Abstract
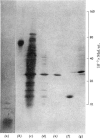
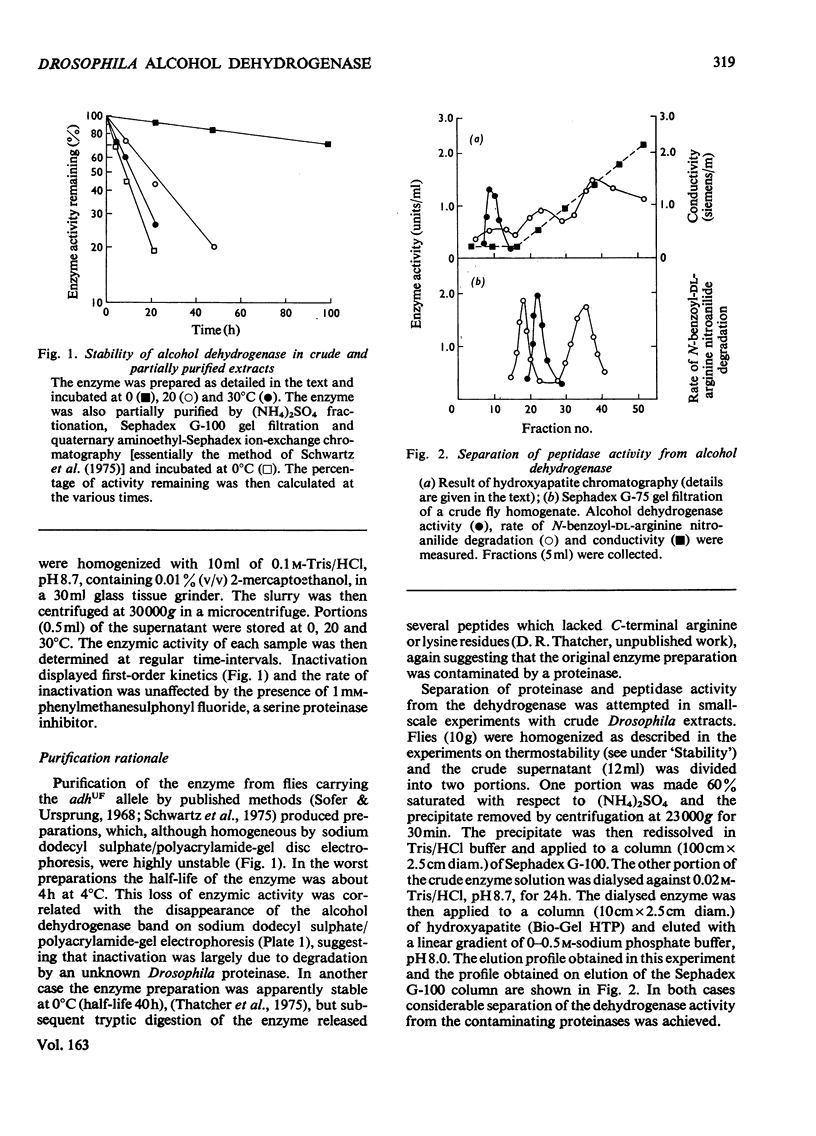
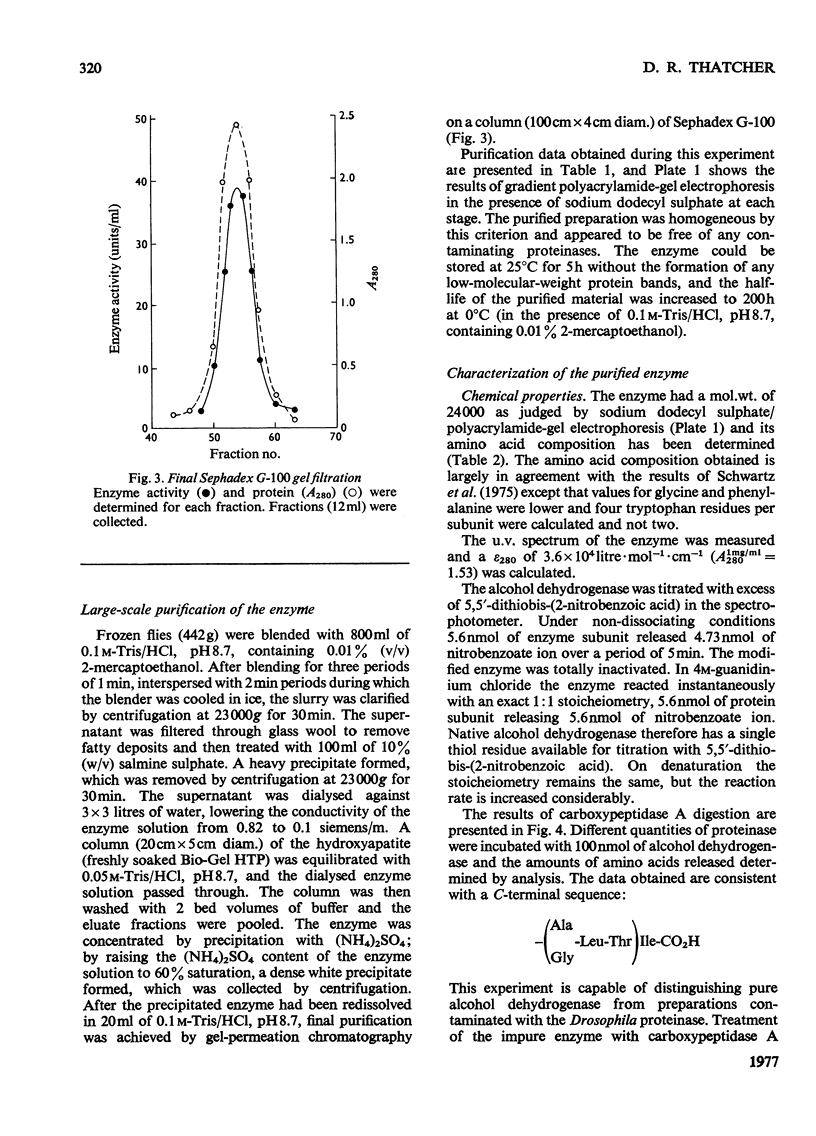
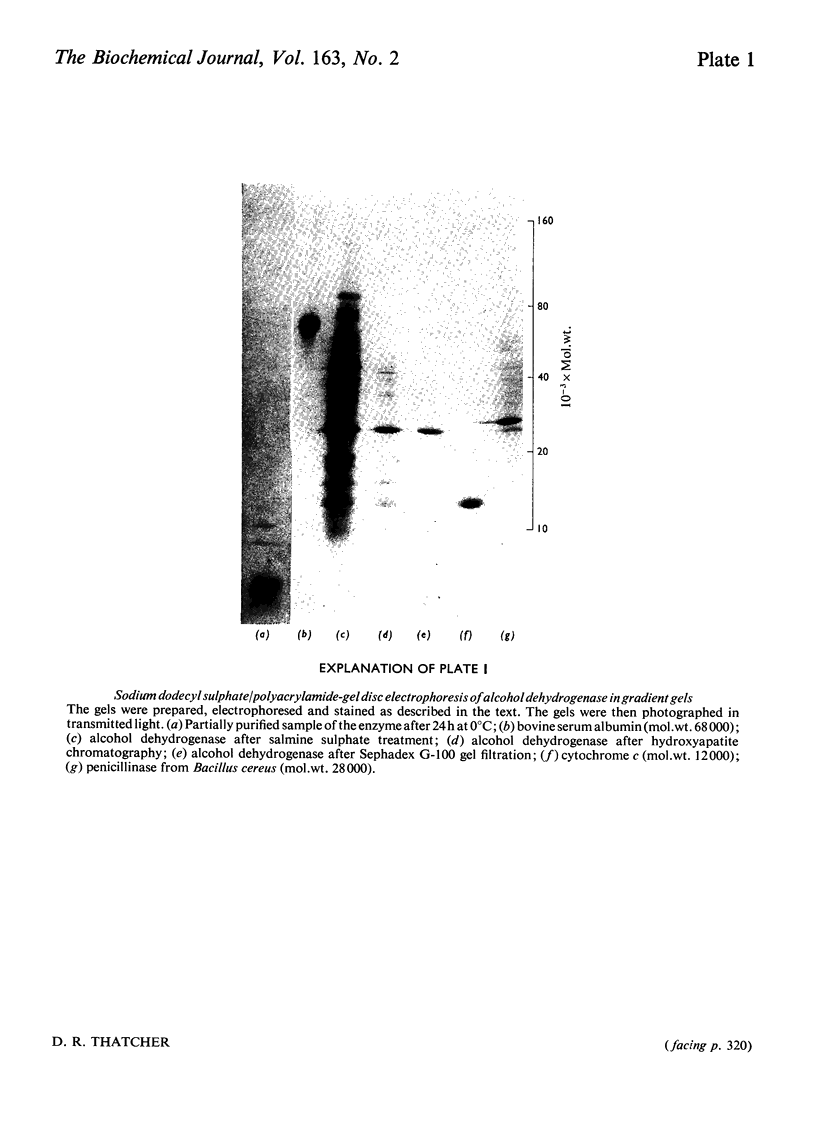
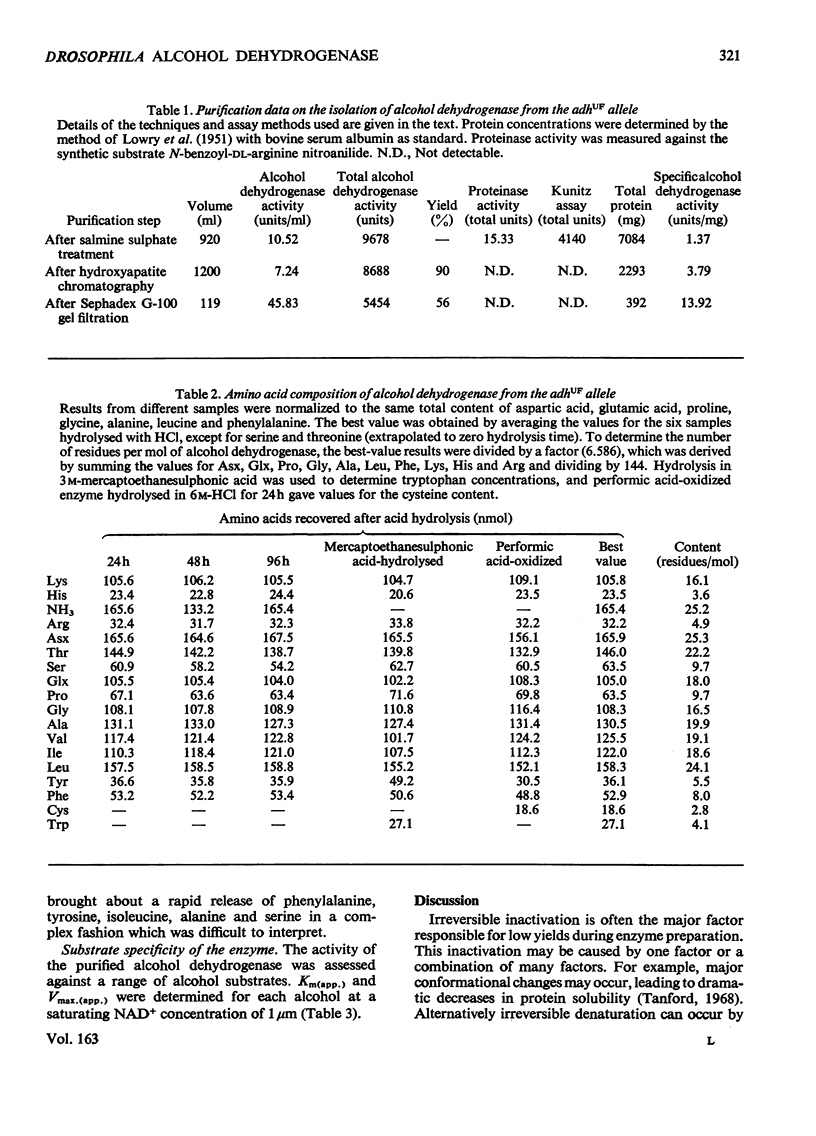
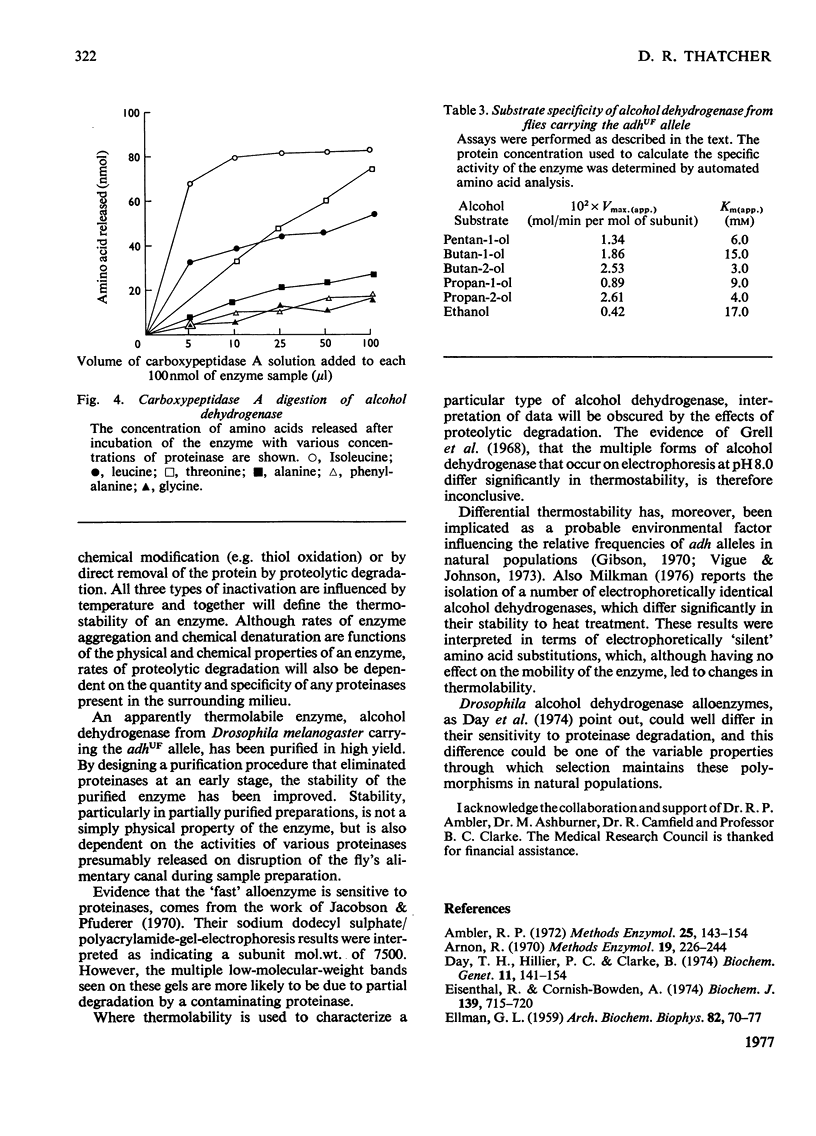
The alcohol dehydrogenase of the Drosophila melanogaster adhUF allele (alloenzyme with ultra-fast electrophoretic mobility) was unstable in crude or partially purified preparations. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis indicated that inactivation was porbably due to proteolytic degradation, and new method of purification of the enzyme was developed. After three steps, namely salmine sulphate precipitation, hydroxyapatite chromatography and Sephadex G-100 gel filtration, a 10-fold purified