Abstract
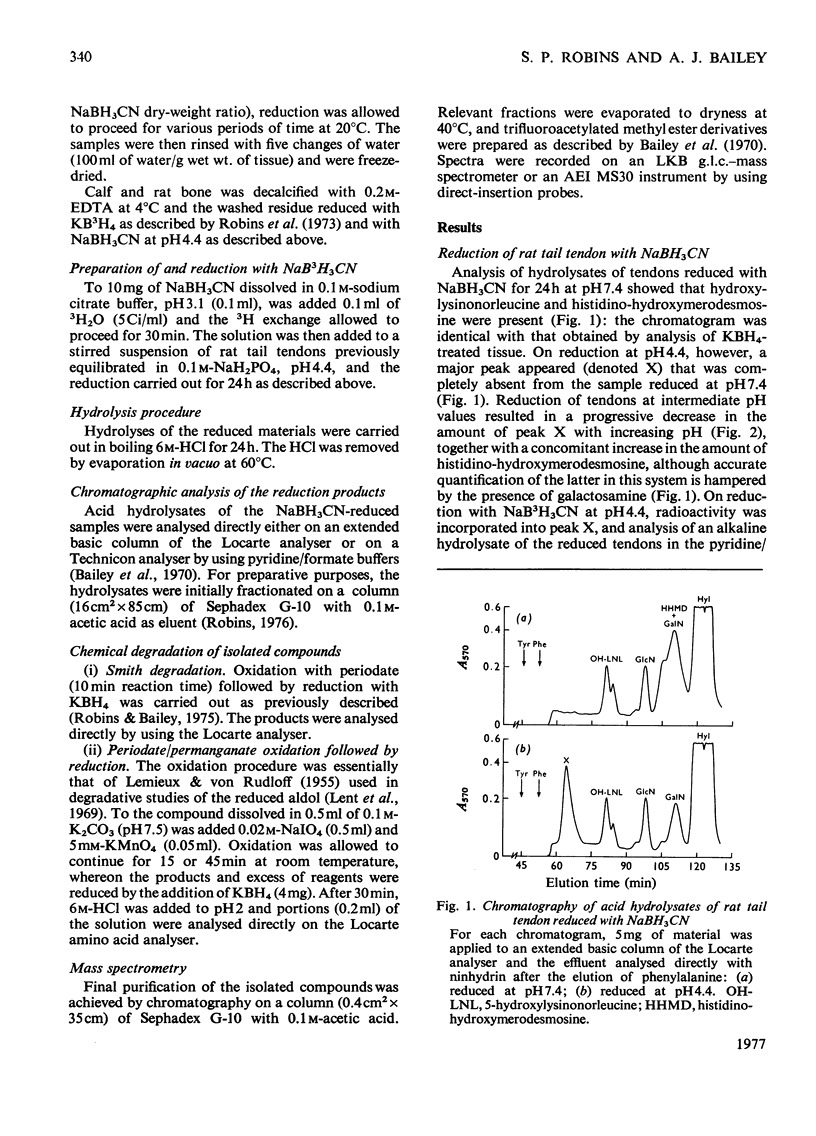
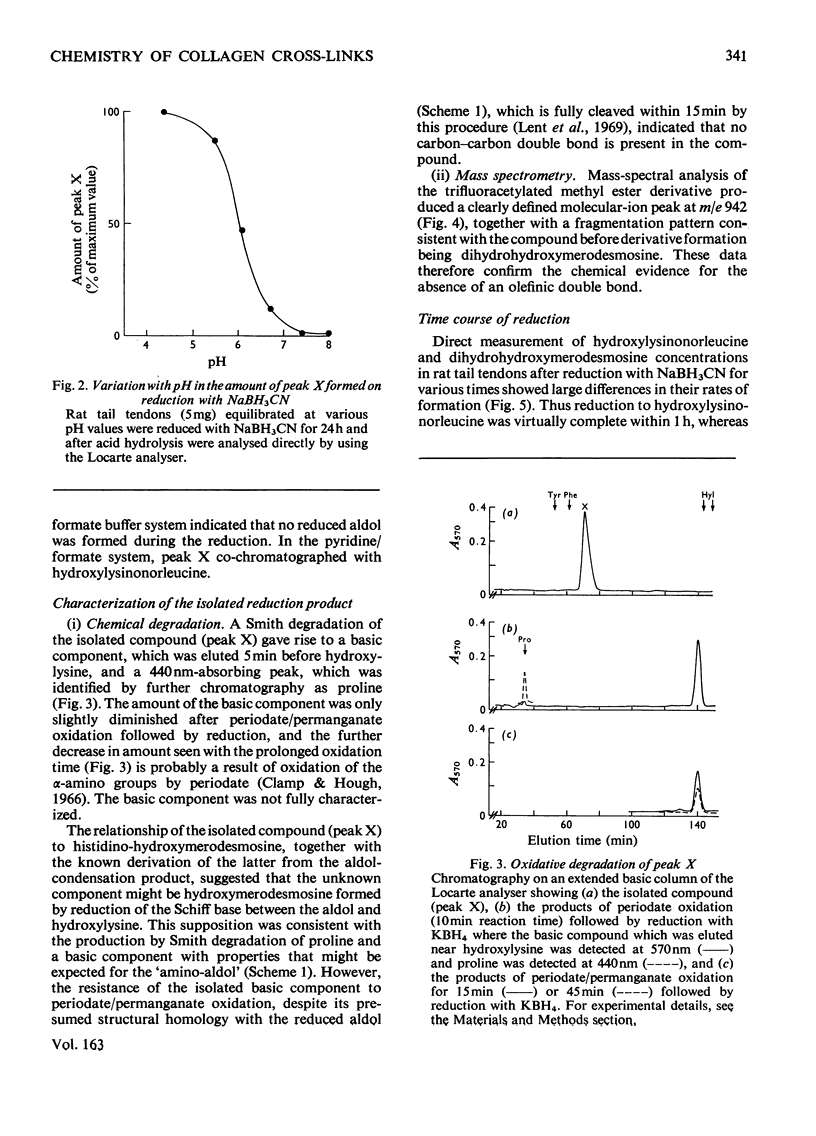
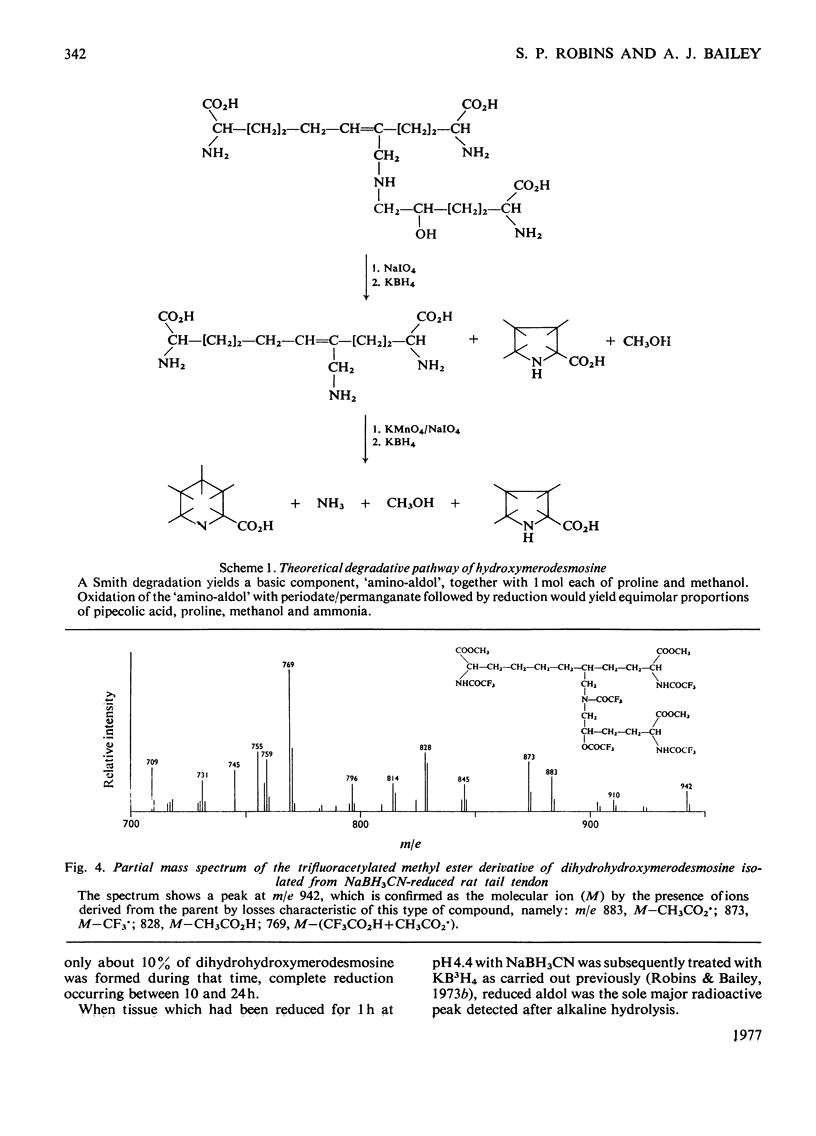
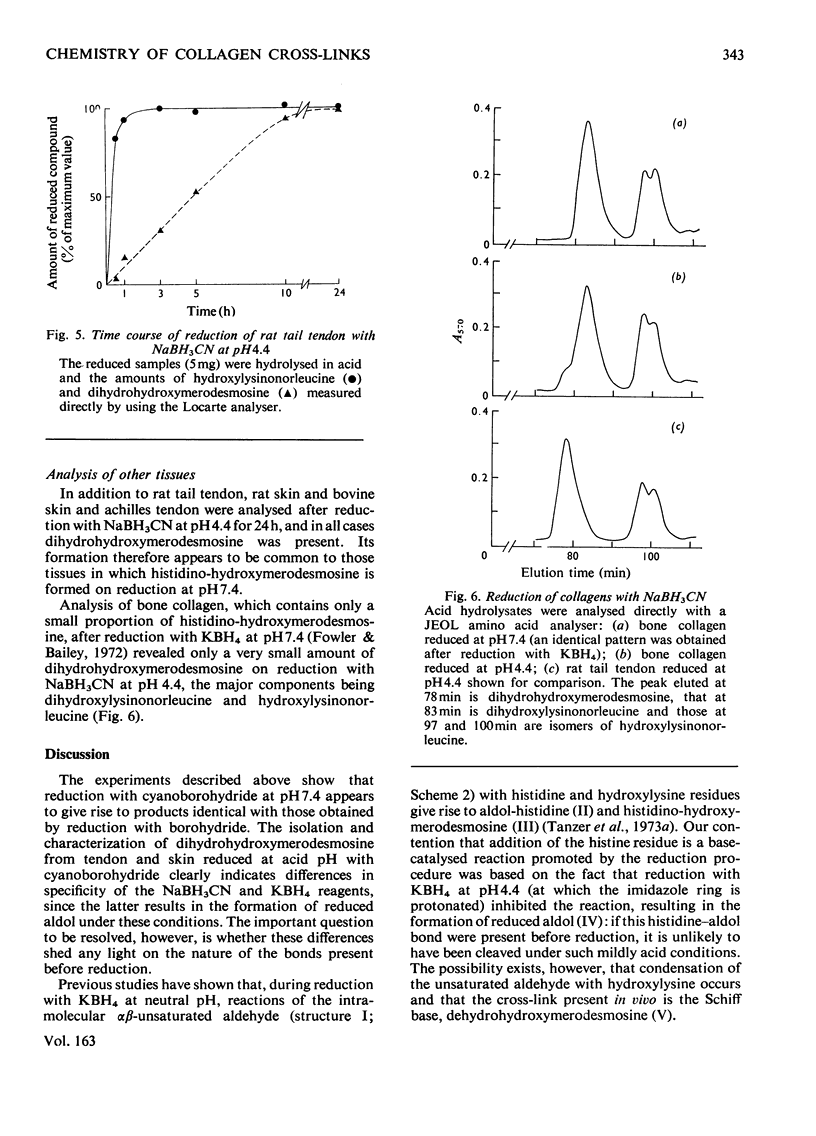
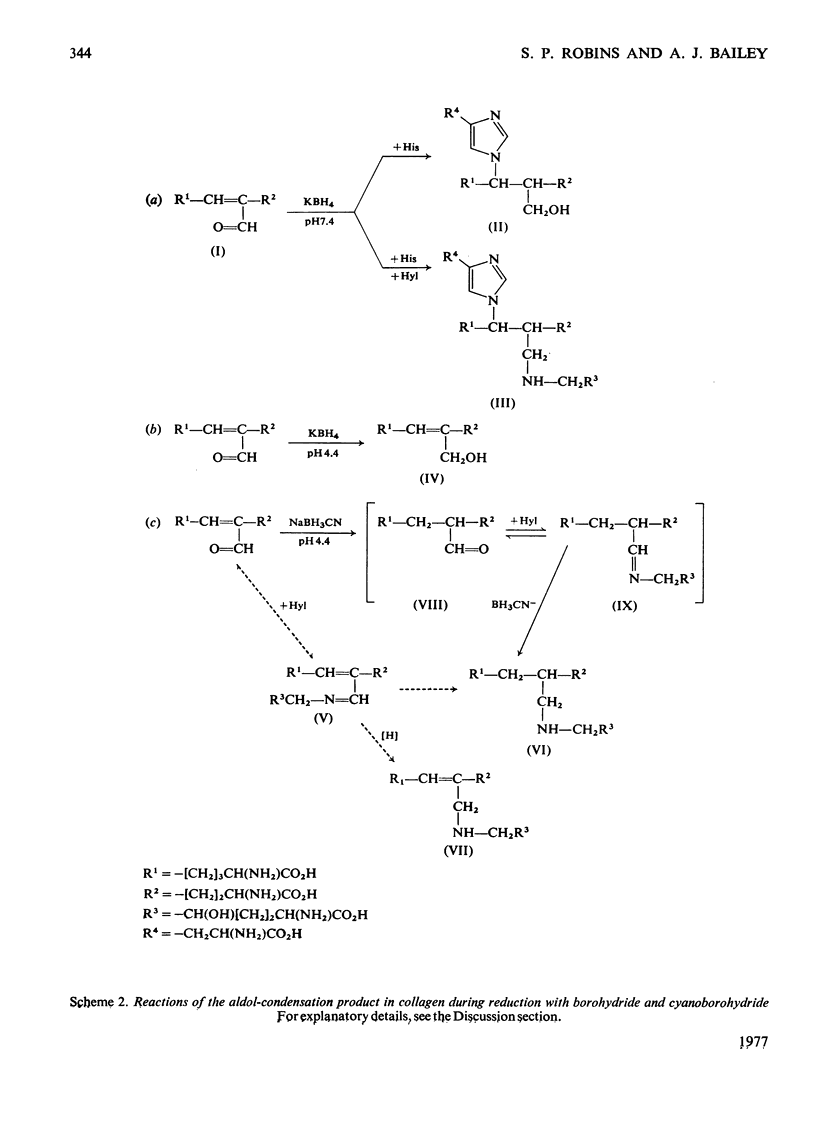
Reduction of tissues with sodium cyanoborohydride at pH7.4 gave results identical with those obtained by KBH4 treatment. On reduction with sodium cyanoborohydride at pH 4.4, however, a previously undetected basic compound was formed and was identified by mass spectrometry and chemical degradation techniques as dihydrohydroxymerodesmosine. Histidino-hydroxymerodesmosine was not present, and further analysis confirmed that reduced aldol, a mojor product of reduction with KBH4 at the lower pH, was also absent. These results, together with an analysis of the time course of the reduction, support previous assertions that histidino-hydroxymerodesmosine is an artifact [robins *Bailey (1973) Biochem. J. 135, 657-665] and suggests that the non-reduced form of hydroxymerodesmosine probably does not constitute