;4(6):533–536. doi: 10.1097/00075197-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Burse R.L. Sex differences in human thermoregulatory response to heat and cold stress. Hum Factors. 1979;21(6):687–699. doi: 10.1177/001872087912210606. [DOI] [PubMed] [Google Scholar]
- 52.Charkoudian N., Stachenfeld N.S. Reproductive hormone influences on thermoregulation in women. Compr Physiol. 2014;4(2):793–804. doi: 10.1002/cphy.c130029. [DOI] [PubMed] [Google Scholar]
- 53.Palm J., Holdenrieder S., Hoffmann G., et al. Predicting major adverse cardiovascular events in children with age-adjusted NT-proBNP. J Am Coll Cardiol. 2021;78(19):1890–1900. doi: 10.1016/j.jacc.2021.08.056. [DOI] [PubMed] [Google Scholar]
- 54.Xu Z., Etzel R.A., Su H., Huang C., Guo Y., Tong S. Impact of ambient temperature on children's health: a systematic review. Environ Res. 2012;117:120–131. doi: 10.1016/j.envres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Li Q., Guo Y., Wei D.M., et al. Does local ambient temperature impact children's blood pressure? A Chinese National Survey. Environ Health. 2016;15:21. doi: 10.1186/s12940-016-0119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Statistics NBo China statistical yearbook. https://www.stats.gov.cn/sj/ndsj/
- 57.Su Z., Zhang Y., Cai X., et al. Improving long-term care and outcomes of congenital heart disease: fulfilling the promise of a healthy life. Lancet Child Adolesc Health. 2023;7(7):502–518. doi: 10.1016/S2352-4642(23)00053-6. [DOI] [PubMed] [Google Scholar]
- 58.Zeger S.L., Thomas D., Dominici F., et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.