preparation was obtained. The enzyme produced was relatively stable compared with alcohol dehydrogenase purified by other methods, and was shown to be proteinase-free. The enzyme had a subunit mol.wt. of 24000 and had a single thiol residue per subunit available for titration with 5,5'-dithiobis-(2-nitrobenzoic acid). The amino acid composition and C-terminal amino acid sequence of the enzyme were determined. The substrate specificity of this alcohol dehydrogenase was also characterized. These results are discussed in relation to experiments on the evolutionary significance of thermostability at the adh locus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Day T. H., Hillier P. C., Clarke B. Properties of genetically polymorphic isozymes of alcohol dehydrogenase in Drosophila melanogaster. Biochem Genet. 1974 Feb;11(2):141–153. doi: 10.1007/BF00485770. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. Enzyme flexibility in Drosophila melanogaster. Nature. 1970 Aug 29;227(5261):959–960. doi: 10.1038/227959a0. [DOI] [PubMed] [Google Scholar]
- Grell E. H., Jacobson K. B., Murphy J. B. Alterations of genetics material for analysis of alcohol dehydrogenase isozymes of Drosophila melanogaster. Ann N Y Acad Sci. 1968 Jun 14;151(1):441–455. doi: 10.1111/j.1749-6632.1968.tb11907.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON F. M., DENNISTON C. GENETIC VARIATION OF ALCOHOL DEHYDROGENASE IN DROSOPHILIA MELANOGASTER. Nature. 1964 Nov 28;204:906–907. doi: 10.1038/204906a0. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Pfuderer P. Interconversion of isoenzymes of Drosophila alcohol dehydrogenase. II. Physical characterization of the enzyme and its subunits. J Biol Chem. 1970 Aug 10;245(15):3938–3944. [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Milkman R. Further evidence of thermostability variation within electrophoretic mobility classes of enzymes. Biochem Genet. 1976 Apr;14(3-4):383–387. doi: 10.1007/BF00484776. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Vigue C. L., Johnson F. M. Isozyme variability in species of the genus Drosophila. VI. Frequency-property-environment relationships of allelic alcohol dehydrogenases in D. melanogaster. Biochem Genet. 1973 Jul;9(3):213–227. doi: 10.1007/BF00485735. [DOI] [PubMed] [Google Scholar]