a major intermolecular bond in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J. Intermediate labile intermolecular crosslinks in collagen fibres. Biochim Biophys Acta. 1968 Aug 13;160(3):447–453. doi: 10.1016/0005-2795(68)90216-x. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Robins S. P., Balian G. Biological significance of the intermolecular crosslinks of collagen. Nature. 1974 Sep 13;251(5471):105–109. doi: 10.1038/251105a0. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Hough L. Some observations on the periodate oxidation of amino compounds. Biochem J. 1966 Oct;101(1):120–126. doi: 10.1042/bj1010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. R., Bailey A. J. Chemical synthesis of the reduced form of an intermolecular crosslink of collagen: a re-evaluation of the structure of syndesine. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1416–1422. doi: 10.1016/0006-291x(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Analysis of a crosslinked peptide from calf bone collagen: evidence that hydroxylysyl glycoside participates in the crosslink. Biochem Biophys Res Commun. 1973 May 15;52(2):663–671. doi: 10.1016/0006-291x(73)90764-x. [DOI] [PubMed] [Google Scholar]
- Fowler L. J., Bailey A. J. Current concepts of the crosslinking in bone collagen. Clin Orthop Relat Res. 1972;85:193–206. doi: 10.1097/00003086-197206000-00035. [DOI] [PubMed] [Google Scholar]
- Lent R. W., Smith B., Salcedo L. L., Faris B., Franzblau C. Studies on the reduction of elastin. II. Evidence for the presence of alpha-aminoadipic acid delta-semialdehyde and its aldol condensation product. Biochemistry. 1969 Jul;8(7):2837–2845. doi: 10.1021/bi00835a022. [DOI] [PubMed] [Google Scholar]
- Masuda M., Karube S., Hayashi Y., Shindo H., Igarashi M. Direct measurement of collagen crosslinks with automatic amino acid analyzer-identification of peaks due to crosslinks. FEBS Lett. 1976 Apr 1;63(2):245–249. doi: 10.1016/0014-5793(76)80104-4. [DOI] [PubMed] [Google Scholar]
- Mechanic G., Gallop P. M., Tanzer M. L. The nature of crosslinking in collagens from mineralized tissues. Biochem Biophys Res Commun. 1971 Nov 5;45(3):644–653. doi: 10.1016/0006-291x(71)90465-7. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Robertson P. B. The stability of collagen cross-links when derived from hydroxylsyl residues. Biochem Biophys Res Commun. 1973 Sep 5;54(1):432–439. doi: 10.1016/0006-291x(73)90940-6. [DOI] [PubMed] [Google Scholar]
- Paz M. A., Henson E., Blumenfeld O. O., Seifter S., Gallop P. M. Dehydromerodesmosine and merodesmosine in elastin. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1518–1523. doi: 10.1016/s0006-291x(71)80258-9. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. Relative stabilities of the intermediate reducible crosslinks present in collagen fibres. FEBS Lett. 1973 Jul 1;33(2):167–174. doi: 10.1016/0014-5793(73)80184-x. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. The chemistry of the collagen cross-links. The characterization of fraction C, a possible artifact produced during the reduction of collagen fibres with borohydride. Biochem J. 1973 Dec;135(4):657–665. doi: 10.1042/bj1350657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Bailey A. J. The chemistry of the collagen cross-links. The mechanism of stabilization of the reducible intermediate cross-links. Biochem J. 1975 Aug;149(2):381–385. doi: 10.1042/bj1490381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer M. L. Cross-linking of collagen. Science. 1973 May 11;180(4086):561–566. doi: 10.1126/science.180.4086.561. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Fairweather R., Gallop P. M. Isolation of the crosslink, hydroxymerodesmosine, from borohydride-reduced collagen. Biochim Biophys Acta. 1973 May 17;310(1):130–136. doi: 10.1016/0005-2795(73)90016-0. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L., Housley T., Berube L., Fairweather R., Franzblau C., Gallop P. M. Structure of two histidine-containing crosslinks from collagen. J Biol Chem. 1973 Jan 25;248(2):393–402. [PubMed] [Google Scholar]


